Continuing Education Activity
Asystole, colloquially referred to as "flatline," represents the cessation of electrical and mechanical cardiac activity. Consequently, blood flow to other vital organs also stops. The condition typically occurs as a deterioration of the initial nonperfusing ventricular rhythms, ventricular fibrillation and pulseless ventricular tachycardia. Without prompt treatment, asystole can quickly lead to irreversible brain damage and death. Management typically involves advanced cardiac life support protocols, including cardiopulmonary resuscitation, administration of fluids and medications like epinephrine, and advanced airway management.
This course aims to equip healthcare professionals with the necessary skills and knowledge to effectively evaluate and manage this life-threatening condition. Participants explore the causes, pathophysiology, clinical presentation, and electrocardiogram findings associated with asystole, enabling them to recognize and respond to this critical situation promptly. Collaboration within an interprofessional team is emphasized throughout the course, highlighting the importance of coordinated care in optimizing patient outcomes during asystole management. By working closely with emergency physicians, cardiologists, nurses, and other healthcare professionals, clinicians gain valuable insights into multidisciplinary approaches for addressing the complexities of asystole.
Objectives:
Differentiate between asystole and other cardiac dysrhythmias, such as ventricular fibrillation or pulseless ventricular tachycardia, through electrocardiogram interpretation and clinical assessment, to tailor appropriate management strategies.
Identify the clinical signs and symptoms indicative of asystole, including the absence of palpable pulses, unresponsiveness, and lack of breathing, in order to initiate timely intervention.
Screen patients at risk of developing asystole, including those with a history of cardiovascular disease, electrolyte imbalances, or drug toxicity, to facilitate early detection and intervention.
Implement effective collaboration and communication among interprofessional team members to improve outcomes for patients in asystole.
Introduction
Asystole Overview
Asystole, informally referred to as "flatline," signifies a complete cessation of the heart's electrical and mechanical activity.[1] The condition frequently begins as a nonperfusing ventricular dysrhythmia, specifically ventricular fibrillation or pulseless ventricular tachycardia (pVT).[2] Pulseless electrical activity (PEA) may likewise progress to asystole.[3] Those with sudden cardiac arrest presenting with asystole as the initial rhythm have an extremely poor prognosis. Return of spontaneous circulation (ROSC) is less often achieved when asystole is the initial cardiac rhythm than a shockable rhythm after out-of-hospital cardiac arrest (OHCA).[4] Patients with asystole as the initial cardiac rhythm after OHCA are less likely to survive after 30 days than if other rhythms are initially detected.[5] Asystole represents the terminal rhythm of a cardiac arrest.
Prolonged resuscitation efforts in a patient in asystole are unlikely to provide a medical benefit in OHCA. Termination of resuscitation efforts should be considered for such individuals in consultation with online medical direction, as local protocols allow. The American College of Emergency Physicians and the National Association of Emergency Medical Services Physicians support emergency medical services protocols allowing providers to cease resuscitation efforts in cases where continued interventions and hospital transport offer no chance of patient survival.[6]
The Heart's Electrical Circuity
Like a complex circuit, the heart's electrical conduction system coordinates the spread of electrical impulses to initiate muscle contractions and propel blood. The sinoatrial node in the right atrium acts as the natural pacemaker, initiating the electrical impulse. This impulse travels through internodal pathways to reach the atrioventricular node lying between the atria and ventricles. The atrioventricular node delays the signal for coordinated atrial contraction before ventricular activation. The impulse is then transmitted through the His bundle, a muscular bridge that splits into right and left bundle branches. These branches further divide into Purkinje fibers, which spread the electrical wavefront throughout the ventricles, triggering synchronized contraction of the heart chambers and blood expulsion.
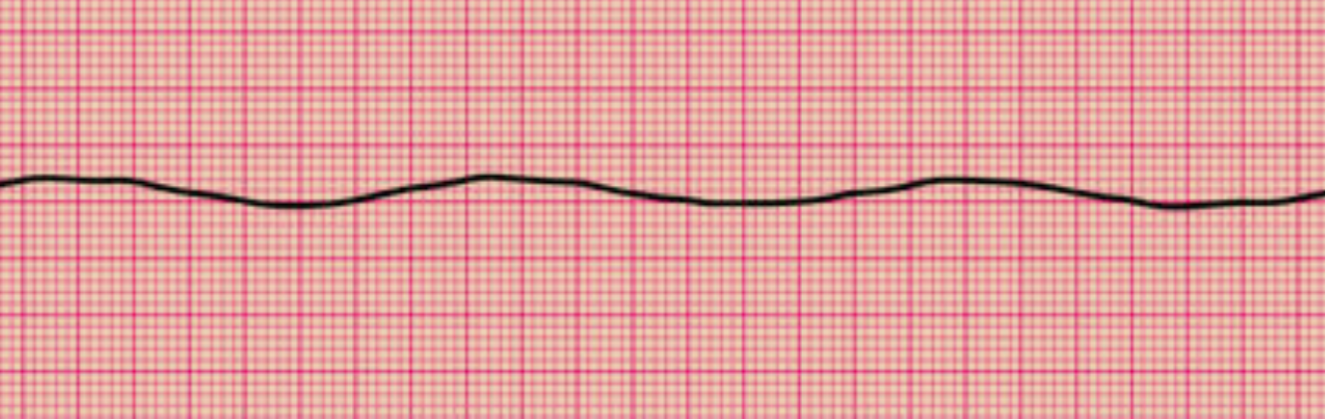
Myocardial voltage-gated channels involved in cardiac pacing include sodium, potassium, and calcium channels. Sodium channels initiate the depolarization phase of the cardiac action potential. Sodium channel activity corresponds to ventricular contraction and the QRS complex on the electrocardiogram (ECG). Potassium channels contribute to repolarization, helping to restore the resting membrane potential. Potassium channel activation corresponds to ventricular relaxation and the T wave on ECG. Calcium channels are involved in both depolarization and repolarization phases and in regulating intracellular calcium levels, which are essential for excitation-contraction coupling and myocardial contraction. Ventricular calcium channel activity contributes to the action potential's plateau phase and the QRS complex. Similar voltage channels exist in the atria. Atrial depolarization is responsible for the P wave on ECG (see Image. Normal Sinus Rhythm on Electrocardiography).[7]
The heart's electrical conduction system is disrupted in cardiac dysrhythmias. This disruption could be due to various factors, including severe ischemia with subsequent myocardial death, electrolyte imbalances hindering electrical flow, or direct myocardial damage from trauma or toxins. The consequence is that the electrical wavefront fails to propagate through the system, leaving the heart in a state of complete electrical and mechanical silence.[8]
Etiology
The causes of asystole in cardiac arrest are wide and varied. Asystole typically results from decompensation of prolonged ventricular fibrillation arrest. Attempted defibrillation of ventricular tachycardia or ventricular fibrillation can also precipitate asystole. However, any cause of cardiac arrest may eventually result in asystole if not promptly treated. Reversible causes must be considered when asystole is the initial cardiac rhythm. The "Hs and Ts" is a useful mnemonic taught in advanced cardiac life support training for quick recollection of the reversible causes of cardiac arrest. The Hs include hypovolemia, hypoxia, hydrogen ion (acidosis), hypokalemia or hyperkalemia, and hypothermia. The Ts include tension pneumothorax, tamponade (cardiac), toxins, and thrombosis (both pulmonary and coronary). These conditions should be immediately treated once identified.[9]
Epidemiology
Each year, over 350,000 out-of-hospital cardiac arrests (OHCAs) occur in the United States, and mortality is extremely high. Data vary in different regions of the country and across studies. Post-OHCA survival remains poor, with less than 23% reaching hospital admission and only 10% surviving to discharge. A Cardiac Arrest Registry to Enhance Survival (CARES) study involving 764 emergency medical services agencies identified 258,342 OHCAs from 2015 to 2019. Asystole was this cohort's most frequently recorded initial rhythm at 51.6%.[10] CARES 2022 data reveal that, of all presenting arrest rhythms, asystole has the lowest rate of survival to hospital admission (16%, compared to 45% in shockable rhythms) and the chance of the patient being discharged alive (2%, compared to 27% in shockable rhythms). (CARES. Survival Outcomes)
By comparison, the yearly incidence of in-hospital cardiac arrests (IHCAs) in the United States was reported to be 300,000. Shockable rhythms comprised only 15.3% of these cases, with nonshockable rhythms, including asystole, constituting the rest. Survival post-IHCA increased from 21.3% to 32.7% between 1999 and 2018. This trend is attributed chiefly to the pulseless electrical activity (PEA)-asystole survival improvement from 18.9% to 30.2% and, to some degree, increased survival after a shockable arrest rhythm, which rose from 29.8% to 39.7%. IHCA survival with shockable rhythms was seen to level off from 2014 to 2018, while PEA-asystole survival slightly increased. These trends are thought to be due to improved resuscitation techniques, wider use of guideline-directed interventions, prompt revascularization if warranted, designated cardiac arrest teams, and protocolized strategies for resuscitative care.[10][11]
Pathophysiology
Asystole arises from disruptions in the heart's electrical conduction system. As mentioned, the condition's etiologies are varied and may be intrinsic or extracardiac. Intrinsic causes include ischemia, cardiac channelopathies, and cardiac disorders that usually lead to an immediate no-flow state like valvular heart disease, myocarditis, hypertensive hypertrophy, and nonischemic cardiomyopathies (dilated, infiltrative, and hypertrophic). Extracardiac causes include the Hs and Ts previously mentioned. Deterioration of a ventricular arrhythmia to asystole is more likely in intrinsic cardiac cases. Progression from bradyarrhythmia or PEA to asystole more commonly arises from decompensation from an extracardiac condition, such as asphyxia and trauma.[11][12] Asystole occurs when the heart fails to generate and propagate normal electrical impulses, resulting in the cessation of effective cardiac contractions and circulation.
History and Physical
Asystole manifests as cardiac arrest, the findings of which are straightforward. A patient in cardiac arrest is unresponsive to all stimuli and is without spontaneous breathing or a palpable pulse. The American Heart Association recommends conducting a quick primary survey to begin evaluating a patient in cardiac arrest. The rescuer should check for any airway obstructions and inspect visually whether the patient is breathing normally. Patients in cardiac arrest may have agonal breathing (gasping) rather than apnea. When assessing circulation, the carotid or brachial pulse may be palpated for no more than 10 seconds. Cardiopulmonary resuscitation (CPR) should be initiated immediately if no pulse is appreciated (see Treatment/Management).
Resuscitating an individual in cardiac arrest necessitates an interprofessional approach. A team member should investigate for reversible causes while the rest are administering basic or advanced cardiac life support. Cardiac causes predominate in older individuals. However, other etiologies must also be considered because as much as 20% to 40% of OHCAs arise from noncardiac pathologies. Providers must have a low threshold for diabetic ketoacidosis, chronic obstructive pulmonary disease, drug toxicity, electrolyte imbalance, and thromboembolism in this cohort.
Noncardiac etiologies are much more frequent in younger patients. Airway obstruction, drowning, trauma, hemorrhage, poisoning, drug overdose, electrocution, and severe dehydration are common. However, hypertrophic cardiomyopathy and channelopathies can cause sudden cardiac arrest in predisposed young individuals.
Information from the patient's caregivers or companions may be able to provide clues about the possible etiology of cardiac arrest. For example, metabolic acidosis from end-stage renal disease must be suspected in an individual with longstanding, poorly controlled diabetes or lupus. Young children may be victims of abuse or neglect in unsafe homes. Although rare, severe decompression sickness must be considered in people with a recent history of prolonged, deep dives, flying in a depressurized aircraft, or space travel. Read StatPearls' article on Decompression Sickness.
A quick physical examination may still be performed on patients in cardiac arrest while resuscitation is ongoing. The examiner may proceed by organ system to look for clues. Head and neck examination may reveal signs of a thyroid storm, traumatic brain injury, tension pneumothorax (tracheal deviation), or asphyxia. Liver disease (fetor hepaticus), methanol poisoning (alcoholic odor), and diabetic ketoacidosis (fruity smell) manifest with characteristic breath odors.
Chest examination may reveal subcutaneous emphysema, usually associated with pneumothorax or pneumomediastinum. A barrel chest or Pickwickian habitus may be clues to a chronic respiratory disease. Abdominal varices, large hernias, and infected surgical wounds may be found on abdominal examination. Genitourinary obstruction, eg, from benign or malignant tumors, may cause urosepsis, renal impairment, and cardiac arrest. Skin and extremity examination may provide physical indicators of cardiac, pulmonary, hematologic, oncologic, thromboembolic, drug-related, or traumatic pathology.
Evaluation
Asystole is identified on cardiac monitoring. The cardiac monitor should show a flatline, meaning the tracings lack P, QRS, and T waves (see Image. Asystole on Electrocardiography). Individuals who temporarily respond to sympathomimetics during resuscitation may occasionally exhibit residual electrical activity, but a significant segment of a 30-second strip often shows flatlining in these patients. People being resuscitated must be linked to a pulse oximeter and cardiac monitor for monitoring vital signs and documenting ROSC.
The following quick tests are helpful when investigating the cause of asystole for possible reversal:
- Echocardiogram: Identifies shockable rhythms, electrolyte imbalance, and cardiac pathology that may have precipitated cardiac arrest
- Capillary blood glucose: Helps detect abnormal glucose levels
- Complete blood count: May show signs of sepsis, bloodborne infections, hemorrhage, and hematologic malignancies
- Complete metabolic panel: Helps assess electrolyte levels and renal and hepatic function
- Coagulation profile: Detects coagulopathy that may contribute to the condition
- Arterial blood gases: For detection and possible classification of acidosis
- Chest and abdominal ultrasound: Can identify cardiac, pulmonary, and abdominal pathology; focused assessment with sonography in trauma (FAST) may be used in acute traumatic settings
Most blood specimens may be obtained during intravenous cannulation. Blood glucose may be determined using a rapid fingerstick test. Quick identification and treatment of asystole's underlying cause may give the patient the best chance of survival.
Treatment / Management
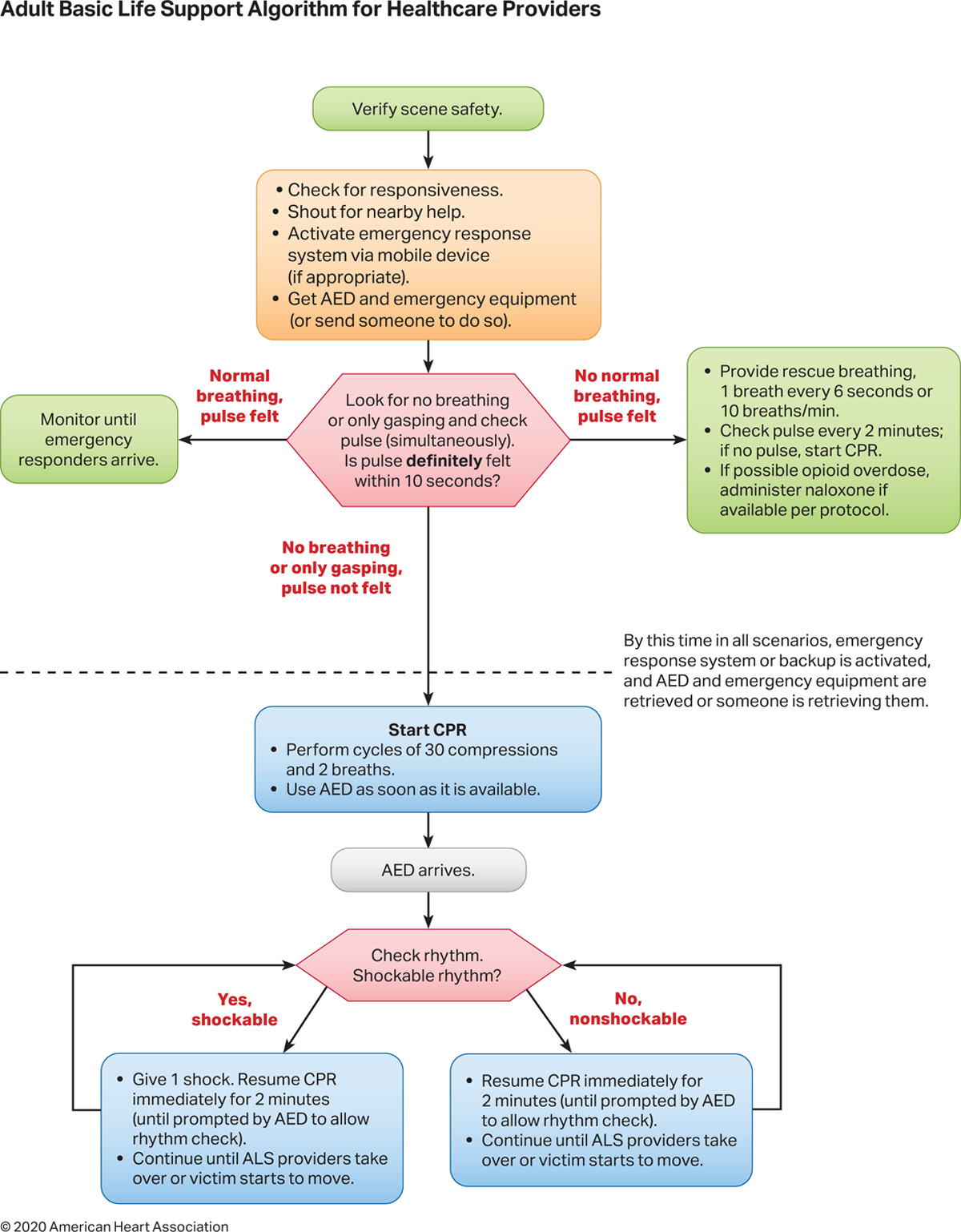
Asystole should be treated following the current American Heart Association BLS and ACLS guidelines. High-quality cardiopulmonary resuscitation (CPR) is the mainstay of treatment and the most important predictor of a favorable outcome. In the 2020 BLS guidelines, the steps in managing cardiac arrest are quickly performed in the following order (see Image. Adult Basic Life Support Algorithm for Healthcare Providers 2020):
- Swiftly surveying the scene for signs that the rescuer should not attempt resuscitation
- Checking for the person's responsiveness
- Shouting for help if no response was elicited from the patient
- Activating the emergency response system, which may be performed simultaneously with the initiation of chest compressions
- Calling for a defibrillator
- Assessing the individual's breathing pattern
- Rapidly evaluating circulation, ie, by palpating for pulses
- Opening the airway using noninvasive techniques if the patient has a pulse. Rescue breaths, 1 every 6 seconds, should be given if the patient has a pulse.
- If the patient is pulseless, initiate high-quality chest compressions (5 cm deep at a rate of 100-120/minute in adults). One CPR cycle consists of 30 compressions and 2 breaths. CPR is performed until an automated external defibrillator (AED) becomes available or the patient is revived.
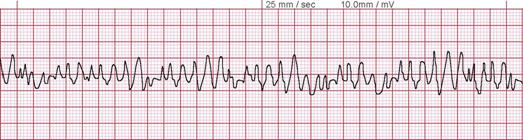
- Use an AED to evaluate the heart rhythm. Defibrillatory shocks are delivered if the cardiac rhythm is shockable, ie, ventricular fibrillation or pVT (see Images. Ventricular Fibrillation and Monomorphic Ventricular Tachycardia on Electrocardiography). A rhythm check must be performed every 2 minutes.
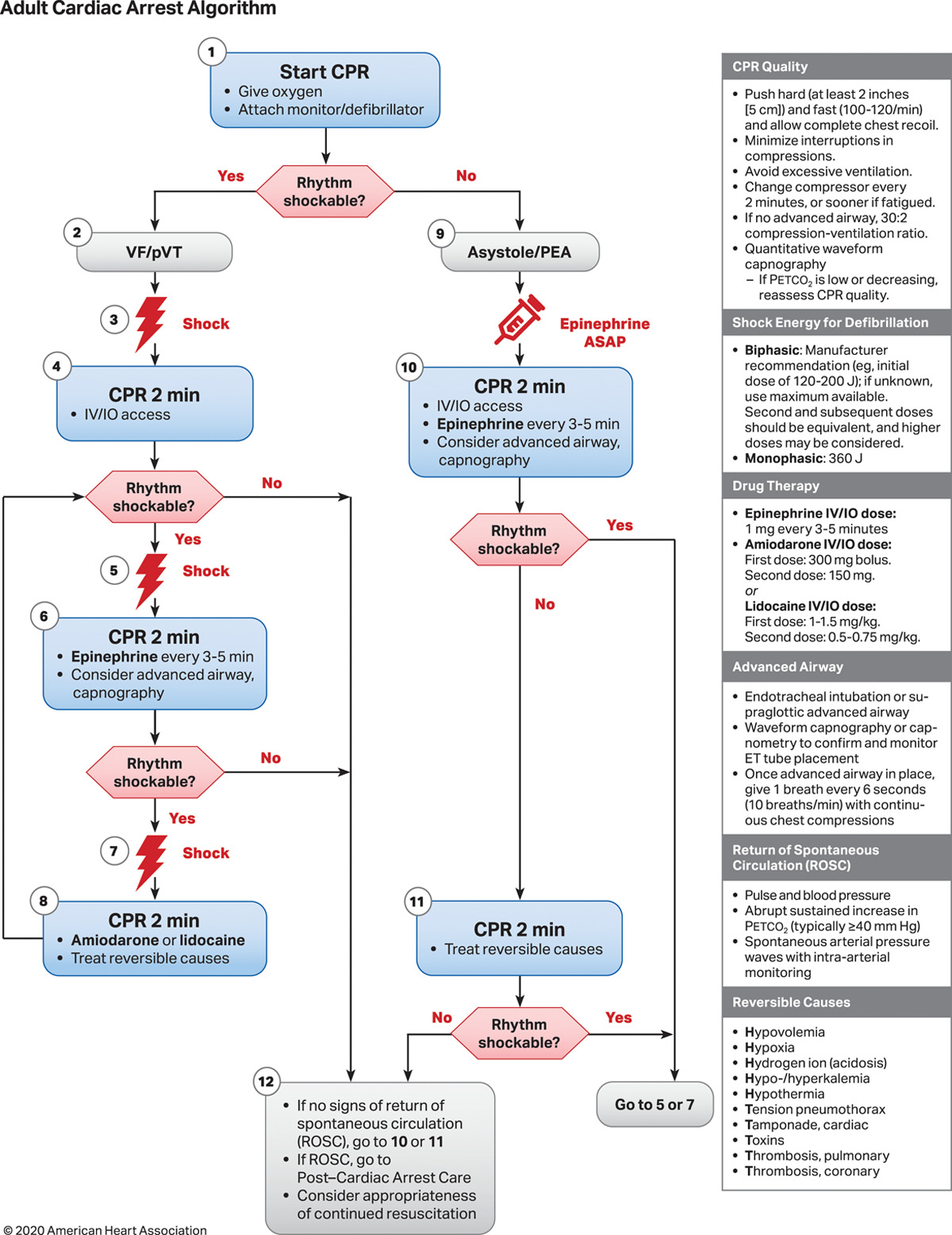
Asystole is a nonshockable rhythm. Therefore, defibrillation should not be attempted if asystole is noted on the cardiac monitor and confirmed on at least 2 leads. High-quality CPR must continue with minimal (<5 seconds) interruption until ACLS is activated, the patient starts moving, or ROSC is documented. CPR should not stop even for endotracheal intubation. Advanced life support should be considered after 2 minutes of performing high-quality CPR without ROSC (see Image. Adult Cardiac Arrest Algorithm 2020). The following are the steps in the ACLS guidelines:
- If asystole persists after 2 minutes of high-quality CPR, intravenous or intraosseous access must be established, and epinephrine must be given through either route 1 mg every 3 to 5 minutes.
- An advanced airway with capnography must be considered if the patient remains unresponsive. The endotracheal route is an alternative for administering medications if intravenous or intraosseous access cannot be established.
- Reversible causes must be treated.
- Give shocks if the rhythm is converted to a shockable rhythm.
- Consider amiodarone or lidocaine if defibrillation is ineffective.
Patients who demonstrate and sustain ROSC must be monitored in the intensive care unit. Hypothermia induction must be considered in all patients who survive cardiac arrest. Clinical trials have not demonstrated improved patient outcomes when administering transcutaneous pacing and vasopressin. Asystole is considered a terminal rhythm of cardiac arrest. Therefore, discussion of termination of resuscitation should be considered during an in-hospital cardiac arrest in the appropriate clinical picture. Out-of-hospital cardiac arrest patients in asystole should also be considered for the cessation of efforts according to local protocol.[13]
Differential Diagnosis
In terms of the presenting cardiac rhythm, the differential diagnosis of asystole includes PEA, ventricular fibrillation, and pVT. These rhythms are managed according to the BLS and ACLS guidelines outlined above, and the underlying cause should be treated as soon as possible. In terms of etiology, various conditions should be considered, including but not limited to the following:
- Hs and Ts: hypovolemia, hypoxia, acidosis, hypokalemia, hyperkalemia, hypothermia, tension pneumothorax, cardiac tamponade, toxins, coronary or pulmonary thrombosis
- Cardiovascular pathology: myocardial infarction, fatal arrhythmias, valvular heart disease, congenital heart disease, hypertrophic cardiomyopathy, aortic dissection
- Pulmonary disease: chronic obstructive pulmonary disease, asthma, aspiration
- Endocrine conditions: adrenal insufficiency, diabetic ketoacidosis, thyrotoxicosis
- Trauma: pneumomediastinum, traumatic brain injury, severe hemorrhage, drowning
- Gastrointestinal disorders: perforated ulcers, gut obstruction, advanced cirrhosis, aortoenteric fistula [14]
- Renal disease: end-stage renal disease, drug-induced nephritis
- Drugs and toxins: β-blockers, quinidine, digoxin, opioids, organophosphates, barbiturates, benzodiazepines, metformin, alcohol, insulin, drugs of abuse like methamphetamine
- Advanced malignancies: lung cancer, breast cancer, brain tumors
- Immune disorders: anaphylaxis, complications of autoimmune disorders like rheumatoid arthritis, polymyositis, and lupus [15]
- Other electrolyte derangements: hypomagnesemia, hypocalcemia
- Infection: pressure ulcers, severe gastroenteritis, pneumonia, urinary tract infections, infectious endocarditis, meningitis, encephalitis
In terms of presentation, the following conditions should be considered:
- Seizure
- Respiratory depression
- Syncope
- Hemorrhagic stroke
- Neuromuscular disorders
- Psychiatric conditions, eg, catatonia and malingering
Despite the limitations, a good clinical investigation and appropriate diagnostic testing can help the interprofessional team diagnose asystole, differentiate it from other conditions, and possibly treat its underlying cause.
Prognosis
Asystole's prognosis depends on several factors, the most important of which are the quality and timeliness of resuscitation. The underlying cause is also an important prognostic determinant, as extracardiac etiologies often have better outcomes than intrinsic cardiac causes, especially if reversed promptly. Age and cardiac function also impact prognosis, as younger individuals with good cardiac function tend to have better odds of long-term survival than older people with preexisting cardiac disorders. Resuscitation efforts for patients in whom good outcomes are anticipated should be aggressive. The availability of resources during resuscitation can also affect patient outcomes. For example, rescuer fatigue can significantly reduce CPR quality, while epinephrine unavailability denies patients with unshockable rhythms the chance for improved survival.
Complications
Death is the ultimate consequence of asystole if CPR fails or is not administered on time. For patients who survive, the sequelae may include neurologic dysfunction and CPR complications like pneumothorax, air embolism, rupture of the spleen, stomach, or colon, hemothorax, liver laceration, rhabdomyolysis, and rib fractures.
Deterrence and Patient Education
Primary prevention of asystole involves strategies aimed at identifying and addressing risk factors and underlying conditions that predispose individuals to cardiac arrest. Preventive measures include promoting healthy lifestyles and avoiding smoking and excessive alcohol consumption. Screening and early identification of cardiovascular risk factors, such as hypertension, hyperlipidemia, and diabetes, are essential for timely intervention and management. Additionally, educating the public about the signs and symptoms of heart disease and encouraging prompt medical evaluation in case of any concerning symptoms can help prevent the progression to cardiac emergencies like asystole.
Safety practices at home, school, work, and on the road must also be emphasized. For example, medications and household chemicals must be kept out of young children's reach. Vulnerable people must be protected from sharps, fires, live wires, and slippery surfaces inside the home. Screens should be built where people may be in danger of falling from a height or into a pool if they cannot swim. The proper attire must be worn to work or when engaging in sports activities. Patients should be counseled about safe driving practices.
Secondary prevention of asystole focuses on early recognition and intervention in individuals at high risk of cardiac arrest, such as those with known cardiovascular disease or a history of arrhythmias. Preventive measures involve regular cardiac function monitoring through electrocardiography or ambulatory cardiac monitoring, allowing for early arrhythmia detection and prompt intervention. Implementing evidence-based therapies, such as medication management, implantable cardioverter-defibrillators, and cardiac resynchronization therapy, can help stabilize cardiac rhythms and reduce the risk of asystole. The importance of bystander CPR in the survival of patients with OHCA must be emphasized to the public. Healthcare professionals should constantly train in BLS and ACLS administration, especially since practices are periodically updated with new evidence. These measures can reduce asystole's mortality rates.
Pearls and Other Issues
Rapid recognition and intervention are paramount in managing asystole. Remembering the Hs and Ts can help identify and address reversible causes contributing to the condition. Prompt initiation of high-quality CPR is crucial, emphasizing adequate chest compressions and minimizing interruptions to maximize perfusion. Integration of ACLS protocols, including early epinephrine administration and airway management, can improve outcomes in patients with asystole. Postresuscitation care focusing on optimizing hemodynamic stability, neuroprotective strategies, and identifying and treating underlying etiologies can enhance survival and mitigate complications following successful resuscitation from asystole.
Enhancing Healthcare Team Outcomes
All healthcare professionals directly engaged in patient care are mandated to learn and constantly train in BLS and ACLS. These management strategies are best administered by an interprofessional team to optimize patient outcomes. Hospitals should have specially assigned interprofessional "rapid response teams," "code teams," or "code blue teams" to manage cardiac arrests.
Code team composition varies widely across sites but typically includes roles such as airway provider, team leader, medication administration nurse, parental support, and documentation. Some less common roles may include defibrillator manager, access provider, CPR coach, and chest compressors. The team leader is often a physician, but some centers have dual physician and nurse team leaders. Physicians from various departments, such as the intensive care unit, emergency department, and cardiology, respond to code events, along with residents. Other teams may include a chaplain, social worker, pharmacist, and occasionally nurse supervisors, paramedics, radiology staff, child-life specialists, and security personnel. Nurses document the resuscitation, particularly the time and interventions given, and provide the necessary supplies.[16]
Interprofessional collaboration should extend beyond the acute resuscitation phase to encompass postresuscitation care, rehabilitation, and discharge planning. Collaborative efforts among healthcare professionals facilitate care continuity, ongoing complication monitoring, and implementation of evidence-based interventions to prevent recurrent cardiac events. By leveraging the interprofessional team's collective expertise and resources, healthcare providers can improve patient outcomes and safety and optimize the quality of care rendered to individuals experiencing cardiac emergencies, including asystole.