Introduction
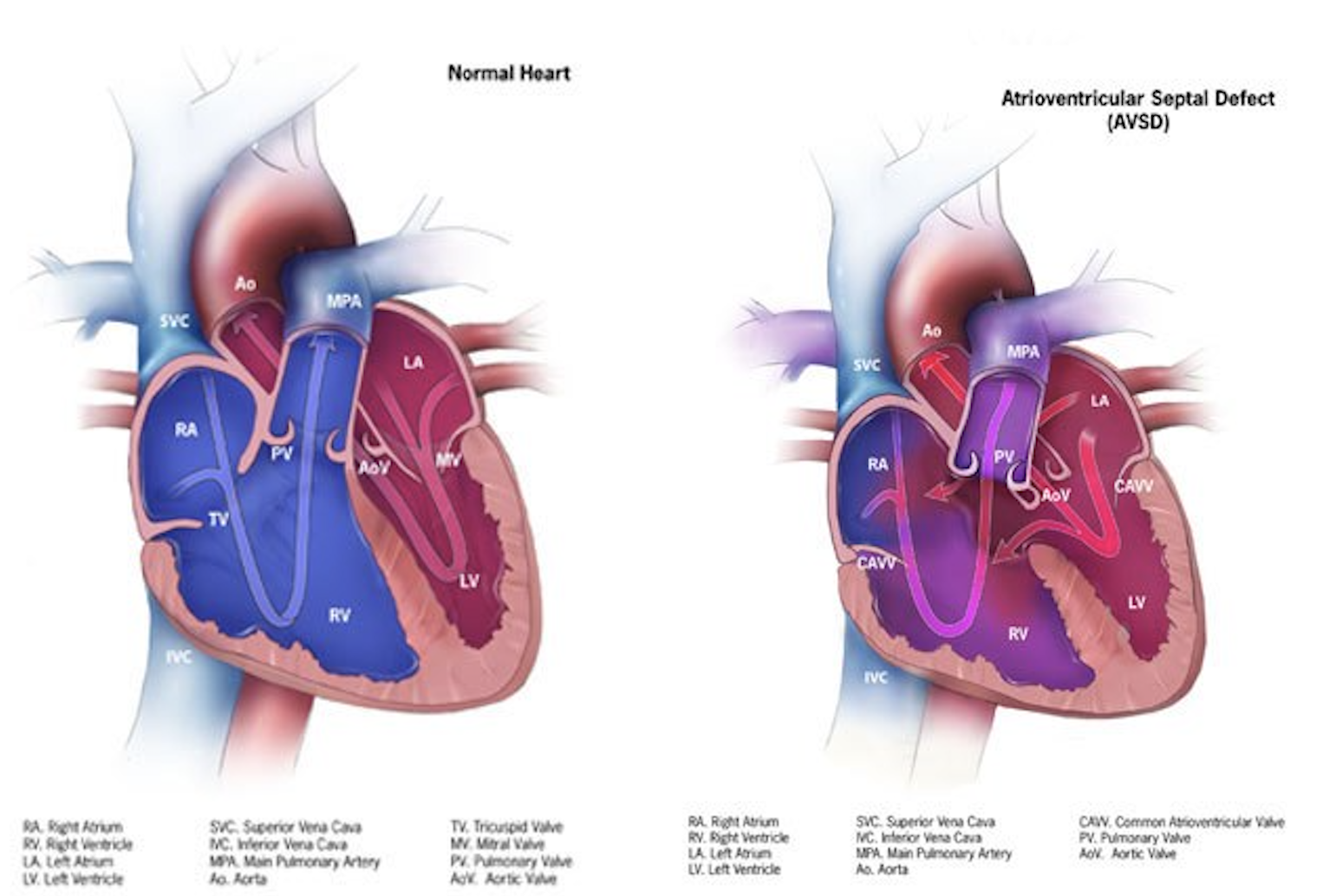
The atrioventricular septal defect is a congenital cardiac malformation that is characterized by a variable degree of the atrial and ventricular septal defect along with a common or partially separate atrioventricular orifice.[1] A partial atrioventricular septal defect is characterized by an ostium primum atrial septal defect, separate atrioventricular valves with a common junction, an inlet ventricular septal defect, and a cleft mitral valve. Whereas the complete form of the atrioventricular septal defect (AVSD) is characterized by a common atrioventricular valve with ostium primum atrial septal defect and an unrestricted ventricular septal defect of inlet type.[2]
The incidence of atrioventricular septal defect has been estimated from 0.24 to 0.31 in 1000 live births with no significant difference in male and female gender, and it has a strong association with Down’s syndrome.[3] Although the long term outcomes of surgical repair in an atrioventricular septal defect are influenced by the presence of associated malformations, such as ventricular hypoplasia, and down`s syndrome, the evolution of the surgical treatment of atrioventricular defects over the last few decades has significantly improved the long term survival. In this review article, we will discuss the etiology, epidemiology, pathophysiology, as well as management, complications, and clinical significance of atrioventricular septal defect.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In almost all patients, the atrioventricular septal defect is caused by genetic mutations, and most of the time, it is associated with syndromes. Every six patients with Down syndrome have associated atrioventricular septal defect, and Down syndrome cell adhesion molecule (DSCAM) gene has been described to be associated with an atrioventricular septal defect and other congenital heart diseases in these patients.[4][5]
The other syndromes associated with the atrioventricular septal defect may include CHARGE, Ellis-van-Creveld, Smith-Lemli-Opitz, and 3p. Other than its association with syndromes, gene mutations associated with the atrioventricular septal defect can also be inherited as an autosomal dominant trait. Gestational diabetes and maternal obesity have also been reported to increase the risk of non-syndromic atrioventricular septal defects.[6]
Epidemiology
The incidence of an atrioventricular septal defect in the general population has been reported to be 0.24 to 0.31 per 1000 live births.[3] It accounts for 3% of all congenital cardiac malformations.[2] Although both male and female genders are affected equally, one of the studies suggests a female-to-male ratio of 1.3 to 1.0, especially in patients with Down syndrome.[7]According to the Society of Thoracic Surgeons congenital database, 4138 cases of complete AVSD were diagnosed between 2013 and 2017. Most of these cases were repaired surgically with low mortality, with or without valvuloplasty (2.6% to 2.9%). Repairs that were not amenable to valvular repair and were addressed with valvular replacement were associated with increased perioperative mortality (16.7%). Of the 2 cases where an attempt at valvuloplasty failed and an intraoperative decision of replacement was taken, the mortality rate was as high as 50% (see images). This demonstrates the importance of careful preoperative visualization & planning in the surgical decision of AVSD interventions.
Pathophysiology
Endocardial cushions are paired (superior and inferior) mesenchymal structures located in the common atrioventricular canal in the early embryonic period, and their growth is of prime importance in the development of atrioventricular septum and atrioventricular valves.[8] These endocardial cushions fuse at the end of 4 weeks of development and form two atria and two ventricles. Failure of fusion results in a variable degree of the atrioventricular septal defect.
The endocardial cushions, derived from the non-chamber myocardium of the atrioventricular canal (AVC), serve as foundational mesenchymal structures in the embryologic formation of cardiac valves and septa. Their morphogenesis relies heavily on signaling pathways driven by vascular endothelial growth factor (VEGF), and variants within genes regulating this pathway have been implicated in congenital malformations affecting the atrioventricular valves and septa.
The notably elevated incidence of AVC anomalies in individuals with trisomy 21 (Down syndrome) provides insight into the genetic underpinnings of endocardial cushion development. A longstanding hypothesis attributes this susceptibility to enhanced cellular adhesiveness observed in trisomy 21-derived cells. Experimental evidence supports this, as fetal fibroblasts from trisomy 21 endocardial-cushion regions exhibit increased in vitro adhesion compared to those from euploid counterparts. If endocardial cushion fusion is contingent on precise temporal and spatial parameters, then delayed cellular migration within the trisomic embryo may significantly disrupt this critical process.[9]
Collagen type VI, particularly its alpha-1 and alpha-2 chains encoded by the COL6A1/COL6A2 gene cluster located in the congenital heart disease–critical region of chromosome 21, is thought to contribute to this pathological mechanism. Fibroblasts from individuals with Down syndrome demonstrate altered adhesion properties to type VI collagen, further implicating matrix interactions in the etiology of these defects. Despite the presence of trisomy, only approximately 50% of individuals with Down syndrome manifest structural heart disease, prompting investigations into genetic modifiers both within and outside the trisomic chromosome that may influence the penetrance of AVC defects.[10][11]
Enrichment of pathogenic variants has been observed in multiple genes—VEGF-A, COL6A1, CRELD1, FBLN1, FRZB, GATA5, NOTCH4, and CEP290—among individuals with Down syndrome and AVC anomalies, suggesting a multifactorial and genetically heterogeneous basis for these defects.[12]
Beyond syndromic associations, familial clustering of AVC anomalies with an autosomal dominant inheritance pattern and incomplete penetrance has been documented, highlighting monogenic contributions. To date, five loci have been definitively associated with non-syndromic atrioventricular septal defects (AVSDs), designated AVSD1 through AVSD5:
-
AVSD1 maps to chromosome 1p31-p21;
-
AVSD2 is attributed to pathogenic mutations in CRELD1 on chromosome 3p25;
-
AVSD3 involves GJA1, encoding connexin 43 (Cx43), on chromosome 6q22;
-
AVSD4 is linked to GATA4 on chromosome 8p23.1;
-
AVSD5 is associated with GATA6 mutations on chromosome 18q11.1.
The CRELD protein family, comprising matricellular proteins implicated in cell-cell interactions, plays a role in these pathologies. Among individuals with AVSDs unrelated to trisomy 21, approximately 6% harbor coding-region missense mutations in CRELD1. The GJA1 gene encodes connexin 43, a gap junction protein highly expressed in the ventricular myocardium, and cardiac neural crest derivatives; compound heterozygous mutations have been reported in AVSD cases.[13][14][13]
The GATA family of transcription factors—known for orchestrating lineage-specific gene expression—also contributes to cardiac morphogenesis. Neural crest-derived ectomesenchymal cells, which originate in the cranial neural folds, are crucial for outflow tract septation and the morphogenesis of branchial arch structures. These cells navigate through pharyngeal arches 3, 4, and 6 to participate in partitioning the conotruncus and aortic sac. Experimental ablation of preotic neural crest populations in animal studies has resulted in a range of conotruncal anomalies, including truncus arteriosus and subarterial ventricular septal defects, underscoring the indispensable role of these cells in cardiovascular development.[14]
Recent research has identified the SOX7 gene as a novel pathogenic candidate in the etiology of atrioventricular septal defects (AVSD). SOX7 appears to modulate the endothelial-to-mesenchymal transition (EndMT) crucial for atrioventricular cushion formation by influencing the WNT4–BMP2 signaling pathway. Specifically, SOX7 deficiency leads to downregulation of WNT4 expression in the endocardium, which in turn diminishes BMP2 expression in the adjacent myocardium. This disruption impairs the EndMT process, ultimately contributing to the pathogenesis of AVSD.[15]
Classification:
Atrioventricular septal defects (AVSDs) represent a spectrum of congenital anomalies characterized by varying degrees of atrial and ventricular septal involvement, as well as abnormalities of the atrioventricular (AV) valves.
Based on atrioventricular valve morphology and its development, atrioventricular septal defects are classified into partial, intermediate, and complete atrioventricular septal defects (AVSD).
These three anatomical variants—partial, intermediate, and complete—represent a phenotypic continuum resulting from a shared developmental defect at the level of the endocardial cushions, reflecting a common genetic and embryologic basis.[16] [17]
In the partial form of AVSD, also termed partial atrioventricular canal, the defining feature is an atrial septal defect located immediately adjacent to the AV valve plane. This defect typically appears as a crescent-shaped gap in the lower segment of the atrial septum and is associated with a morphologically trifoliate left AV valve, which frequently results in variable degrees of valvular regurgitation. Although historically referred to as an ostium primum atrial septal defect, this terminology is anatomically misleading, as the lesion does not originate from the septum primum.
The intermediate or transitional subtype of AVSD occupies a position between the partial and complete forms in terms of structural complexity. It is characterized by the presence of two distinct AV valve orifices—right and left—alongside a premum-type atrial septal defect and a ventricular septal defect situated beneath the AV valve plane. The ventricular component of the defect is typically localized to the inlet portion of the septum. Unlike in complete AVSD, there is no exposed or "bare" crest at the superior margin of the interventricular septum in these cases.
In complete AVSD, also referred to as complete atrioventricular canal defects, the malformation comprises both an interatrial and interventricular communication beneath a single, undivided common AV valve. This valve bridges the ventricular septum, incorporating both superior (anterior) and inferior (posterior) bridging leaflets. A characteristic feature of this form is the presence of an uncovered or "bare" zone at the crest of the ventricular septum, representing the common atrioventricular junction.
In the complete form of the atrioventricular septal defect, a common atrioventricular valve has five leaflets, including superior bridging, inferior bridging, left mural, right mural, and anterosuperior. Rastelli divided complete atrioventricular septal defect into three anatomical subgroups, based solely on the degree of attachment and bridging of the superior leaflet to the ventricular septum.[18]
In type A, the superior bridging leaflet of the common atrioventricular valve is equally divided but firmly attached to the crest of the interventricular septum with the help of chordae (incidence 69%). (See image)
In type B, an aberrant insertion of a papillary muscle originates from the right aspect of the interventricular septum and extends to the left portion of the shared anterior bridging leaflet (incidence 9%). (See image)
In type C, the superior bridging leaflet is not divided and does not have an attachment to the interventricular septum, thus providing a large unrestricted atrioventricular septal defect (incidence 22%). (See image)
Rastelli type A is associated with left-sided obstruction, type C is associated with tetralogy of Fallot and other complex congenital heart diseases, and type B is the least common form of the complete atrioventricular septal defect.[19]
The Concept of Balance in Atrioventricular Septal Defects
Approximately one-tenth of atrioventricular canal defects (AVCDs) exhibit disproportionate commitment of the common atrioventricular valve (AVV) to one ventricle, resulting in what is termed an unbalanced AVCD. In these cases, the AVV preferentially directs inflow toward either the right or the left ventricle, giving rise to right-dominant or left-dominant anatomical configurations, respectively. This imbalance is closely associated with underlying disparities in ventricular size and development, with unequal AVV inflow serving as a cardinal diagnostic feature.
Right ventricular (RV) dominance is observed approximately twice as often as left ventricular (LV) dominance. It frequently presents alongside hypoplasia of the left ventricle, a parachute-like configuration of the left AV valve, closely approximated LV papillary muscles with a diminutive or absent mural leaflet, and may coexist with other structural anomalies such as a bicuspid aortic valve or coarctation of the aorta. In contrast, AV canal defects with left ventricular dominance are typically accompanied by right ventricular hypoplasia and obstructive lesions of the right ventricular outflow tract, including pulmonary stenosis or pulmonary atresia.
Efforts to objectively assess the degree of AVV and ventricular imbalance have led to the development of various echocardiographic indices aimed at stratifying patients for biventricular versus univentricular surgical strategies. Among these tools is the atrioventricular valve index (AVVI)—which quantifies the relative area of the AVV committed to each ventricle—as well as assessments of the angular alignment of AVV inflows and measurements relating valve inflow dimensions to the size of the ventricular septal defect. Despite their theoretical utility, these metrics often suffer from limited reproducibility and have not gained widespread clinical adoption.
In recent years, advanced imaging modalities such as cardiac magnetic resonance (CMR) and ECG-gated cine computed tomography (CT) have emerged as valuable adjuncts for detailed anatomical assessment. These techniques offer superior spatial resolution and enhanced accuracy in quantifying ventricular volumes and AVV geometry, thus playing an increasingly critical role in preoperative planning for patients with unbalanced AV canal anatomy.[20]
History and Physical
Clinical presentation of atrioventricular septal defects is influenced by the type of atrioventricular septal defect, the magnitude of the intracardiac shunt, and other associated cardiac malformations.[3] In patients with complete atrioventricular septal defect, signs of pulmonary congestion, and right heart failure develop in early infancy due to significant left to right shunt as pulmonary vascular resistance drops after birth. Heart failure and Eisenminger may develop even earlier if these patients have associated atrioventricular valve regurgitation, ventricular imbalance, or coarctation of the aorta.[21]
Patients with the partial atrioventricular septal defect without other complex congenital cardiac malformations and minimal atrioventricular valve regurgitation, usually remain asymptomatic in infancy and early childhood. They are diagnosed on the bases of incidental findings, including pulmonary or tricuspid flow murmur and a fixed splitting due to atrial septal defect.
Symptoms of heart failure in infants may include difficulty in feeding, sleepiness, lethargy, and failure to thrive, while children may have a complaint of dyspnea.
Signs of heart failure include:
- Tachypnea, tachycardia
- S3 gallop
- Rales on chest auscultation
- Raised jugular venous pressure
- Tender hepatomegaly
- Wide fixed splitting due to atrial septal defect
- Pansystolic murmur due to atrioventricular valve regurgitation
- Pulmonary flow murmur due to increased flow through the pulmonary valve
- Mid diastolic flow murmur due to increased flow through the tricuspid valve
General physical examination may reveal cyanosis when there is a reversal of shunt (Eisenmenger syndrome) and dysmorphic features in case of associated syndromes.
Evaluation
Antenatal Evaluation
Antenatal ultrasonography with a four-chamber view is the commonly used diagnostic test for the atrioventricular septal defect. The most common findings include a common atrioventricular valve and a defect in the atrial or ventricular septum. However, the sensitivity of antenatal ultrasound for the atrioventricular septal defect is very low.[22]
Postnatal Evaluation
Chest Radiograph: It demonstrates cardiomegaly and pulmonary plethora, especially in those cases with associated atrioventricular valve regurgitation.
Electrocardiogram: The characteristic electrocardiographic findings include a superior axis in the frontal plan, right ventricular hypertrophy, and atrioventricular block. Other findings may include superior p wave axis and partial right bundle branch block.
Echocardiogram: Echocardiographic findings include:[23]
- Abnormal configuration of atrioventricular valve
- Loss of normal offset of atrioventricular valve
- Abnormal position of papillary muscles
- Disproportion in left ventricular inlet and outlet
- Ostium primum atrial septal defect
- Ventricular septal defect of inlet type
- And other associated cardiac malformations.
Cardiac Magnetic Resonance Imaging: Magnetic resonance imaging reveals findings similar to an echocardiogram. It is more accurate in measuring the size of defects and regurgitation fraction through the atrioventricular valve.
Treatment / Management
The management of atrioventricular septal defects can be divided into medical and surgical treatment.[24]
Medical Treatment
It includes diuretics and vasodilators to reduce the preload and afterload to relieve the symptoms associated with pulmonary congestion and heart failure. Associated feeding problems and failure to thrive are managed by tube feeding and providing extra calories. In atrioventricular septal defects, medical treatment is usually directed at optimizing the condition of the patient for surgery.[3]
Surgical Treatment
Surgical correction is the ultimate treatment of atrioventricular septal defect. Atrioventricular septal repair is a complex surgical procedure and carries operative mortality of more than 3% even in the contemporary era of advanced surgical techniques.[25] It also carries significant postoperative mortality and morbidity due to residual intracardiac shunts, atrioventricular valve regurgitation, left ventricular outflow tract obstruction, and arrhythmias.[26]
The assessment of pre-operative imaging and hemodynamic data is essential for the optimal selection of surgical procedures to reduce the need for recurrent surgery and postoperative complications.[27] In previous studies, the requirement of the recurrent procedure is reported as high as 18.2% at 15 years after surgical correction, and left atrioventricular valve dysplasia, absence of cleft closure, and associated cardiac malformations are found to increase the rate of recurrent procedures.[28](B2)
Surgical Approaches for Repairing Complete Atrioventricular Septal Defects
The operative correction of complete atrioventricular septal defects (AVSDs) may be undertaken using one of several established techniques, each aimed at reconstructing the atrioventricular septum while preserving valvular function and minimizing postoperative complications.
1. Single-Patch Repair:This method involves the use of a single patch to simultaneously close both the atrial and ventricular septal components of the defect. Following the division of the common atrioventricular valve (AVV), the right and left valve components are reattached to the newly created septal structure. Closure of the left AVV cleft is routinely performed as part of this reconstruction. Untreated autologous pericardium is commonly utilized as the patch material. Careful attention is given to anchoring the AVV tissue close to the crest of the ventricular septum, which facilitates optimal leaflet coaptation and function.
2. Two-Patch Technique:This approach employs separate patches to address the atrial and ventricular septal defects individually. Typically, a Dacron patch is used to close the ventricular component, while the atrial septum is reconstructed with a patch fashioned from autologous pericardium. As with the single-patch technique, the cleft in the left AV valve is closed primarily. It is critical to undersize the ventricular patch in the anteroposterior axis and maintain a low patch profile to prevent excessive displacement of valve tissue into the atrial chamber, which has been correlated with an increased risk of postoperative left AVV regurgitation.
3. Modified Single-Patch ("Australian") Technique:In this variation, the ventricular component is eliminated by directly suturing the common AVV leaflets to the crest of the ventricular septum. A separate pericardial patch is then used to close the primum atrial septal defect. Closure of the left AVV cleft is carried out similarly to the aforementioned techniques.
All three strategies have demonstrated favorable early outcomes; however, none entirely eliminates the possibility of late reoperation, particularly in the context of residual or recurrent left AVV regurgitation. The likelihood of requiring reintervention appears to be higher in patients presenting with significant preoperative valve insufficiency. Accordingly, comprehensive echocardiographic assessment is imperative before hospital discharge and throughout long-term follow-up.[29][30][31]
In complete AVSD, surgical closure should be performed in early infancy to reduce the pulmonary vascular disease, whereas, in incomplete atrioventricular septal defect, a repair can be slightly delayed if the patient is not symptomatic. For partial AVSD, the primary repair is preferred with patch closure and atrioventricular valvuloplasty.
For balanced complete AVSD, early primary repair with two patch closure techniques is preferred over one patch closure, as one patch closure is associated with an increased rate of recurrent procedures due to patch dehiscence and residual shunt. Pulmonary artery banding is no longer used as a routine procedure in complete AVSD repair. For unbalanced complete AVSD, the repair technique may include single ventricle palliation with the staged biventricular repair or primary biventricular repair.[26]
Differential Diagnosis
Common differential diagnoses of AVSD include ostium secondum atrial septal defect, isolated ventricular septal defect and tetralogy of Fallot. The symptoms of heart failure and enlargement of cardiac chambers are common in these malformations, and echocardiogram plays a major role in differentiating AVSD from the aforementioned conditions.
Prognosis
The prognosis of untreated atrioventricular septal defect is dismal. Around 50% of the patients die during infancy, either due to heart failure or pulmonary infections.[2] Those who survive beyond one year, they develop the irreversible pulmonary vascular disease and later on the reversal of the shunt.
Patients undergoing surgical repair have 15 years of survival of around 90%, and 9% to 10% of those require reoperation within 15 years.[32]
Complications
Most of the complications of AVSD are related to intracardiac shunts or atrioventricular valve regurgitation. In complete AVSD, shunting of blood from left to right leads to right-sided overload and signs of heart failure and pulmonary congestion at a very early age, which contributes to significant mortality during infancy. If the shunt is not corrected, it causes an irreversible pulmonary vascular disease that leads to pulmonary hypertension and Eisenmenger syndrome.
Regurgitation of blood from the ventricle to atria through the atrioventricular valve leads to pulmonary congestion and enlargement of the atrium. Enlargement of the atrium can lead to supraventricular arrhythmias. Other complications are related to poor feeding, which may include malnutrition and failure to thrive.
Deterrence and Patient Education
How can an atrioventricular septal defect be diagnosed?
It is usually diagnosed by a cardiologist (who is a specialist in congenital cardiac diseases). He makes the diagnosis based on symptoms of heart failure and examination findings, including a murmur. Then he may advise a few tests to confirm the diagnosis. These tests may include:
- Chest radiograph: It is used to take a picture of the lungs and heart.
- Electrocardiogram (ECG): It detects any abnormality in the electrical activity of the heart.
- Echocardiogram: It uses sound waves and creates a picture of the internal structure and parts of the heart.
- Cardiac catheterization: It measures blood pressure and concentration of oxygen inside the heart chambers and helps the doctor detect Intracardiac shunting of blood.
How can an atrioventricular septal defect be treated?
It is treated by surgically repairing the defect in the first year of life. Sometimes medications are given by the doctor to reduce the symptoms of heart failure.
These medications may include pills to excrete extra water from the body via urine (diuretics) and pills, which dilate the blood vessels and decrease the peripheral vascular resistance.
Pearls and Other Issues
Diagnosis of AVSD in fetal life or early neonatal period is essential in order to initiate appropriate medical treatment and to plan early surgical repair. In the contemporary era of advanced surgical techniques, the operative mortality of atrioventricular septal defect repair is low with excellent long term outcomes even in patients with Down syndrome.
Atrioventricular valve regurgitation is the most common reason for reoperation, that’s why it is mandatory to assess the imaging data before surgical repair and pay detailed attention to atrioventricular valve repair at the time of primary repair. Postoperatively patients should be followed regularly for atrioventricular valve regurgitation and left ventricular outflow tract obstruction.[3]
Enhancing Healthcare Team Outcomes
An atrioventricular septal defect is a complex congenital cardiac malformation, which requires a multidisciplinary and interprofessional approach in order to optimize medical treatment, decrease surgical mortality, and improve long term outcomes. A multidisciplinary team is the cornerstone of a multi-professional approach, which may include a pediatric cardiologist, a cardiac imaging expert, a pediatric cardiac surgeon, a cardiac anesthesiologist, a cardiac nurse, a nutritionist, and a cardiac pharmacist.
During fetal life and early infancy, a detailed screening for congenital cardiac malformations is mandatory in order to make an early diagnosis of the atrioventricular septal defect. After an early diagnosis, it is essential to institute appropriate medical therapy and plan surgical repair. Assessment of imaging and hemodynamic data is essential for planning a surgical repair.
In the postoperative period, wound care, appropriate nutrition, and nursing care are important to reduce the duration of hospitalization and promote early postoperative recovery. Meticulous postoperative follow up is required to monitor and assess the long-term complications of surgical repair and the need for recurrent surgery.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Jacobs JP, Burke RP, Quintessenza JA, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project: atrioventricular canal defect. The Annals of thoracic surgery. 2000 Apr:69(4 Suppl):S36-43 [PubMed PMID: 10798414]
Calabrò R, Limongelli G. Complete atrioventricular canal. Orphanet journal of rare diseases. 2006 Apr 5:1():8 [PubMed PMID: 16722604]
Craig B. Atrioventricular septal defect: from fetus to adult. Heart (British Cardiac Society). 2006 Dec:92(12):1879-85 [PubMed PMID: 17105897]
Tubman TR, Shields MD, Craig BG, Mulholland HC, Nevin NC. Congenital heart disease in Down's syndrome: two year prospective early screening study. BMJ (Clinical research ed.). 1991 Jun 15:302(6790):1425-7 [PubMed PMID: 1829969]
Level 1 (high-level) evidenceBarlow GM, Chen XN, Shi ZY, Lyons GE, Kurnit DM, Celle L, Spinner NB, Zackai E, Pettenati MJ, Van Riper AJ, Vekemans MJ, Mjaatvedt CH, Korenberg JR. Down syndrome congenital heart disease: a narrowed region and a candidate gene. Genetics in medicine : official journal of the American College of Medical Genetics. 2001 Mar-Apr:3(2):91-101 [PubMed PMID: 11280955]
Level 3 (low-level) evidenceAgopian AJ, Moulik M, Gupta-Malhotra M, Marengo LK, Mitchell LE. Descriptive epidemiology of non-syndromic complete atrioventricular canal defects. Paediatric and perinatal epidemiology. 2012 Nov:26(6):515-24. doi: 10.1111/ppe.12006. Epub 2012 Sep 24 [PubMed PMID: 23061687]
Santoro M, Coi A, Spadoni I, Bianchi F, Pierini A. Sex differences for major congenital heart defects in Down Syndrome: A population based study. European journal of medical genetics. 2018 Sep:61(9):546-550. doi: 10.1016/j.ejmg.2018.05.013. Epub 2018 May 9 [PubMed PMID: 29753092]
Markwald RR, Krook JM, Kitten GT, Runyan RB. Endocardial cushion tissue development: structural analyses on the attachment of extracellular matrix to migrating mesenchymal cell surfaces. Scanning electron microscopy. 1981:(Pt 2):261-74 [PubMed PMID: 7034167]
Level 3 (low-level) evidencePelleri MC, Gennari E, Locatelli C, Piovesan A, Caracausi M, Antonaros F, Rocca A, Donati CM, Conti L, Strippoli P, Seri M, Vitale L, Cocchi G. Genotype-phenotype correlation for congenital heart disease in Down syndrome through analysis of partial trisomy 21 cases. Genomics. 2017 Oct:109(5-6):391-400. doi: 10.1016/j.ygeno.2017.06.004. Epub 2017 Jun 23 [PubMed PMID: 28648597]
Level 3 (low-level) evidenceReeser RS, Salazar AK, Prutton KM, Roede JR, VeDepo MC, Jacot JG. Trisomy 21 Alters Cell Proliferation and Migration of iPSC-Derived Cardiomyocytes on Type VI Collagen. Cellular and molecular bioengineering. 2024 Feb:17(1):25-34. doi: 10.1007/s12195-023-00791-x. Epub 2024 Jan 3 [PubMed PMID: 38435791]
Gittenberger-de Groot AC, Bartram U, Oosthoek PW, Bartelings MM, Hogers B, Poelmann RE, Jongewaard IN, Klewer SE. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003 Dec:275(2):1109-16 [PubMed PMID: 14613310]
Trevino CE, Holleman AM, Corbitt H, Maslen CL, Rosser TC, Cutler DJ, Johnston HR, Rambo-Martin BL, Oberoi J, Dooley KJ, Capone GT, Reeves RH, Cordell HJ, Keavney BD, Agopian AJ, Goldmuntz E, Gruber PJ, O'Brien JE Jr, Bittel DC, Wadhwa L, Cua CL, Moskowitz IP, Mulle JG, Epstein MP, Sherman SL, Zwick ME. Identifying genetic factors that contribute to the increased risk of congenital heart defects in infants with Down syndrome. Scientific reports. 2020 Oct 22:10(1):18051. doi: 10.1038/s41598-020-74650-4. Epub 2020 Oct 22 [PubMed PMID: 33093519]
Pugnaloni F, Digilio MC, Putotto C, De Luca E, Marino B, Versacci P. Genetics of atrioventricular canal defects. Italian journal of pediatrics. 2020 May 13:46(1):61. doi: 10.1186/s13052-020-00825-4. Epub 2020 May 13 [PubMed PMID: 32404184]
Chaithra S, Agarwala S, Ramachandra NB. High-risk genes involved in common septal defects of congenital heart disease. Gene. 2022 Oct 5:840():146745. doi: 10.1016/j.gene.2022.146745. Epub 2022 Jul 18 [PubMed PMID: 35863714]
Hong N, Zhang E, Xie H, Jin L, Zhang Q, Lu Y, Chen AF, Yu Y, Zhou B, Chen S, Yu Y, Sun K. The transcription factor Sox7 modulates endocardiac cushion formation contributed to atrioventricular septal defect through Wnt4/Bmp2 signaling. Cell death & disease. 2021 Apr 12:12(4):393. doi: 10.1038/s41419-021-03658-z. Epub 2021 Apr 12 [PubMed PMID: 33846290]
Anderson RH, Ho SY, Falcao S, Daliento L, Rigby ML. The diagnostic features of atrioventricular septal defect with common atrioventricular junction. Cardiology in the young. 1998 Jan:8(1):33-49 [PubMed PMID: 9680269]
Kim JS, Virágh S, Moorman AF, Anderson RH, Lamers WH. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circulation research. 2001 Mar 2:88(4):395-402 [PubMed PMID: 11230106]
Rastelli G, Kirklin JW, Titus JL. Anatomic observations on complete form of persistent common atrioventricular canal with special reference to atrioventricular valves. Mayo Clinic proceedings. 1966 May:41(5):296-308 [PubMed PMID: 5932615]
Backer CL, Mavroudis C, Alboliras ET, Zales VR. Repair of complete atrioventricular canal defects: results with the two-patch technique. The Annals of thoracic surgery. 1995 Sep:60(3):530-7 [PubMed PMID: 7677476]
Banka P, Schaetzle B, Komarlu R, Emani S, Geva T, Powell AJ. Cardiovascular magnetic resonance parameters associated with early transplant-free survival in children with small left hearts following conversion from a univentricular to biventricular circulation. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014 Oct 7:16(1):73. doi: 10.1186/s12968-014-0073-1. Epub 2014 Oct 7 [PubMed PMID: 25314952]
Berger TJ, Blackstone EH, Kirklin JW, Bargeron LM Jr, Hazelrig JB, Turner ME Jr. Survival and probability of cure without and with operation in complete atrioventricular canal. The Annals of thoracic surgery. 1979 Feb:27(2):104-11 [PubMed PMID: 453968]
ter Heide H, Thomson JD, Wharton GA, Gibbs JL. Poor sensitivity of routine fetal anomaly ultrasound screening for antenatal detection of atrioventricular septal defect. Heart (British Cardiac Society). 2004 Aug:90(8):916-7 [PubMed PMID: 15253968]
Smallhorn JF, Sutherland GR, Anderson RH, Macartney FJ. Cross-sectional echocardiographic assessment of conditions with atrioventricular valve leaflets attached to the atrial septum at the same level. British heart journal. 1982 Oct:48(4):331-41 [PubMed PMID: 7126385]
Level 2 (mid-level) evidenceBacker CL, Stewart RD, Mavroudis C. What is the best technique for repair of complete atrioventricular canal? Seminars in thoracic and cardiovascular surgery. 2007 Fall:19(3):249-57 [PubMed PMID: 17983953]
St Louis JD, Jodhka U, Jacobs JP, He X, Hill KD, Pasquali SK, Jacobs ML. Contemporary outcomes of complete atrioventricular septal defect repair: analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. The Journal of thoracic and cardiovascular surgery. 2014 Dec:148(6):2526-31. doi: 10.1016/j.jtcvs.2014.05.095. Epub 2014 Jul 21 [PubMed PMID: 25125206]
Chauhan S. Atrioventricular septal defects. Annals of cardiac anaesthesia. 2018 Jan-Mar:21(1):1-3. doi: 10.4103/aca.ACA_219_17. Epub [PubMed PMID: 29336382]
Overman DM, Baffa JM, Cohen MS, Mertens L, Gremmels DB, Jegatheeswaran A, McCrindle BW, Blackstone EH, Morell VO, Caldarone C, Williams WG, Pizarro C. Unbalanced atrioventricular septal defect: definition and decision making. World journal for pediatric & congenital heart surgery. 2010 Apr:1(1):91-6. doi: 10.1177/2150135110363024. Epub [PubMed PMID: 23804728]
Hoohenkerk GJ, Bruggemans EF, Rijlaarsdam M, Schoof PH, Koolbergen DR, Hazekamp MG. More than 30 years' experience with surgical correction of atrioventricular septal defects. The Annals of thoracic surgery. 2010 Nov:90(5):1554-61. doi: 10.1016/j.athoracsur.2010.06.008. Epub [PubMed PMID: 20971263]
Level 2 (mid-level) evidenceFong LS, Betts K, Bell D, Konstantinov IE, Nicholson IA, Winlaw DS, Orr Y, Australian CAVSD Study Group. Complete atrioventricular septal defect repair in Australia: Results over 25 years. The Journal of thoracic and cardiovascular surgery. 2020 Mar:159(3):1014-1025.e8. doi: 10.1016/j.jtcvs.2019.08.005. Epub 2019 Aug 30 [PubMed PMID: 31590953]
Juaneda I, Pizzulli L, Ferrari P, Molinas R, Diaz J, Azar I, Allub A, Juaneda E, Louis JS, Peirone A, Kathy J. Outcomes of Atrioventricular Septal Defect Repair: Two-Institutions, 10-Year Experience in Cordoba, Argentina. World journal for pediatric & congenital heart surgery. 2025 Jan 30:():21501351241305135. doi: 10.1177/21501351241305135. Epub 2025 Jan 30 [PubMed PMID: 39885724]
Reynen S, Hövels-Gürich HH, Vazquez-Jimenez JF, Messmer BJ, Sachweh JS. Long-Term Outcome Up To 40 Years after Single Patch Repair of Complete Atrioventricular Septal Defect in Infancy or Childhood. The Thoracic and cardiovascular surgeon. 2021 Dec:69(S 03):e68-e75. doi: 10.1055/s-0041-1740070. Epub 2021 Dec 25 [PubMed PMID: 34953470]
Crawford FA Jr, Stroud MR. Surgical repair of complete atrioventricular septal defect. The Annals of thoracic surgery. 2001 Nov:72(5):1621-8; discussion 1628-9 [PubMed PMID: 11722055]