Introduction
Ocular tuberculosis (TB) is a clinical disease caused by or associated with Mycobacterium tuberculosis (MTB). MTB has various modes of transmission and can infect virtually any ocular tissue. Much like syphilis' ability to mimic multiple skin conditions, ocular TB should be thought of as "the great imitator" of ocular pathologies.[1] Choroidal tubercles were first anatomically described in 1855 and identified with an ophthalmoscope in 1867. A year after discovering the organism, MTB was identified in the eye in 1883.[2] An autopsy study of miliary TB in 1950 even reported that eye examination exceeded chest radiography in diagnostic sensitivity.[3]
Since this time, TB has become increasingly rare in Western nations, and advancements in laboratory diagnostic tests have led eye examinations for choroidal tubercles to fall out of favor in current guideline recommendations. Globally, more than 1.7 billion people are estimated to be infected with TB.[4] Additionally, MTB is the leading cause of death from a single infectious agent globally and the leading cause of death among persons living with human immunodeficiency infection.[5]
Recognition of ocular TB as a clinical manifestation of extrapulmonary TB is critical, as a timely diagnosis can lead to early initiation of antituberculosis therapy and prevent poor patient outcomes.[6] Most cases of ocular TB are presumptive, as getting histological or microbiological evidence of infection is extremely difficult. Also, the ocular disease may be due to direct infection or an immune reaction to MTB. Uniform diagnostic criteria are lacking, and the interpretation of diagnostic modalities may vary globally. However, early detection and prompt management of cases with presumed ocular tuberculosis may lead to good outcomes.[7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
MTB can affect the eyes in multiple ways, including:
- Direct ocular infection from an exogenous source, such as contact with the eyelids or conjunctiva (primary ocular TB)
- Hematogenous spread of MTB from a pulmonary focus or extrapulmonary site (secondary ocular TB)
- A hypersensitivity reaction in eye structures exposed to MTB antigens
The most common mechanism of ocular involvement is hematogenous spread from pulmonary TB.[7] Seeding may occur from primary infection or the reactivation of a dormant lesion. MTB spreads by aerosolized droplets. Airborne bacteria are inhaled into the respiratory alveoli, where they encounter alveolar macrophages. Approximately 90% of these infected individuals never develop clinical disease and remain asymptomatic, a state termed latent TB.[8] Of the remaining 10%, approximately 5% will develop the disease within the first few years of exposure. The last 5% may develop symptoms several years later as host immunity wanes.[9] Alveolar macrophages phagocytize the bacteria and release cytokines to recruit circulating monocytes to the site of infection, but MTB escapes eradication by inhibiting the fusion of the macrophage phagolysosome.[10] This allows the bacteria to proliferate in nonactivated and partly-activated macrophages. Eventually, bacteria-laden macrophages disseminate into the lymphatics and venous circulation through erosions in the alveolar epithelium and migrate to oxygen-rich regions of the body, including the lung apex, various organs, and the eye.[11]
Latent TB infection is defined as evidence of TB infection without evidence of active TB disease clinical manifestations. Detection of latent infection is only achieved by indirect testing of an individual’s immune response to MTB antigen using a tuberculin skin test (TST) or an interferon-gamma release assay (IGRA).[6] Active TB is defined by the British Thoracic Society (BTS) as the range of clinical manifestations that occur in symptomatic individuals infected with MTB. Active TB is distinct from latent TB infection, which is asymptomatic. The BTS has clarified this disease process further, stating that latent TB infection and active TB are a continuum rather than distinct clinical entities.[12][6] This clinical definition suggests that all cases of ocular TB should be considered and managed as active TB disease.[12][6]
Epidemiology
According to the World Health Organization (WHO), about 10.4 million people fall ill, and 1.8 million people die from TB each year.[13] The burden of the disease varies considerably among countries, being 8 to 12 times higher in low-resource countries compared to industrialized countries. Geographically, the majority of TB cases in 2018 were in Southeast Asia (44%), and globally, the top 5 nations were India (27%), China (9%), Indonesia (8%), the Philippines (6%), and Pakistan (6%).[14] Ocular TB cases have been estimated to be less than 1% in the United States, 4% in China, 6% in Italy, and 16% in Saudi Arabia.[15]
Historically, epidemiologic data on ocular TB has varied widely due to the lack of specific diagnostic criteria. In 1967, a study of 10,524 patients at a TB sanitarium reported an incidence of ocular TB in 1.4% of patients.[16] A 1997 study of 100 randomly selected Spanish patients with TB reported that 18% had ocular TB.[17] In patients presenting with uveitis in North India from 1996 to 2001, 9.86% of cases were caused by TB.[18] A prospective case series of 126 patients from Japan with uveitis from 1998 to 2000 reported that 7.9% were due to intraocular TB.[19]
Pathophysiology
The mode of infection can characterize the pathophysiologic effects of MTB on the eye. Patients who present with involvement of lids, lacrimal apparatus, adnexa, sclera, conjunctiva, or cornea are likely to have a primary ocular infection due to direct contact with the eye.[2][20] Hematogenous spread of MTB from pulmonary or extrapulmonary sites most commonly affects the uveal tract, consisting of the iris and ciliary body (anteriorly) and the choroid (posteriorly). Therefore, tubercular uveitis may present as anterior, intermediate, posterior, or panuveitis (granulomatous or nongranulomatous). The choroid receives the highest blood flow per unit of tissue in the body and creates an oxygen-rich environment analogous to the apex of the lung.[21] MTB-laden macrophages deposit in the first available capillary beds upon entering the eye, which leads to posterior uveitis being the most common presentation of ocular TB.[22] Bacilli multiply and incite local inflammation, which manifests as a choroidal tubercle.[23][24]
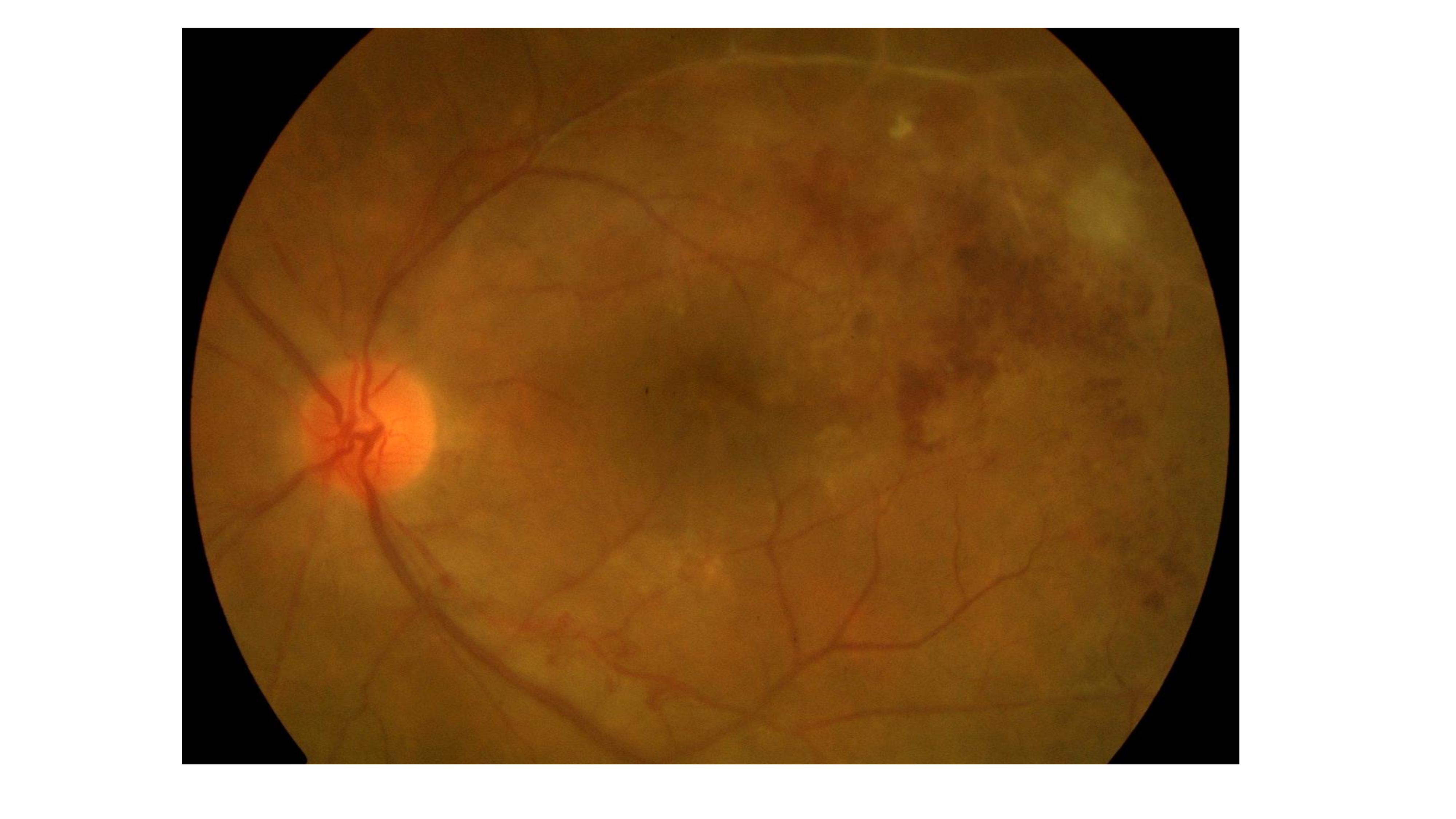
Tubercles that coalesce or grow large are reclassified as tuberculoma.[25][26] The tubercles and tuberculoma involve all layers of choroidal tissue and are surrounded by obliterated choroidal blood vessels. The lesion may liquefy into a subretinal abscess (see Image. Choroidal Tuberculosis Abscess).[26] The overlying retinal pigment epithelium remains normal in the early stages, but later, it can become disrupted with pigmentation, and the overlying retina may detach. Early intervention may completely resolve the lesion or result in an atrophic scar.[23] Hypersensitivity reactions involving the eye, including phlyctenular keratoconjunctivitis or Eales disease, are theorized to result from an immunologic response to nonviable MTB organisms in ocular tissue.[27] However, the pathophysiologic mechanism is poorly understood.[28][29]
Histopathology
In most cases, intraocular tissue involvement makes biopsy risky and impractical. In rare cases of external disease or anterior segment involvement, a biopsy may be taken for histopathologic analysis. Ziehl-Neelsen acid-fast stained bacilli or caseous necrosis with epithelioid cells and Langerhans giant cells suggests ocular TB.[30] Bacteria in multinucleated giant cells are inconsistently detected by histologic staining.[29] As a result, biopsy results and reliability may be variable.
History and Physical
Clinical History
In recent years, those at the most significant risk of developing ocular TB are immunocompromised individuals. Extrapulmonary involvement is seen in more than 50% of patients who have acquired immunodeficiency syndrome and TB.[31] Others at risk include individuals taking immunosuppressive therapy, healthcare workers, homeless and prisoner populations, immigrants from endemic countries, and patients with comorbid alcohol use disorder, chronic liver disease, chronic hemodialysis, diabetes, malignancy, or silicosis.[32]
Therefore, clinicians should obtain a comprehensive medical and social history, specifically inquiring about the patient's human immunodeficiency virus status when suspecting ocular TB. This information is especially critical when interpreted in conjunction with a history of nonspecific symptoms of pulmonary TB (eg, chest pain, chronic cough, hemoptysis, fever, anorexia, night sweats, and unexplained weight loss), travel to a TB-endemic country, interaction with an active TB patient, or prior positive radiographic findings, tuberculin skin test, or interferon-gamma release assay.
Ocular Tuberculosis Presentation
Ocular TB can have multiple manifestations. Patients may report acute or chronic inflammation that is unilateral or bilateral. Patients may also report headaches, flashes, floaters, or red eyes. Choroidal tubercles near the macula may present with diminished visual acuity and photosensitivity. The lack of visual symptoms does not rule out ocular TB, as small tubercles in the peripheral fundus may be asymptomatic.[23][33]
Ocular TB is a great imitator of various ocular pathologies. Therefore, a clinician should consider ocular TB along with other differential diagnoses in patients presenting with ocular symptoms. Ocular TB may be extraocular, involving structures on or around the eye, or intraocular, involving structures inside the eye. Extraocular and intraocular clinical signs of ocular TB are findings that may be observed according to the anatomical location.
Extraocular Tuberculosis
Orbit clinical features
The involvement of the orbit is most common in children.[20] Patients may present with proptosis, eyelid swelling, intermittent periorbital swelling, headache, epistaxis, decreased vision, visual field abnormalities, chemosis, Marcus Gunn pupil(s), epiphora, and increased orbital resistance to retropulsion.[34][35] Other orbital features include cold abscess, orbital periostitis, bony destruction, extraocular muscle involvement, soft tissue tuberculoma, and orbital apex syndrome.[36][37]
Eyelid and lacrimal gland clinical features
Eyelid involvement is also most common in children. Eyelid TB can present as lupus vulgaris with reddish-brown "apple-jelly" nodules, a lid abscess, chronic blepharitis, or atypical chalazion.[15][38] Other features include recurrent chalazion, chronic blepharitis, and diffuse infiltration simulating preseptal cellulitis.[37] TB involving the lacrimal gland presents as symptomatic dacryoadenitis or lacrimal gland abscess indistinguishable from other bacterial infections.[39]
Conjunctivae clinical features
Primary tuberculous conjunctivitis is a chronic disease leading to scarring. Patients present with ocular redness, discomfort, and mucopurulent discharge with regional lymphadenopathy.[40] Other conjunctival manifestations include subconjunctival nodules, phlycten, ulcers, tuberculomas, and polyps.[39]
Corneal and scleral clinical features
Phlyctenular keratoconjunctivitis, an inflammatory nodule at the limbus, or interstitial keratitis are frequent clinical presentations associated with corneal TB. The phlyctenule is believed to be a hypersensitivity reaction to MTB antigen, and it may erode the epithelia and create photophobia, redness, and tearing. Tuberculous interstitial keratitis presents as a unilateral sectoral, peripheral stromal infiltrate with vascularization.[41] Other features include disciform keratitis, corneal erosion, and corneal ulcer.[42]
Tuberculous scleritis is challenging to diagnose outside the context of active systemic TB. Scleritis is usually chronic, does not respond to anti-inflammatory treatment, can be necrotizing, and usually presents anteriorly; posterior scleritis involvement is rare.[43] Other manifestations include nodular or diffuse anterior scleritis, sclerouveitis, sclerokeratitis, and scleromalacia.[39]
Intraocular Tuberculosis
Anterior and intermediate uveitis clinical features
TB involving the uvea is usually granulomatous. Anterior uveitis may present with iris granulomas with broad-based posterior synechiae, Koeppe and Busacca nodules, mutton-fat keratic precipitates on the posterior aspect of the cornea, or a complicated cataract.[44][45] In children, band keratopathy can occur. Intermediate uveitis usually presents with unilateral or bilateral asymmetry and resembles par planitis.[46] Features include mild-to-moderate vitritis with snowballs, cystoid macular edema, snow banking, peripheral vascular sheathing, or peripheral retinochoroidal granulomas.[23][47]
Posterior uveitis clinical features
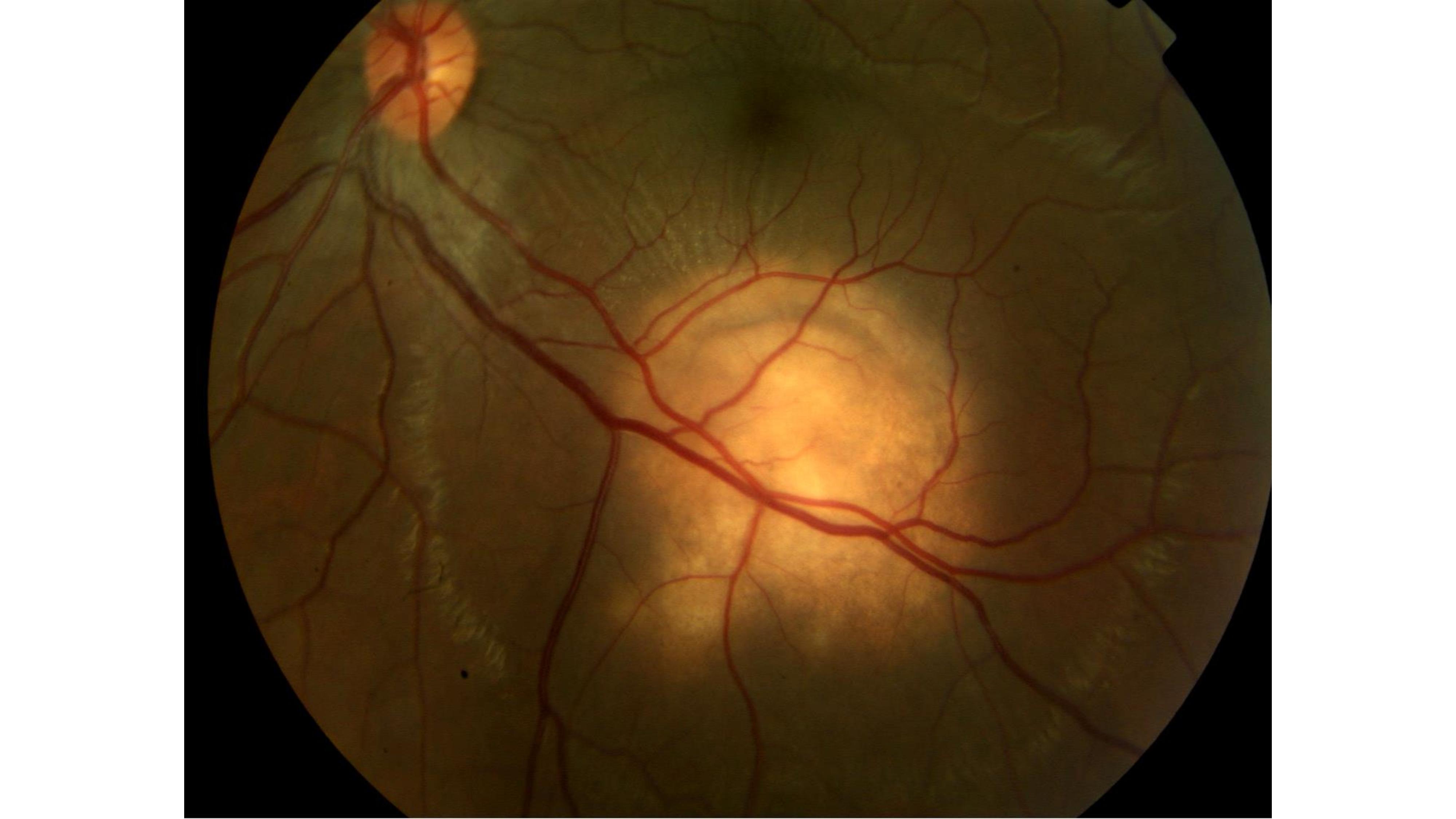
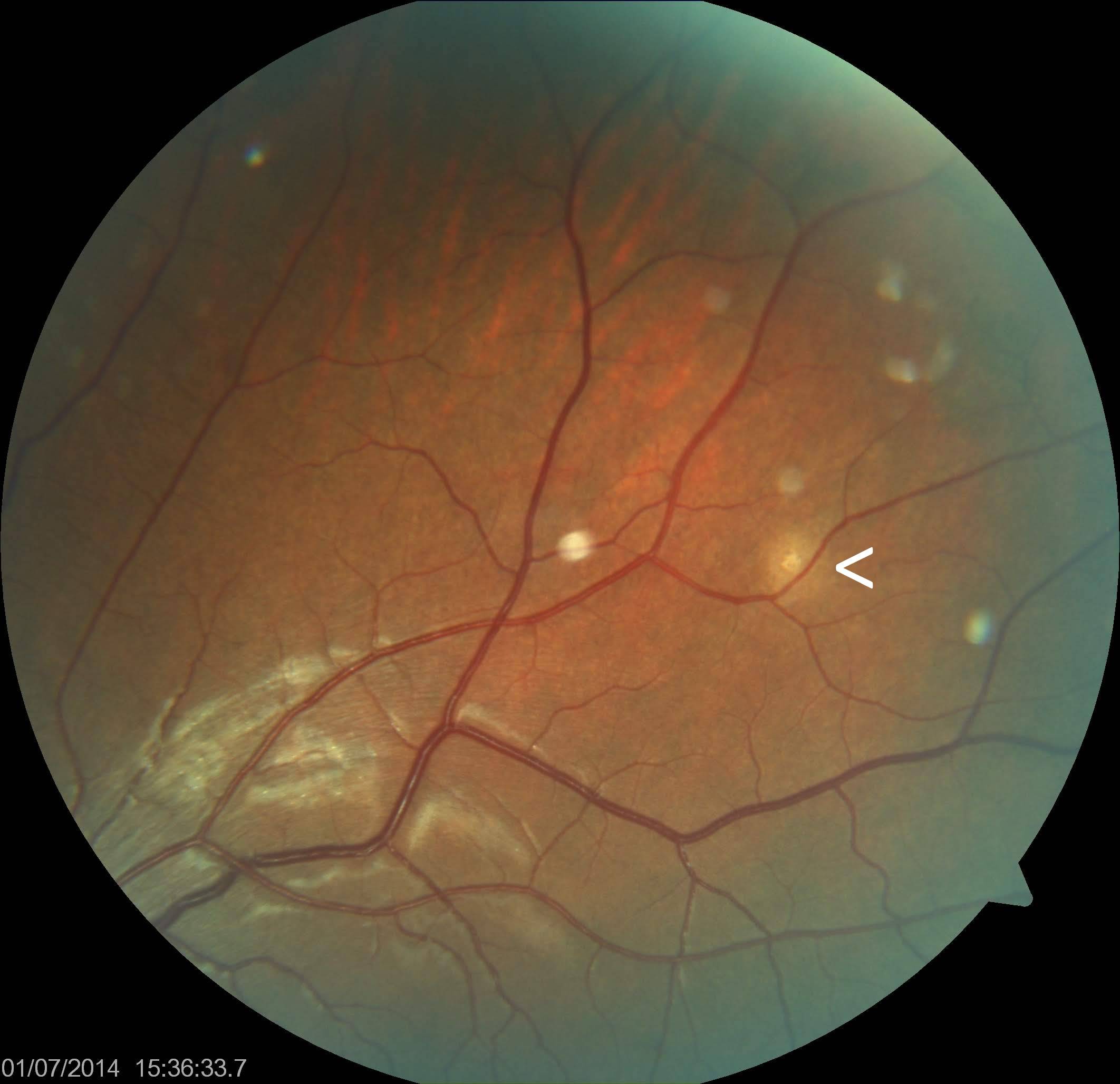
Ocular TB most commonly presents as posterior uveitis. Common patterns include a solitary tubercle (see Image. Choroidal Tubercle), multiple tubercles, miliary choroidal tubercles, tuberculoma, tubercular subretinal abscess, and multifocal choroiditis (see Image. Tubercular Choroidal Granuloma). Tubercles are white-yellow nodules that typically reside in the posterior pole. Usually, fewer than 5 tubercles are present, but there may be as many as 50 or 60. Choroidal tubercles, tuberculoma, and subretinal abscess are thought to be caused by direct infection by MTB.[46] A patient with choroidal tuberculomas due to military TB, diabetic macular edema, and proliferative diabetic retinopathy who responded well to anti-vascular endothelial growth factor therapy, pan-retinal photocoagulation, and ATT has been reported.[24]
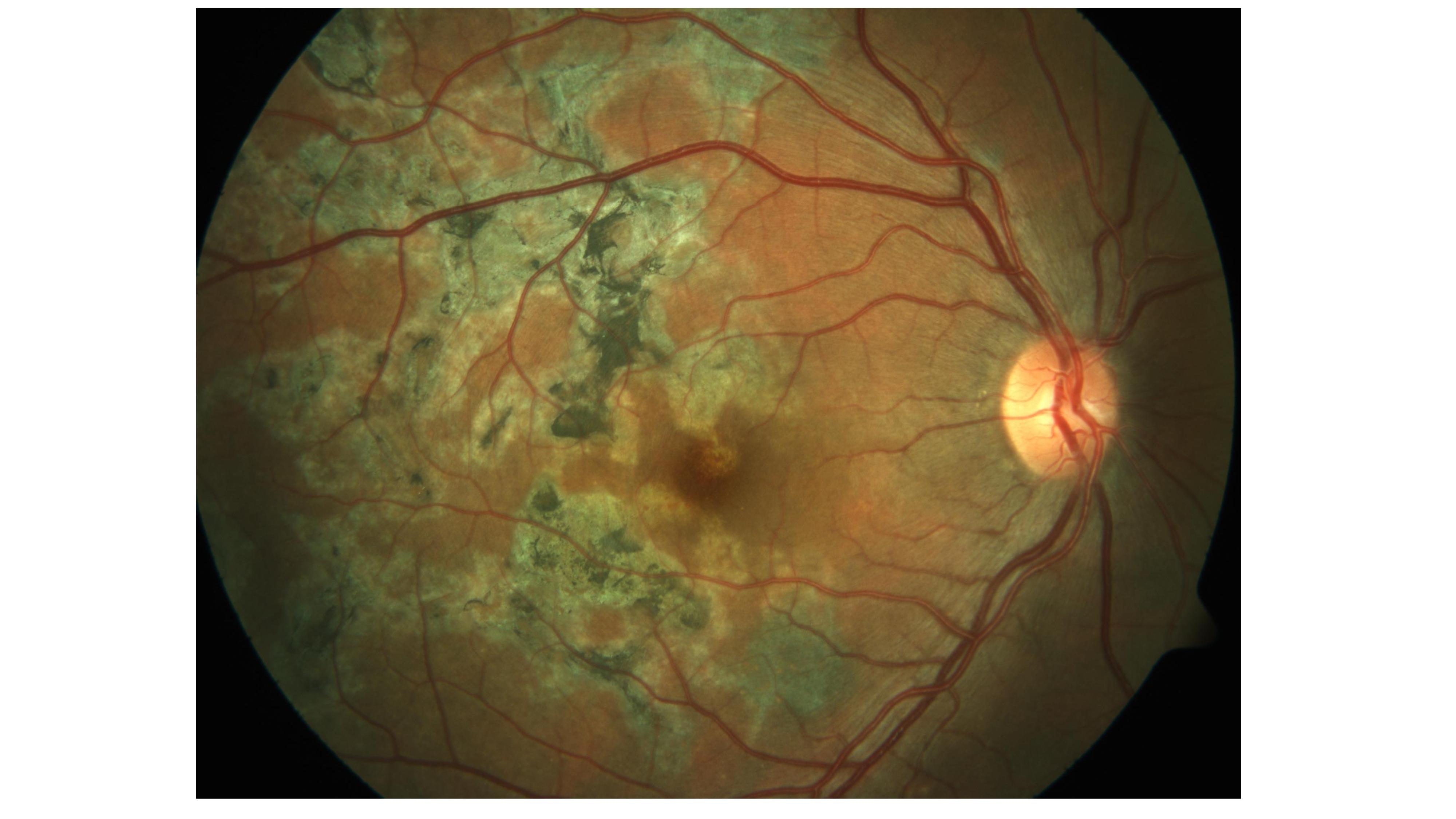
Noncontiguous, multifocal choroiditis may progress to a diffuse, contiguous pattern called a serpiginous-like lesion. This lesion resembles serpiginous choroiditis (multifocal serpiginous choroiditis); in contrast to classic serpiginous choroiditis, it does not extend to the disc, tends to spare the fovea even when the macula is involved, and it is more multifocal and pigmented (see Image. Tubercular Serpigionous-Like Choroiditis). The vitreous in tuberculous serpiginous-like choroiditis (TBSLC) is usually inflamed, whereas no vitreous inflammation is present in serpiginous choroiditis.[7][48] Gupta and colleagues described presumed TBSLC as having 3 morphological variants.[49] The "multifocal progressive choroiditis" started as multiple distinct patches of choroiditis that had "wave-like progression," and these eventually coalesced, resulting in diffuse choroiditis simulating serpiginous choroiditis. Some of these cases had retinal vasculitis.[49]
The second variant of presumed TBSLC manifested with diffuse plaque-like or ameboid choroiditis that initially resembled serpiginous choroiditis. Some of these patients may have optic disc edema and vitreous snowballs.[49] The mixed variant has a different presentation in the opposite eye. All such patients had strong TST, an induration of 20 mm or more after the Mantoux test using 5 tuberculin units or necrosis, and chest x-ray findings such as hilar lymphadenopathy or infiltrates.[49] A few such patients also had acid-fast bacilli on sputum examination, and histopathology of biopsied hilar or cervical lymph nodes showed caseation necrosis. The polymerase chain reaction for IS6110 in aqueous (ie, eyes with significant anterior chamber reaction) or vitreous was positive in patients with presumed TBSLC who underwent this test.[49] All such patients responded favorably with antituberculin therapy and oral corticosteroids.[49] However, whether TBSLC is due to direct infection of choroid by MTB (needing ATT) or a hypersensitivity reaction to TB (needing systemic steroids and or immunomodulatory therapy) is still being explored.[50] Choroiditis and tuberculoma may be associated with choroidal neovascular membranes.[51]
Retina clinical features
TB of the retina is almost always a result of the extension from choroidal disease. Rarely does hematogenous spread affect the retina before the choroid. Retinal lesions may include focal tubercles, subretinal abscesses, focal retinitis, or diffuse retinitis. Occlusive retinal vasculitis may occur and induce neovascularization. Exudative retinal hemorrhagic periphlebitis with uveitis is highly suggestive of intraocular TB.[52] Gupta et al reported approximately 50% of patients with tubercular retinal vasculitis have active or healed choroiditis patches under retinal vessels. Patients may also have snowball opacities in the inferior vitreous cavity, optic disc edema, or macular star.[40] Patients with tubercular retinitis may have periarterial plaques (ie, Kyrieleis arteriolitis).[53]
Optic nerve clinical features
Optic neuropathy develops from direct infection induced by TB or a hypersensitivity reaction to the infectious agent. This condition may present as an optic nerve tubercle, papillitis, neuroretinitis, or papilledema. Optic nerve swelling and posterior tuberculous scleritis have been reported.[54] The optic nerves of both eyes may be affected by toxic optic neuropathy due to ethambutol or isoniazid.[55] Papilledema and secondary optic atrophy in both eyes may result from increased intracranial pressure due to tubercular meningitis, obstructive hydrocephalus, or edema due to multiple tuberculomas in the brain.[56]
Endophthalmitis
Tubercular subretinal abscesses may burst into the vitreous cavity and present as endophthalmitis. Panophthalmitis and orbital cellulitis may also occur.[23][33][23]
Evaluation
Due to the various ocular TB manifestations, a clinical diagnosis is quite challenging. Diagnosing ocular TB begins with a complete history, physical examination, and fundoscopic exam. Gupta et al identified broad-based posterior synechiae, retinal vasculitis without choroiditis, retinal vasculitis with choroiditis, and serpiginous-like choroiditis as features of ocular TB with specificities of 93%, 97%, 99%, and 98%, respectively. However, all these findings have poor sensitivities.[52] Other highly suspicious lesions include choroidal granulomas (granulomatous uveitis), multifocal serpiginous choroiditis, occlusive retinal periphlebitis, or vasculitis.[7][41][43][48][52][54][57][58]
The evaluation of ocular TB should include:
- Ocular investigations: Ocular imaging includes anterior segment photography, optical coherence tomography of the macula, fundus fluorescein angiography (FFA), indocyanine green angiography, and ocular ultrasound.[59][60] Diagnosing ocular tuberculosis often depends heavily on the clinical features and the ocular findings.
- Optical coherence tomography of the macula can reveal intraretinal fluid in cystoid macular edema associated with intermediate uveitis. Inflammatory choroidal neovascular membranes may be associated with subretinal fluid and finger-like projection from choroidal neovascular membranes to the outer retina (pitchfork sign).[61] Bacillary layer detachment has also been reported in TBSLC.[62] The tubercular choroidal granuloma may show the characteristic contact sign adjacent to it, denoted by contact between the outer retina and the retinal pigment epithelium near an area with subretinal fluid.[63]
- FFA shows early hypofluorescence and late hyperfluorescence in active choroiditis. Window defects and block fluorescence denote healed choroiditis. FFA helps to rule out other differential diagnoses.
- Ocular ultrasound helps rule out posterior scleritis (subtenon fluid) and ocular malignancies (eg, amelanotic choroidal melanoma).
- Fundus autofluorescence shows hyperautofluorescence with ill-defined margins in areas of active choroiditis, whereas healed choroiditis shows hypoautofluorescence with sharp borders.[64][65]
- Anterior chamber fluid or vitreous humor sample can be used in molecular diagnostic testing via polymerase chain reaction (PCR). The most common target for PCR to diagnose tuberculosis is IS6110. Other targets include MPB64 or MPT64 and protein B.[66][67] PCR is becoming the testing method of choice because of better accuracy and faster test results than culture. Results from a case-control study of 22 patients with known TB uveitis demonstrated a 77.2% sensitivity and 92.1% specificity for PCR detection of MTB in aqueous and vitreous aspirates.[68] The positivity of PCR may not signify the presence of actively multiplying live microorganisms. PCR for tuberculosis was positive in the subretinal fluid of cases with retinal detachment and those with tuberculin skin test (TST) positivity.[69] Thus, the results of PCR have to be correlated with clinical features, specifically in countries with high endemicity.
- Laboratory studies: TST and IGRA evaluate the patient's cellular immune response to MTB; both tests have strengths and limitations.[70]
- TST can be falsely positive after Bacille Calmette-Guérin (BCG) vaccinations and other mycobacteria infections.[71] Skin induration at the injection site is checked 48 to 72 hours after intradermal injection of tuberculin.
- Tests for interferon-gamma release assay (eg, T-SPOT and QauntiFERON-TB Gold) are more specific, but false-positive cases can occur in low-endemic areas.[72] Contrary to the tuberculin skin test, IGRA is an in vitro test. The WHO noted that "there is insufficient data and low-quality evidence on the performance of IGRAs in low- and middle-income countries, typically those with a high TB and/or HIV burden."[73] The same WHO policy statement also mentions that "neither IGRAs nor the TST should be used for the diagnosis of active TB disease."[73]
- In patients from tuberculosis-endemic countries, the role of TST and IGRA is debatable as most of such patients already have exposure to MTB and BCG vaccination. The positivity of these tests suggests latent TB with exposure to MTB and does not denote a diagnosis of active tuberculosis in a patient from an endemic country.[73]
- The WHO recommends using GeneXpert MTB/RIF assay, an automated real-time PCR, to rapidly and simultaneously detect TB and rifampicin resistance in <2 hours. This is a nucleic acid amplification technique. Xpert MTB/RIF assay is currently used with sputum samples; the Food and Drug Administration has not approved it for use with ocular fluids.[20][58]
- Imaging studies: Up to 60% of cases with extrapulmonary TB may not have evidence of lung TB and may have a normal chest x-ray.[74][75][50] Chest x-ray or high-resolution computed tomography (CT) of the chest demonstrates findings of pulmonary TB, including hilar lymph node enlargement, pulmonary infiltrates, and cavitation.[76]
- High-resolution computed tomography (HRCT) chest has a better sensitivity (96%) of picking up active pulmonary tuberculosis than chest x-rays (48%).[77][78] Another study noted that "the sensitivity, specificity, positive predictive value, negative predictive values of thorax HRCT in determining the activity of the illness (pulmonary tuberculosis) were found as 97%, 86.7%, 94.2%, and 92.9%, respectively."[77]
- Magnetic resonance imaging is preferred for TB of central nervous system and tuberculous spondylitis.[78]
- Positron emission tomography-computed tomography (PET-CT) may help in evaluating the response of pulmonary and extrapulmonary TB to antitubercular therapy.[79]
- Neuroimaging should be done in patients with choroidal tuberculoma to rule out brain involvement.[80]
- Biopsy: A fine-needle aspiration biopsy or tissue biopsy attempt may be made as the culture of MTB is the gold-standard diagnosis.[23] Demonstration of acid-fast bacilli also confirms the diagnosis of tuberculosis. However, samples are difficult to collect, impose significant risk on patients, and culture results may take up to 10 weeks.[81]
Presumptive Intraocular Tuberculosis Criteria
A definitive diagnosis of TB uveitis is only made when MTB, or its deoxyribonucleic acid, is isolated from ocular fluids. In most cases, ophthalmologists are unable to make a definitive diagnosis. Still, the patient may have ophthalmological features consistent with ocular TB, confirmed TB exposure (positive TST or IGRA), or evidence of a tubercular lesion on a chest x-ray or CT scan. If 1 of these features is present, the diagnosis of "presumed ocular TB" should be made, and treatment should be offered.[19][70][82][83] However, a negative result on these tests does not rule out the disease. TSTs often yield negative results in patients with disseminated TB [84] and about 60% of patients with extrapulmonary TB have no evidence of pulmonary TB.[85] Gupta and colleagues classified intraocular TB into 3 types of presumptive intraocular TB, comprising confirmed, probable, and possible criteria.
Confirmed intraocular tuberculosis
Both of the following findings should be present:
- A minimum of 1 clinical sign suggestive of intraocular tuberculosis should be present. The clinical signs include:
- Anterior chamber or vitreous cells with or without posterior synechia
- Vitreous snowballs
- Perivascular cuffing
- Single or multiple choroidal tuberculoma with or without subretinal fluid
- Optic nerve head granuloma with or without neuroretinitis
- Subretinal abscess
- Microbiological confirmation of tuberculosis bacilli in the sample for ocular fluids or tissue [57]
Probable intraocular tuberculosis
All 3 criteria must be present together, including:
- A minimum of 1 clinical sign suggestive of intraocular tuberculosis should be present. Other causes should be excluded.
- Evidence of chest x-ray consistent with TB infection or clinical evidence of extraocular TB or microbiological confirmation from sputum or extraocular sites
- At least 1 of the following:
- Documented exposure to TB
- Immunological evidence of TB infection [57]
Possible intraocular tuberculosis
This category requires the presence of all of the following first, second, and third criteria or both the first and fourth criteria:
- At least 1 clinical sign suggestive of intraocular TB with other etiologies excluded
- Chest x-ray not consistent with TB infection and no clinical evidence of extraocular TB
- At least 1 of the following:
- Documented exposure to TB
- Immunological evidence of TB infection
- Evidence of chest x-ray consistent with TB infection or clinical evidence of extraocular TB but none of the characteristics given in the third criterion [57]
Presumptive intraocular tuberculosis treatment
Treatment of presumed ocular tuberculosis begins with an ATT trial. Patients are evaluated for response to a 4-drug course of isoniazid, rifampicin, ethambutol, and pyrazinamide. After 4 to 6 weeks of treatment, a positive response is taken as evidence for ocular TB, and treatment should be continued.[23] The possible side effects of ATT, including hepatotoxicity and toxic optic neuropathy, make the clinical decision to start ATT challenging, particularly when a definitive diagnosis of TB is not reached.[86]
Treatment / Management
In general, treating ocular tuberculosis is the same as treating extrapulmonary TB. Treatment consists of a 4-drug regimen administered in 2 phases: rifampicin, isoniazid, pyrazinamide, and ethambutol daily for 2 months, followed by rifampicin and isoniazid for 4 or more months. The total duration of ATT for ocular TB varies from 6 to 19 months.[87] ATT for at least 9 months in ocular TB may reduce the likelihood of recurrence by 11 times.[88] Bansal and colleagues evaluated 360 patients with active uveitis (ie, cellular reaction in the anterior chamber with or without keratic precipitates and active vitreous inflammation, retinal vasculitis, choroiditis, or neuro-retinitis) and positive TST (induration of ≥10 mm), for whom causes other than TB were excluded.[89] They found that adding corticosteroids to ATT reduced the chances of recurrence of ocular inflammation.[89](A1)
If the patient fails to respond in 3 to 4 weeks, multidrug-resistant TB should be considered, and management should continue in conjunction with an infectious disease specialist.[90] Multidrug-resistant TB is defined as resistance to at least isoniazid and rifampicin. Preextensively drug-resistant tuberculosis is defined by the United States Centers for Disease Control (CDC) as TB "caused by an organism that is resistant to isoniazid, rifampin, and a fluoroquinolone OR by an organism that is resistant to isoniazid, rifampin, and a second-line injectable (eg, amikacin, capreomycin, and kanamycin)." Extensively drug-resistant tuberculosis is defined by CDC as TB "caused by an organism that is resistant to isoniazid, rifampin, a fluoroquinolone, and a second-line injectable (eg, amikacin, capreomycin, and kanamycin) OR by an organism that is resistant to isoniazid, rifampin, a fluoroquinolone, and bedaquiline or linezolid."(B3)
Most authorities agree that choroidal tubercle, tuberculoma, and subretinal abscess need ATT as they are considered direct infections by MTB.[7][91] Usually, these will regress with ATT alone.[92] Sometimes, oral corticosteroids are added to reduce inflammation. However, whether other forms of presumed ocular TB, including TBSLC, vitritis, retinal vasculitis, and neuroretinitis, are due to active invasion by MTB or a hypersensitivity reaction to MTB or due to remote immune priming is still being explored.[93] Rao et al described the acid-fast bacilli in the retinal pigment epithelium in a patient with panuveitis; the real-time PCR was positive for IS6110.[94](B3)
Steroids are given to reverse insult from granulomatous inflammation and to help prevent a delayed-type hypersensitivity response to TB antigens.[95] They must be used judiciously with ATT, not alone, due to the concern of inducing latent disease reactivation or prolonging the active growth of bacilli in the eye.[23] Rifampicin induces hepatic steroid metabolizing enzymes and will suppress the therapeutic effect of corticosteroids. Increasing the corticosteroid dosage when in concomitant use with rifampicin may be necessary to maintain effectiveness.[96](B3)
Paradoxical worsening after initiation of ATT has been reported. This phenomenon is thought to result from a Jarisch-Herxheimer-like reaction as the immune system gains increased exposure to bacterial antigens after treatment, releasing pro-inflammatory cytokines.[97] Cotreatment with corticosteroids can circumvent this phenomenon.[23] (B3)
Agarwal et al found that tubercular serpiginous-like choroiditis with poor initial best-corrected visual acuity and foveal and optic disc involvement was associated with inadequate response to therapy. They also noted that a higher grade of lesion opacity at baseline may be associated with a higher risk of poor therapeutic response and paradoxical worsening with treatment.[98] In addition to ATT, TB delayed hypersensitivity retinal vasculitis (Eales disease) patients with retinal new vessels also benefit from peripheral scatter retinal laser treatment to decrease the ischemic drive for retinal neovascularization.[27] Choroidal neovascular membranes associated with choroidal tuberculosis may respond well to anti-vascular endothelial growth factor agents.[51] Large choroidal tuberculomas may also respond well to intravitreal anti-vascular endothelial growth factor agents.[99](B3)
The Collaborative Ocular Tuberculosis Study Recommendations
Due to a lack of high-level evidence to guide clinicians in the management of tubercular uveitis, the Collaborative Ocular Tuberculosis Study (COTS), an international expert-led consensus initiative, developed evidence- and experience-based recommendations regarding initiation of antitubercular treatment ATT and adjunctive anti-inflammatory and immunosuppressive therapy for the various subtypes of tubercular choroiditis (eg, TBSLC, tuberculoma, and tubercular unifocal or multifocal choroiditis). However, it must be noted that most of these are opinions of various experts and are not a result of randomized control trials or studies.[100][101][102][103][104](B3)
Due to its high association with TB, TBSLC can be treated with ATT with only a single positive immunologic test, either a TST or IGRA, without radiologic evidence of TB. However, the patient's background should be factored into the clinical evaluation. In a TB-endemic region, an isolated positive TST, despite negative IGRA, is sufficient to initiate ATT. This is a testament to the strong predictive value of a positive TST for ocular TB in patients of endemic areas with the serpiginous-like choroiditis phenotype. However, the positivity rate of TST in such areas may be too high, and the side effects of ATT must be considered before starting.[50][72][105] Another factor that complicates the clinical judgment to start ATT in TBSLC is its very similar clinical appearance to serpiginous choroiditis, which is an autoimmune phenomenon and needs immunosuppression alone as therapy.(B2)
In non endemic regions, however, a positive IGRA is required to initiate ATT because of its increased specificity for TB. COTS guidelines further suggest that a tuberculoma is highly representative of tubercular uveitis. As such, ATT should be initiated if there is any immunologic evidence for TB. In endemic regions, radiographic evidence alone may be sufficient to support treatment initiation.[104] In contrast to TBSLC and tuberculoma, the unifocal and multifocal choroiditis phenotypes have a relatively weaker association with TB. As such, immunologic evidence and radiologic findings suggestive of healed or active pulmonary TB are necessary to support the initiation of ATT for these phenotypes.[104](B3)
Furthermore, the COTS recommends that oral corticosteroids be started concomitantly with or soon after initiation of ATT to control the inflammatory response of infection in patients with TBSLC, tuberculoma (except in the setting of active systemic TB), and tubercular unifocal or multifocal choroiditis. Clinicians can justify starting systemic corticosteroid-sparing immunosuppressive therapy for patients with TBSLC and tubercular unifocal or multifocal choroiditis who have recurrent inflammation while tapering the dose of oral corticosteroids. Potential drug interactions when combining ATT with immunosuppressive agents must always be considered.[104](B3)
The COTS developed the COTS Calculator, an online clinical scoring system for initiation of ATT in patients with ocular TB, which was derived from expert consensus data. This scoring system can be accessed online at https://www.oculartb.net/cots-calc. The calculator will generate a score of 1 to 5 by inputting patient details and relevant clinical findings.[100] Interpretation of generated scores is as follows:(B3)
- 1: Very low probability for most experts to initiate ATT (<20%)
- 2: Low probability for most experts to initiate ATT (21% to 40%)
- 3: Mixed probability for most experts to initiate ATT (41% to 60%)
- 4: High probability for most experts to initiate ATT (61% to 80%)
- 5: Very high probability for most experts to initiate AT (81% to 100%)
Differential Diagnosis
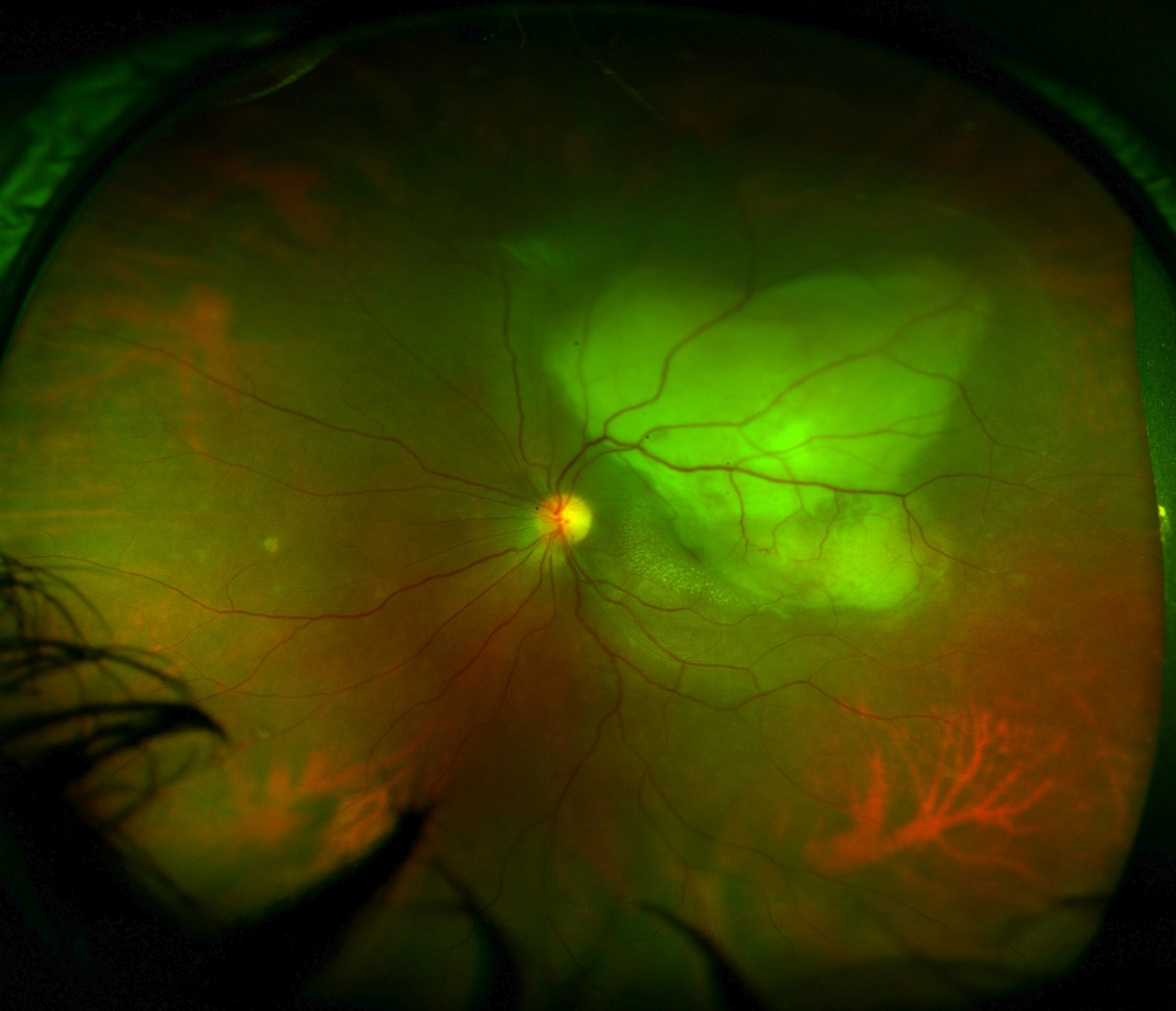
Sarcoidosis, syphilis, toxoplasmosis, toxocariasis, and fungal infections can present as panuveitis with choroidal involvement and should be considered in the differential diagnosis of tubercular uveitis.[106] Tubercular anterior uveitis in children may mimic juvenile idiopathic uveitis. In patients presenting predominantly with retinal vasculitis, Eales disease should be considered. Eales disease is characterized by overlapping stages of retinal vasculitis, vascular occlusion, and retinal neovascularization (see Image. Eales Disease). The cause of Eales disease remains unknown, but there is speculation it is a delayed retinal hypersensitivity reaction to MTB. Prior study results have shown patients with Eales disease have consistently been found more likely to test positive for MTB than control populations.[107]
Ophthalmologists should also consider the diagnosis of acute posterior multifocal placoid pigment epitheliopathy (APMPPE) or classic serpiginous choroiditis in patients presenting with TBSC. APMPPE is a rare and poorly understood inflammatory chorioretinopathy that predominantly affects the macula. Evidence supports a primary vasculitis or a delayed-type hypersensitivity reaction to various pathogens, including MTB.[108] The acute phase of APMPPE presents with multifocal flat, gray-white lesions with a placoid appearance found at the level of the posterior pole in the retinal pigmented epithelium.[109]
Fluorescein angiography reveals early hypofluorescence and late, irregular hyperfluorescence. Similarly, the early stages of TBSC can resemble APMPPE as it may present with yellowish-white plaque-like lesions that appear hypofluorescent early and hyperfluorescent late on fluorescein angiography.[49] However, the individual discrete lesions of TBSC progressively enlarge and converge into diffuse, contiguous choroiditis with an active leading edge.
Prognosis
The immediate use of ATT in ocular TB is associated with good outcomes. ATT was noted to reduce the recurrence of TBSLC in a study.[48] Most lesions completely regress and leave minimal residual damage. Depending on the ocular TB, some lesions resolve as focal chorioretinal scars. However, lesions in the context of acquired immunodeficiency syndrome have been reported to progress despite ATT.[106]
Complications
Clinicians should also know that ATT medications are associated with adverse ocular effects. Ethambutol causes dose-dependent ocular toxicity, eg, optic neuritis, photophobia, extraocular muscle paresis, red-green dyschromatopsia, central scotomas, and disc edema.[110] Patients receiving doses of more than 15 mg/kg/day require an ophthalmic evaluation every 4 weeks. Symptoms that occur typically resolve in 3 to 12 months.[34] Most patients with TBSLC have pigmented scars over the posterior pole after healing from choroiditis. However, the visual acuity and the fovea may be preserved till late disease. Recurrence is another issue with TBSLC. Healed ocular choroidal tuberculosis may be associated with a choroidal neovascular membrane that may respond well to anti-VEGF agents.[51]
Deterrence and Patient Education
Patients diagnosed with ocular TB should be counseled on the 6-month duration of treatment and the health consequences if not completed. Directly observed treatment is a strategy WHO recommends to increase treatment adherence, where a clinician or family member witnesses the patient taking treatment.
Pearls and Other Issues
Clinicians should bear in mind the following factors when managing ocular TB:
- The most common mechanism of ocular involvement is hematogenous spread from pulmonary TB.
- Ocular TB can involve any part of the eye and can occur with or without evidence of pulmonary or extrapulmonary TB disease.
- The most common ocular TB manifestation is granulomatous uveitis; the most common presentation is posterior uveitis.
- Choroidal granulomas, occlusive retinal vasculitis, and multifocal serpiginous-like choroiditis are the most typical lesions related to ocular TB.
- The gold standard for diagnosis is the identification of MTB in culture. However, this is rarely possible. PCR of ocular fluid is a recent alternative to cultures but is not widely available. Diagnosis, in most cases, is “presumed ocular TB.” Ocular findings must be supported in context with risk factor history, chest radiography findings, and TST or IGRA testing. Negative tests do not rule out ocular TB.
- Treatment of ocular TB is the same as for pulmonary TB, and concomitant steroid therapy is often required.
Enhancing Healthcare Team Outcomes
A management approach involving physicians, advanced practitioners, nurses, pharmacists, and other health professionals is essential to enhance patient-centered care, outcomes, patient safety, and team performance related to ocular TB. Given that MTB is the leading infectious disease killer of individuals with human immunodeficiency virus/acquired immunodeficiency syndrome worldwide, healthcare professionals should be cognizant of the potential extrapulmonary complication of TB, particularly its spread to ocular tissue.
Physicians and ophthalmologists play a pivotal role in educating fellow clinicians and healthcare professionals about the possibility of ocular TB and its visual implications if undiagnosed. They should ensure that the retinal examination becomes a part of the initial workup for all patients with a CD4 count of less than 100 cells/µL. Furthermore, interprofessional communication and care coordination among healthcare clinicians are critical in referring TB and HIV-infected individuals to infectious disease specialists for managing medical treatment. Nurses and pharmacists also have vital roles in administering TST to HIV-infected patients and should be actively involved in the care team.
Local health officials should be contacted if an individual with HIV or any patient tests positive for TB infection, as the majority of states mandate reporting confirmed TB to the proper authorities within 24 hours. By leveraging the expertise of physicians, ophthalmologists, advanced clinicians, nurses, pharmacists, and other health professionals, a collaborative and proactive approach can be established to enhance patient-centered care, improve outcomes, prioritize patient safety, and optimize overall team performance in addressing ocular TB and its implications for individuals coinfected with HIV/AIDS.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Koundanya VV, Tripathy K. Syphilis Ocular Manifestations. StatPearls. 2024 Jan:(): [PubMed PMID: 32644383]
Helm CJ, Holland GN. Ocular tuberculosis. Survey of ophthalmology. 1993 Nov-Dec:38(3):229-56 [PubMed PMID: 8310395]
Level 3 (low-level) evidenceEMERY JL, LORBER J. Radiological and pathological correlation of miliary tuberculosis of lungs in children, with special reference to choroidal tubercles. British medical journal. 1950 Sep 23:2(4681):702-4 [PubMed PMID: 14772458]
Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS medicine. 2016 Oct:13(10):e1002152. doi: 10.1371/journal.pmed.1002152. Epub 2016 Oct 25 [PubMed PMID: 27780211]
MacNeil A, Glaziou P, Sismanidis C, Maloney S, Floyd K. Global Epidemiology of Tuberculosis and Progress Toward Achieving Global Targets - 2017. MMWR. Morbidity and mortality weekly report. 2019 Mar 22:68(11):263-266. doi: 10.15585/mmwr.mm6811a3. Epub 2019 Mar 22 [PubMed PMID: 30897077]
Kon OM, Beare N, Connell D, Damato E, Gorsuch T, Hagan G, Perrin F, Petrushkin H, Potter J, Sethi C, Stanford M. BTS clinical statement for the diagnosis and management of ocular tuberculosis. BMJ open respiratory research. 2022 Mar:9(1):. doi: 10.1136/bmjresp-2022-001225. Epub [PubMed PMID: 35379660]
Albert DM, Raven ML. Ocular Tuberculosis. Microbiology spectrum. 2016 Nov:4(6):. doi: 10.1128/microbiolspec.TNMI7-0001-2016. Epub [PubMed PMID: 27837746]
Jilani TN, Avula A, Zafar Gondal A, Siddiqui AH. Active Tuberculosis. StatPearls. 2024 Jan:(): [PubMed PMID: 30020618]
Dannenberg AM Jr. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunology today. 1991 Jul:12(7):228-33 [PubMed PMID: 1822092]
Level 3 (low-level) evidenceJamwal SV, Mehrotra P, Singh A, Siddiqui Z, Basu A, Rao KV. Mycobacterial escape from macrophage phagosomes to the cytoplasm represents an alternate adaptation mechanism. Scientific reports. 2016 Mar 16:6():23089. doi: 10.1038/srep23089. Epub 2016 Mar 16 [PubMed PMID: 26980157]
Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland). 2010 Nov:90(6):361-6. doi: 10.1016/j.tube.2010.08.005. Epub 2010 Sep 9 [PubMed PMID: 20829117]
Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM, Sherman DR. Incipient and Subclinical Tuberculosis: a Clinical Review of Early Stages and Progression of Infection. Clinical microbiology reviews. 2018 Oct:31(4):. doi: 10.1128/CMR.00021-18. Epub 2018 Jul 18 [PubMed PMID: 30021818]
Holmes KK, Bertozzi S, Bloom BR, Jha P, Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, Salomon J, Vassall A, Volchenkov G, White R, Wilson D, Yadav P. Tuberculosis. Major Infectious Diseases. 2017 Nov 3:(): [PubMed PMID: 30212088]
Fukunaga R, Glaziou P, Harris JB, Date A, Floyd K, Kasaeva T. Epidemiology of Tuberculosis and Progress Toward Meeting Global Targets - Worldwide, 2019. MMWR. Morbidity and mortality weekly report. 2021 Mar 26:70(12):427-430. doi: 10.15585/mmwr.mm7012a4. Epub 2021 Mar 26 [PubMed PMID: 33764960]
Alvarez GG, Roth VR, Hodge W. Ocular tuberculosis: diagnostic and treatment challenges. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2009 Jul:13(4):432-5. doi: 10.1016/j.ijid.2008.09.018. Epub 2009 Apr 22 [PubMed PMID: 19386531]
Donahue HC. Ophthalmologic experience in a tuberculosis sanatorium. American journal of ophthalmology. 1967 Oct:64(4):742-8 [PubMed PMID: 6061532]
Bouza E, Merino P, Muñoz P, Sanchez-Carrillo C, Yáñez J, Cortés C. Ocular tuberculosis. A prospective study in a general hospital. Medicine. 1997 Jan:76(1):53-61 [PubMed PMID: 9064488]
Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian journal of ophthalmology. 2004 Jun:52(2):121-5 [PubMed PMID: 15283216]
Level 2 (mid-level) evidenceMorimura Y, Okada AA, Kawahara S, Miyamoto Y, Kawai S, Hirakata A, Hida T. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology. 2002 May:109(5):851-7 [PubMed PMID: 11986087]
Pillai S, Malone TJ, Abad JC. Orbital tuberculosis. Ophthalmic plastic and reconstructive surgery. 1995 Mar:11(1):27-31 [PubMed PMID: 7748819]
Level 3 (low-level) evidenceHeiden D, Saranchuk P, Keenan JD, Ford N, Lowinger A, Yen M, McCune J, Rao NA. Eye examination for early diagnosis of disseminated tuberculosis in patients with AIDS. The Lancet. Infectious diseases. 2016 Apr:16(4):493-9. doi: 10.1016/S1473-3099(15)00269-8. Epub 2016 Feb 18 [PubMed PMID: 26907735]
Ludi Z, Sule AA, Samy RP, Putera I, Schrijver B, Hutchinson PE, Gunaratne J, Verma I, Singhal A, Nora RD, van Hagen PM, Dik WA, Gupta V, Agrawal R. Diagnosis and biomarkers for ocular tuberculosis: From the present into the future. Theranostics. 2023:13(7):2088-2113. doi: 10.7150/thno.81488. Epub 2023 Apr 1 [PubMed PMID: 37153734]
Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Survey of ophthalmology. 2007 Nov-Dec:52(6):561-87 [PubMed PMID: 18029267]
Level 3 (low-level) evidenceChawla R, Venkatesh P, Tripathy K, Chaudhary S, Sharma SK. Successful Management of Proliferative Diabetic Retinopathy and Multiple Choroidal Tubercles in a Patient with Miliary Tuberculosis. Journal of ophthalmic & vision research. 2018 Apr-Jun:13(2):210-211. doi: 10.4103/jovr.jovr_203_16. Epub [PubMed PMID: 29719654]
Tripathy K, Chawla R, Sharma YR. Intravitreal Bevacizumab for Choroidal Neovascular Membrane at the Edge of a Healed Choroidal Tuberculoma. Ocular immunology and inflammation. 2018:26(2):239-241. doi: 10.1080/09273948.2016.1206205. Epub 2016 Aug 19 [PubMed PMID: 27541084]
Tripathy K, Chawla R. Choroidal tuberculoma. The National medical journal of India. 2016 Mar-Apr:29(2):106 [PubMed PMID: 27586221]
Raizada K,Tripathy K, Eales Disease StatPearls. 2022 Jan; [PubMed PMID: 32644547]
Biswas J, Therese L, Madhavan HN. Use of polymerase chain reaction in detection of Mycobacterium tuberculosis complex DNA from vitreous sample of Eales' disease. The British journal of ophthalmology. 1999 Aug:83(8):994 [PubMed PMID: 10413707]
Level 3 (low-level) evidenceMadhavan HN, Therese KL, Gunisha P, Jayanthi U, Biswas J. Polymerase chain reaction for detection of Mycobacterium tuberculosis in epiretinal membrane in Eales' disease. Investigative ophthalmology & visual science. 2000 Mar:41(3):822-5 [PubMed PMID: 10711699]
Bayot ML, Mirza TM, Sharma S. Acid Fast Bacteria. StatPearls. 2024 Jan:(): [PubMed PMID: 30725806]
Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American family physician. 2005 Nov 1:72(9):1761-8 [PubMed PMID: 16300038]
Level 3 (low-level) evidenceKhan FY. Review of literature on disseminated tuberculosis with emphasis on the focused diagnostic workup. Journal of family & community medicine. 2019 May-Aug:26(2):83-91. doi: 10.4103/jfcm.JFCM_106_18. Epub [PubMed PMID: 31143078]
Chawla R, Garg S, Venkatesh P, Kashyap S, Tewari HK. Case report of tuberculous panophthalmitis. Medical science monitor : international medical journal of experimental and clinical research. 2004 Oct:10(10):CS57-9 [PubMed PMID: 15448600]
Level 3 (low-level) evidenceThompson MJ, Albert DM. Ocular tuberculosis. Archives of ophthalmology (Chicago, Ill. : 1960). 2005 Jun:123(6):844-9 [PubMed PMID: 15955987]
Simakurthy S, Tripathy K. Marcus Gunn Pupil. StatPearls. 2025 Jan:(): [PubMed PMID: 32491607]
Dhakal S, Neupane S, Arjyal Kafle P, Badhu BP, Upadhyaya Kafle S. Primary Orbital Tuberculosis with Cold Abscess: A Case Report. JNMA; journal of the Nepal Medical Association. 2024 Feb 24:62(270):148-151. doi: 10.31729/jnma.8441. Epub 2024 Feb 24 [PubMed PMID: 38409977]
Level 3 (low-level) evidenceDalvin LA, Smith WM. Orbital and external ocular manifestations of Mycobacterium tuberculosis: A review of the literature. Journal of clinical tuberculosis and other mycobacterial diseases. 2016 Aug:4():50-57. doi: 10.1016/j.jctube.2015.11.001. Epub 2015 Nov 27 [PubMed PMID: 31723688]
Thomas S, Suhas S, Pai KM, Raghu AR. Lupus vulgaris--report of a case with facial involvement. British dental journal. 2005 Feb 12:198(3):135-7 [PubMed PMID: 15706374]
Level 3 (low-level) evidenceMadhukar K, Bhide M, Prasad CE, Venkatramayya. Tuberculosis of the lacrimal gland. The Journal of tropical medicine and hygiene. 1991 Jun:94(3):150-1 [PubMed PMID: 2051518]
Level 3 (low-level) evidenceGupta A, Gupta V, Arora S, Dogra MR, Bambery P. PCR-positive tubercular retinal vasculitis: clinical characteristics and management. Retina (Philadelphia, Pa.). 2001:21(5):435-44 [PubMed PMID: 11642371]
Goyal JL, Jain P, Arora R, Dokania P. Ocular manifestations of tuberculosis. The Indian journal of tuberculosis. 2015 Apr:62(2):66-73. doi: 10.1016/j.ijtb.2015.04.004. Epub 2015 Jun 16 [PubMed PMID: 26117474]
Latiff N, Lakshmipathy M, Janani MK, Dutta Majumder P. Tuberculous corneal ulcer with hypopyon: A case report. Indian journal of ophthalmology. 2020 May:68(5):922-924. doi: 10.4103/ijo.IJO_1368_19. Epub [PubMed PMID: 32317491]
Level 3 (low-level) evidenceNanda M, Pflugfelder SC, Holland S. Mycobacterium tuberculosis scleritis. American journal of ophthalmology. 1989 Dec 15:108(6):736-7 [PubMed PMID: 2512813]
Level 3 (low-level) evidenceTabbara KF. Ocular tuberculosis: anterior segment. International ophthalmology clinics. 2005 Spring:45(2):57-69 [PubMed PMID: 15791158]
Gurnani B, Kim J, Tripathy K, Mahabadi N, Edens MA. Iritis. StatPearls. 2024 Jan:(): [PubMed PMID: 28613659]
Geetha R, Tripathy K. Chorioretinitis. StatPearls. 2024 Jan:(): [PubMed PMID: 31869169]
Kohli P, Tripathy K, Patel BC. Macular Edema. StatPearls. 2024 Jan:(): [PubMed PMID: 35015421]
Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012 Nov:119(11):2334-42. doi: 10.1016/j.ophtha.2012.05.034. Epub 2012 Aug 11 [PubMed PMID: 22892153]
Level 2 (mid-level) evidenceGupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginouslike choroiditis: clinical presentations and management. Ophthalmology. 2003 Sep:110(9):1744-9 [PubMed PMID: 13129872]
Level 2 (mid-level) evidenceShakarchi FI. Ocular tuberculosis: current perspectives. Clinical ophthalmology (Auckland, N.Z.). 2015:9():2223-7. doi: 10.2147/OPTH.S65254. Epub 2015 Nov 26 [PubMed PMID: 26648690]
Level 3 (low-level) evidenceTripathy K. Choroidal neovascular membrane in intraocular tuberculosis. GMS ophthalmology cases. 2017:7():Doc24. doi: 10.3205/oc000075. Epub 2017 Sep 1 [PubMed PMID: 28944155]
Level 3 (low-level) evidenceGupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. American journal of ophthalmology. 2010 Apr:149(4):562-70. doi: 10.1016/j.ajo.2009.11.020. Epub 2010 Feb 10 [PubMed PMID: 20149341]
Level 2 (mid-level) evidenceChawla R, Tripathy K, Sharma YR, Venkatesh P, Vohra R. Periarterial Plaques (Kyrieleis' Arteriolitis) in a Case of Bilateral Acute Retinal Necrosis. Seminars in ophthalmology. 2017:32(2):251-252. doi: 10.3109/08820538.2015.1045153. Epub 2015 Jul 10 [PubMed PMID: 26161821]
Level 3 (low-level) evidenceMINTON J. Ocular manifestations of tuberculous meningitis: a pictorial survey. Transactions. Ophthalmological Society of the United Kingdom. 1956:76():561-79 [PubMed PMID: 13409572]
Level 3 (low-level) evidenceSharma P, Sharma R. Toxic optic neuropathy. Indian journal of ophthalmology. 2011 Mar-Apr:59(2):137-41. doi: 10.4103/0301-4738.77035. Epub [PubMed PMID: 21350283]
Zuhaimy H, Leow SN, Vasudevan SK. Optic disc swelling in a patient with tuberculous meningitis: a diagnostic challenge. BMJ case reports. 2017 Aug 9:2017():. pii: bcr-2017-221170. doi: 10.1136/bcr-2017-221170. Epub 2017 Aug 9 [PubMed PMID: 28794092]
Level 3 (low-level) evidenceGupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocular immunology and inflammation. 2015 Feb:23(1):7-13. doi: 10.3109/09273948.2014.967358. Epub 2014 Oct 14 [PubMed PMID: 25314361]
Gupta A, Gupta V. Tubercular posterior uveitis. International ophthalmology clinics. 2005 Spring:45(2):71-88 [PubMed PMID: 15791159]
Muraleedharan S, Tripathy K. Indocyanine Green (ICG) Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35593804]
Ruia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Hoang QV, Cunningham ET Jr, Sorenson JA, Freund KB. The "pitchfork sign" a distinctive optical coherence tomography finding in inflammatory choroidal neovascularization. Retina (Philadelphia, Pa.). 2013 May:33(5):1049-55. doi: 10.1097/IAE.0b013e31827e25b8. Epub [PubMed PMID: 23514797]
Socci da Costa D, Gomes E Silva A, Melichar A, Neves DB, Correa PA, Moraes RT. Bacillary layer detachment in serpiginous-like choroiditis of presumed intraocular tuberculosis: Report of two cases. American journal of ophthalmology case reports. 2022 Sep:27():101653. doi: 10.1016/j.ajoc.2022.101653. Epub 2022 Jul 7 [PubMed PMID: 35845750]
Level 3 (low-level) evidenceSalman A, Parmar P, Rajamohan M, Vanila CG, Thomas PA, Jesudasan CA. Optical coherence tomography in choroidal tuberculosis. American journal of ophthalmology. 2006 Jul:142(1):170-2 [PubMed PMID: 16815274]
Lee CS, Lee AY, Forooghian F, Bergstrom CS, Yan J, Yeh S. Fundus autofluorescence features in the inflammatory maculopathies. Clinical ophthalmology (Auckland, N.Z.). 2014:8():2001-12. doi: 10.2147/OPTH.S68446. Epub 2014 Sep 29 [PubMed PMID: 25302012]
Gupta A, Bansal R, Gupta V, Sharma A. Fundus autofluorescence in serpiginouslike choroiditis. Retina (Philadelphia, Pa.). 2012 Apr:32(4):814-25. doi: 10.1097/IAE.0b013e3182278c41. Epub [PubMed PMID: 22080913]
Raveendran R, Wattal C. Utility of multiplex real-time PCR in the diagnosis of extrapulmonary tuberculosis. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2016 May-Jun:20(3):235-41. doi: 10.1016/j.bjid.2016.01.006. Epub 2016 Mar 26 [PubMed PMID: 27020707]
Sehgal V, Sharma M, Lnu P, Sharma K, Sharma A, Sharma N, Modi M. Comparison of Protein B Polymerase Chain Reaction (PCR) With IS6110 PCR for Diagnosis of Tuberculous Meningitis Patients. Cureus. 2023 Jan:15(1):e33783. doi: 10.7759/cureus.33783. Epub 2023 Jan 15 [PubMed PMID: 36798623]
Ortega-Larrocea G, Bobadilla-del-Valle M, Ponce-de-León A, Sifuentes-Osornio J. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Archives of medical research. 2003 Mar-Apr:34(2):116-9 [PubMed PMID: 12700006]
Level 2 (mid-level) evidenceBajgai P, Sharma K, Bansal R, Gupta N, Sharma A, Gupta A. Detection of Mycobacterium tuberculosis Genome in Subretinal Fluid of Patients with Latent Tuberculosis Infection. Ocular immunology and inflammation. 2016 Dec:24(6):615-620 [PubMed PMID: 26645647]
Vasconcelos-Santos DV, Zierhut M, Rao NA. Strengths and weaknesses of diagnostic tools for tuberculous uveitis. Ocular immunology and inflammation. 2009 Sep-Oct:17(5):351-5. doi: 10.3109/09273940903168688. Epub [PubMed PMID: 19831571]
Okafor CN, Rewane A, Momodu II. Bacillus Calmette Guerin. StatPearls. 2024 Jan:(): [PubMed PMID: 30844212]
Tripathy K. Comment on Trad et al.'s "Update on Immunological Test (Quantiferon-TB Gold) Contribution in the Management of Tuberculosis-Related Ocular Inflammation". Ocular immunology and inflammation. 2019:27(1):138-139. doi: 10.1080/09273948.2017.1371766. Epub 2017 Oct 11 [PubMed PMID: 29020492]
Level 3 (low-level) evidence. Use of Tuberculosis Interferon-Gamma Release Assays (IGRAs) in Low- and Middle- Income Countries: Policy Statement. 2011:(): [PubMed PMID: 26269875]
M A, El-Asrar A, Abouammoh M, Al-Mezaine HS. Tuberculous uveitis. Middle East African journal of ophthalmology. 2009 Oct:16(4):188-201. doi: 10.4103/0974-9233.58421. Epub [PubMed PMID: 20404986]
Cimino L, Herbort CP, Aldigeri R, Salvarani C, Boiardi L. Tuberculous uveitis, a resurgent and underdiagnosed disease. International ophthalmology. 2009 Apr:29(2):67-74 [PubMed PMID: 17486298]
Ganesh SK, Roopleen, Biswas J, Veena N. Role of high-resolution computerized tomography (HRCT) of the chest in granulomatous uveitis: a tertiary uveitis clinic experience from India. Ocular immunology and inflammation. 2011 Feb:19(1):51-7. doi: 10.3109/09273948.2010.525680. Epub [PubMed PMID: 21250925]
Balkan A, Balci E, Yüksekol I, Ozkan M, Taşan Y, Papuşcu Y, Ekiz K, Bilgiç H, Demirci N, Seber O. [The role of high resolution computerized tomography (HRCT) in the diagnosis and treatment of pulmonary tuberculosis]. Tuberkuloz ve toraks. 2004:52(1):38-46 [PubMed PMID: 15143371]
Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2015 Mar:32():87-93. doi: 10.1016/j.ijid.2014.12.007. Epub [PubMed PMID: 25809762]
Malherbe ST, Chen RY, Dupont P, Kant I, Kriel M, Loxton AG, Smith B, Beltran CGG, van Zyl S, McAnda S, Abrahams C, Maasdorp E, Doruyter A, Via LE, Barry CE 3rd, Alland D, Richards SG, Ellman A, Peppard T, Belisle J, Tromp G, Ronacher K, Warwick JM, Winter J, Walzl G. Quantitative 18F-FDG PET-CT scan characteristics correlate with tuberculosis treatment response. EJNMMI research. 2020 Feb 10:10(1):8. doi: 10.1186/s13550-020-0591-9. Epub 2020 Feb 10 [PubMed PMID: 32040770]
Khatri GD, Krishnan V, Antil N, Saigal G. Magnetic resonance imaging spectrum of intracranial tubercular lesions: one disease, many faces. Polish journal of radiology. 2018:83():e524-e535. doi: 10.5114/pjr.2018.81408. Epub 2018 Dec 29 [PubMed PMID: 30800191]
Heemskerk D, Caws M, Marais B, Farrar J. Tuberculosis in Adults and Children. 2015:(): [PubMed PMID: 26937536]
Figueira L, Fonseca S, Ladeira I, Duarte R. Ocular tuberculosis: Position paper on diagnosis and treatment management. Revista portuguesa de pneumologia. 2017 Jan-Feb:23(1):31-38. doi: 10.1016/j.rppnen.2016.10.004. Epub 2016 Dec 14 [PubMed PMID: 27988134]
Sanghvi C, Bell C, Woodhead M, Hardy C, Jones N. Presumed tuberculous uveitis: diagnosis, management, and outcome. Eye (London, England). 2011 Apr:25(4):475-80. doi: 10.1038/eye.2010.235. Epub 2011 Feb 4 [PubMed PMID: 21293496]
Level 2 (mid-level) evidenceKhan FY, Aladab AH. Role of Fiberoptic Bronchoscopy in the Rapid Diagnosis of Sputum Smear-negative Disseminated Tuberculosis with Pulmonary Miliary Infiltrates. Oman medical journal. 2020 Jan:35(1):e87. doi: 10.5001/omj.2020.05. Epub 2020 Jan 15 [PubMed PMID: 31993225]
Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984 Jan:63(1):25-55 [PubMed PMID: 6419006]
Saxena R, Singh D, Phuljhele S, Kalaiselvan V, Karna S, Gandhi R, Prakash A, Lodha R, Mohan A, Menon V, Garg R. Ethambutol toxicity: Expert panel consensus for the primary prevention, diagnosis and management of ethambutol-induced optic neuropathy. Indian journal of ophthalmology. 2021 Dec:69(12):3734-3739. doi: 10.4103/ijo.IJO_3746_20. Epub [PubMed PMID: 34827033]
Level 3 (low-level) evidenceKee AR, Gonzalez-Lopez JJ, Al-Hity A, Gupta B, Lee CS, Gunasekeran DV, Jayabalan N, Grant R, Kon OM, Gupta V, Westcott M, Pavesio C, Agrawal R. Anti-tubercular therapy for intraocular tuberculosis: A systematic review and meta-analysis. Survey of ophthalmology. 2016 Sep-Oct:61(5):628-53. doi: 10.1016/j.survophthal.2016.03.001. Epub 2016 Mar 10 [PubMed PMID: 26970263]
Level 1 (high-level) evidenceAng M, Hedayatfar A, Wong W, Chee SP. Duration of anti-tubercular therapy in uveitis associated with latent tuberculosis: a case-control study. The British journal of ophthalmology. 2012 Mar:96(3):332-6. doi: 10.1136/bjophthalmol-2011-300209. Epub 2011 Jun 30 [PubMed PMID: 21719564]
Level 2 (mid-level) evidenceBansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. American journal of ophthalmology. 2008 Nov:146(5):772-9. doi: 10.1016/j.ajo.2008.06.011. Epub 2008 Aug 16 [PubMed PMID: 18708180]
Seung KJ,Keshavjee S,Rich ML, Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harbor perspectives in medicine. 2015 Apr 27; [PubMed PMID: 25918181]
Level 3 (low-level) evidenceLyon CE, Grimson BS, Peiffer RL Jr, Merritt JC. Clinicopathological correlation of a solitary choroidal tuberculoma. Ophthalmology. 1985 Jun:92(6):845-50 [PubMed PMID: 3897938]
Young L, Yakin M, Sen HN. Choroidal granuloma resolution with tuberculosis treatment. American journal of ophthalmology case reports. 2020 Dec:20():100969. doi: 10.1016/j.ajoc.2020.100969. Epub 2020 Oct 16 [PubMed PMID: 33117917]
Level 3 (low-level) evidenceBasu S, Elkington P, Rao NA. Pathogenesis of ocular tuberculosis: New observations and future directions. Tuberculosis (Edinburgh, Scotland). 2020 Sep:124():101961. doi: 10.1016/j.tube.2020.101961. Epub 2020 Jul 24 [PubMed PMID: 33010848]
Level 3 (low-level) evidenceRao NA, Saraswathy S, Smith RE. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Dec:124(12):1777-9 [PubMed PMID: 17159041]
Sharma A, Thapa B, Lavaju P. Ocular tuberculosis: an update. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2011 Jan-Jun:3(1):52-67. doi: 10.3126/nepjoph.v3i1.4280. Epub [PubMed PMID: 21587325]
Schutz C, Davis AG, Sossen B, Lai RP, Ntsekhe M, Harley YX, Wilkinson RJ. Corticosteroids as an adjunct to tuberculosis therapy. Expert review of respiratory medicine. 2018 Oct:12(10):881-891. doi: 10.1080/17476348.2018.1515628. Epub 2018 Sep 6 [PubMed PMID: 30138039]
Ganesh SK, Abraham S, Sudharshan S. Paradoxical reactions in ocular tuberculosis. Journal of ophthalmic inflammation and infection. 2019 Sep 6:9(1):19. doi: 10.1186/s12348-019-0183-x. Epub 2019 Sep 6 [PubMed PMID: 31493128]
Agarwal A, Marchese A, Rabiolo A, Agrawal R, Bansal R, Gupta V. Clinical and Imaging Factors Associated With the Outcomes of Tubercular Serpiginous-like Choroiditis. American journal of ophthalmology. 2020 Dec:220():160-169. doi: 10.1016/j.ajo.2020.07.024. Epub 2020 Jul 23 [PubMed PMID: 32710829]
Jain S, Agarwal A, Gupta V. Resolution of Large Choroidal Tuberculoma following Monotherapy with Intravitreal Ranibizumab. Ocular immunology and inflammation. 2020 Apr 2:28(3):494-497. doi: 10.1080/09273948.2019.1582786. Epub 2019 Apr 15 [PubMed PMID: 30986122]
Agrawal R, Ludi Z, Betzler BK, Testi I, Mahajan S, Rousellot A, Kempen JH, Smith JR, McCluskey P, Nguyen QD, Pavesio C, Gupta V. The Collaborative Ocular Tuberculosis Study (COTS) calculator-a consensus-based decision tool for initiating antitubercular therapy in ocular tuberculosis. Eye (London, England). 2023 May:37(7):1416-1423. doi: 10.1038/s41433-022-02147-7. Epub 2022 Jun 28 [PubMed PMID: 35764876]
Level 3 (low-level) evidenceAgrawal R, Testi I, Bodaghi B, Barisani-Asenbauer T, McCluskey P, Agarwal A, Kempen JH, Gupta A, Smith JR, de Smet MD, Yuen YS, Mahajan S, Kon OM, Nguyen QD, Pavesio C, Gupta V, Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 2: Guidelines for Initiating Antitubercular Therapy in Anterior Uveitis, Intermediate Uveitis, Panuveitis, and Retinal Vasculitis. Ophthalmology. 2021 Feb:128(2):277-287. doi: 10.1016/j.ophtha.2020.06.052. Epub 2020 Jun 27 [PubMed PMID: 32603726]
Level 3 (low-level) evidenceShukla D. Re: Agrawal et al.: Collaborative Ocular Tuberculosis Study consensus guidelines on the management of tubercular uveitis - Report 2: guidelines for initiating antitubercular therapy in anterior uveitis, intermediate uveitis, panuveitis, and retinal vasculitis (Ophthalmology. 2021;128:277-287). Ophthalmology. 2021 Jul:128(7):e34-e35. doi: 10.1016/j.ophtha.2021.03.024. Epub 2021 Apr 15 [PubMed PMID: 33865623]
Level 3 (low-level) evidenceChawla R, Venkatesh P, Kumar V, Azad S. Re: Agrawal et al.: Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 1 (Ophthalmology. 2021;128:266-276). Ophthalmology. 2021 Dec:128(12):e217. doi: 10.1016/j.ophtha.2021.08.008. Epub 2021 Sep 6 [PubMed PMID: 34503846]
Level 3 (low-level) evidenceAgrawal R, Testi I, Mahajan S, Yuen YS, Agarwal A, Kon OM, Barisani-Asenbauer T, Kempen JH, Gupta A, Jabs DA, Smith JR, Nguyen QD, Pavesio C, Gupta V, Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 1: Guidelines for Initiating Antitubercular Therapy in Tubercular Choroiditis. Ophthalmology. 2021 Feb:128(2):266-276. doi: 10.1016/j.ophtha.2020.01.008. Epub 2020 Jan 11 [PubMed PMID: 32115264]
Level 3 (low-level) evidenceGülbay BE, Gürkan OU, Yildiz OA, Onen ZP, Erkekol FO, Baççioğlu A, Acican T. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respiratory medicine. 2006 Oct:100(10):1834-42 [PubMed PMID: 16517138]
Level 2 (mid-level) evidenceGriffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American journal of respiratory and critical care medicine. 2007 Feb 15:175(4):367-416 [PubMed PMID: 17277290]
Madhavan HN, Therese KL, Doraiswamy K. Further investigations on the association of Mycobacterium tuberculosis with Eales' disease. Indian journal of ophthalmology. 2002 Mar:50(1):35-9 [PubMed PMID: 12090085]
Abu El-Asrar AM, Aljazairy AH. Acute posterior multifocal placoid pigment epitheliopathy with retinal vasculitis and papillitis. Eye (London, England). 2002 Sep:16(5):642-4 [PubMed PMID: 12194084]
Level 3 (low-level) evidenceCozubas R, Ungureanu E, Instrate SL, Alexandrescu C, Nanu RV, Carstocea L, Voinea LM, Ciuluvica R. Similarities and differences between three different types of white dot syndrome and the therapeutic possibilities. Romanian journal of ophthalmology. 2018 Jul-Sep:62(3):183-187 [PubMed PMID: 30505986]
Barron GJ, Tepper L, Iovine G. Ocular toxicity from ethambutol. American journal of ophthalmology. 1974 Feb:77(2):256-60 [PubMed PMID: 4204592]