Ophthalmic Manifestations of Coronavirus (COVID-19)
Ophthalmic Manifestations of Coronavirus (COVID-19)
Introduction
Beginning in December 2019, COVID-19 developed into a global pandemic caused by the highly transmissible SARS-CoV-2.[1] Numerous anecdotal and published reports initially indicated eye redness and irritation in patients with COVID-19, supporting conjunctivitis as an ocular manifestation of SARS-CoV-2 infection. Ongoing reports reveal associations between COVID-19 and uveitic, retinovascular, and neuro-ophthalmic disease.
During the 2003 severe acute respiratory syndrome (SARS) outbreak, a study identified SARS-CoV in tear samples collected in Singapore from patients with SARS.[2] The absence of eye protection emerged as a primary risk factor for SARS-CoV transmission from patients to healthcare workers in Toronto, raising concerns about the potential for respiratory illnesses to be transmitted through ocular secretions.[3][4] Similar concerns have been expressed regarding SARS-CoV-2, particularly among eye care providers and frontline personnel responsible for assessing patients with the potential initial symptoms of COVID-19.
Dr Li Wenliang, an opthalmologist, was among the first to express apprehensions about the coronavirus transmission in Chinese patients. Unfortunately, he succumbed to COVID-19, and it was suspected that he contracted the virus from an asymptomatic glaucoma patient in his clinic.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
SARS-CoV-2 is an enveloped, positive single-stranded RNA beta coronavirus responsible for causing COVID-19 (see Image. Structure of COVID-19). It was initially associated with an outbreak in Wuhan, located in China's Hubei province.[1] Direct contact with mucous membranes, including the eye, is a suspected transmission route.
Coronaviruses have been known to cause severe ocular diseases in animals, leading to anterior uveitis, retinitis, vasculitis, and optic neuritis in feline and murine species. In contrast, ocular manifestations in humans are typically mild and rare, although there is a growing number of reported ocular findings in individuals who test positive for COVID-19.[6]
No ocular manifestations of Middle East respiratory syndrome (MERS) or SARS have been reported. However, as mentioned earlier, SARS-CoV was identified in ocular secretions.[2] Other coronaviruses have been found to cause viral conjunctivitis in humans.[7]
Epidemiology
As of April 4, 2020, the World Health Organization (WHO) reported 1,272,953 confirmed cases and 69,428 deaths worldwide due to COVID-19, with an additional 79,332 new cases confirmed in the preceding 24 hours. Meanwhile, the Centers for Disease Control and Prevention (CDC) had documented 337,278 cases and 9637 deaths in the United States up to that date.
On April 16, 2021, slightly over a year later, the global death toll surpassed the 3 million mark. The gravity of the pandemic is underscored by noting the rate of fatalities: it took 8.5 months from the first fatality in China to reach the initial 1 million lives lost, 3.5 months to reach 2 million, and just 3 months to exceed the 3 million lives lost milestone.
As of December 23, 2021, the US has reported 51,574,787 confirmed cases of COVID-19 and 809,300 deaths. Globally, the WHO has documented over 276 million confirmed cases and 5,374,744 deaths due to COVID-19. Additionally, 8,649,057,088 vaccine doses have been administered worldwide by that date. The United States leads in the number of infections, followed by India, Brazil, the United Kingdom, Russia, Turkey, and France.
Viral mutations leading to variants of SARS-CoV-2 have been identified globally, including the B.1.1.7 variant in the United Kingdom in early 2020, the B.1.526 variant in the United States in November 2020, the B.1.525 variant in the United Kingdom and Nigeria in December 2020, and the B.1.351 variant in South Africa in late 2020. The Delta variant, B.1.617.2, was first identified in India in December 2020 and rapidly spread across over 60 countries due to a 40% to 60% increase in transmissibility, becoming the predominant strain worldwide by August 2021.[8] Most recently, the Omicron variant, B.1.1.529, was designated a variant of concern in late November 2021 following cases that emerged from Botswana and South Africa, showing rapid and exponential spread.[9]
Initial studies postulated that ocular manifestations of COVID-19 were infrequent. Only 9 (0.8%) out of 1099 patients from 552 hospitals across 30 provinces in China were reported to exhibit conjunctival congestion from December 2019 through January 2020.[10] Recent data, however, indicate a significantly higher incidence of ocular signs and symptoms. A 2021 meta-analysis by Nasiri et al reported a pooled prevalence of all ocular manifestations among 7300 COVID-19 patients as 11.03%, with conjunctivitis being the most frequent ocular disease (88.8%).[11] In the same meta-analysis, other common symptoms included dry eye or foreign body sensation (16%), eye redness (13.3%), tearing (12.8%), and itching (12.6%).
In a case series involving 38 hospitalized patients with COVID-19 in Hubei province, China, ocular symptoms were reported in 12 individuals (31.6%).[12] These patients exhibited conjunctival hyperemia (3 patients), chemosis (7 patients), epiphora (7 patients), or increased secretions (7 patients). Notably, only 1 patient presented with epiphora as the initial symptom of COVID-19. Among those with ocular manifestations, 2 patients (16.7%) tested positive for SARS-CoV-2 through reverse-transcriptase polymerase chain reaction (RT-PCR) using a conjunctival swab and nasopharyngeal swabs. Only 1 patient in the study presented with conjunctivitis as the first symptom.[12] The authors observed that patients with ocular symptoms had higher white blood cell and neutrophil counts, C-reactive protein, and elevated levels of procalcitonin and lactate dehydrogenase than patients without ocular abnormalities.
In a study by Xia et al involving 30 hospitalized patients with COVID-19, 1 patient tested positive for SARS-CoV-2 in ocular secretions through a conjunctival swab. This patient did not present with severe fever or respiratory symptoms during testing.[13]
Among ocular manifestations of the disease, conjunctivitis and keratoconjunctivitis are the most prevalent ocular symptoms in adults.[14] The most common ophthalmological findings include conjunctival irritation followed by diplopia and cotton wool patches.[14]
Pathophysiology
The pathogenesis and tissue tropism of SARS-CoV-2 are linked to the interaction between the viral spike protein and its cognate receptor on human host cells— the angiotensin-converting enzyme 2 (ACE-2) receptor. Efficient cell entry requires cleavage by the protein transmembrane serine protease 2 (TMPRSS2). ACE-2 expression is predominant in respiratory mucosal and alveolar epithelial cells but has also been identified in other tissues, including the gastrointestinal tract, kidney, vascular endothelial cells, immune cells, and even neurons. Virulence is achieved through direct cellular invasion and death and the induction of widespread cytokine-driven inflammation and vascular leakage.[15] Additionally, immune cell and complement debris may contribute to an increased thromboembolic state.
The potential for infection through ocular secretions is currently unknown, and the mechanisms by which SARS-CoV-2 accumulates in ocular secretions are not fully understood. Several theories have been proposed, including direct inoculation of ocular tissues from respiratory droplets or aerosolized viral particles, migration from the nasopharynx from the nasolacrimal duct, or even hematogenous spread through the lacrimal gland.[6]
Data surrounding the expression of ACE-2 and TMPRSS2 on the ocular surface are varied. While a study showed the presence of both proteins on the cornea and limbus, low levels were noted in the conjunctiva.[16] Lange et al similarly reported low levels of ACE-2 in the human conjunctiva.[17]
A case report from Rome, Italy, documented the isolation of SARS-CoV-2 by RT-PCR from conjunctival swabs in a patient with COVID-19 exhibiting ocular symptoms.[18] Conjunctival swabs were collected from the hospital on days 3 to 27. Despite clinical resolution of conjunctivitis by day 20, the patient had detectable SARS-CoV-2 RNA in conjunctival samples on day 21 and subsequently on day 27, even after SARS-CoV-2 was no longer detectable by nasopharyngeal swab. Notably, the viability and transmissibility of SARS-CoV-2 in human ocular secretions remain uncertain since the virus has not been successfully cultured from human tears or conjunctival swabs.[19] Limited reports suggest that tears can serve as both an early and late source of infection transmission, even after the patient becomes asymptomatic.[18][20]
In a study by Azzolini et al, SARS-CoV-2 was identified on the ocular surface using RT-PCR in 52 of 91 patients with COVID-19 (57.1%).[5] The virus was detected on the ocular surface in 10 of 17 patients, even when the nasopharyngeal swab results were negative. Viral particles in tears have been postulated to originate from the lacrimal gland through diffusion from a systemic viral load or direct contagion from airborne droplets.[5]
History and Physical
The prevalence of ocular manifestations in patients with COVID-19 varies widely, ranging from 2% to 32% in reported studies.[21][22][23] These variations underscore the importance of ongoing research to understand better the frequency and nature of ocular involvement in patients with COVID-19.
Conjunctiva
Individuals infected with SARS-CoV-2 may present with acute conjunctivitis symptoms, including eye redness, ocular irritation, soreness, foreign body sensation, tearing, mucoid discharge, eyelid swelling, congestion, and chemosis. While these symptoms more commonly occur in patients with severe systemic symptoms of COVID-19, they can occasionally present as an initial manifestation of the disease.[12]
Notably, unremitting conjunctivitis was identified as the sole manifestation of COVID-19 in 5 patients with confirmed SARS-CoV-2 infection through nasopharyngeal RT-PCR. These patients never developed fever, general malaise, or respiratory symptoms throughout their illness.[24]
Examination findings may include features consistent with mild follicular conjunctivitis, such as unilateral or bilateral bulbar conjunctiva injection, follicular reaction of the palpebral conjunctiva, watery discharge, and mild eyelid edema. Bilateral chemosis alone may represent third-spacing in a critically ill patient rather than a true ocular manifestation of the virus.
A case report by Cheema et al documented the first keratoconjunctivitis as the presenting manifestation of COVID-19 in North America.[25] The patient primarily presented with symptoms of eye redness and tearing. The examination revealed conjunctival injection, a follicular reaction of the palpebral conjunctiva, and corneal findings that developed rapidly over 3 days. These corneal findings included transient pseudodendritic lesions and diffuse subepithelial infiltrates with overlying epithelial defects.
Severe hemorrhagic conjunctivitis and pseudomembrane formation have also manifested 19 days after the onset of COVID-19 systemic symptoms and 11 days after admission to the intensive care unit.[26] Notably, in pediatric patients, COVID-19 has been strongly associated with the Kawasaki-like illness known as multisystem inflammatory syndrome in children (MIS-C). Several ocular manifestations, including papilledema, iritis, and keratitis, have been reported in this syndrome, with conjunctivitis being the most prevalent ocular manifestation.[27]
Sclera/Episclera
Epsicleritis and scleritis are findings associated with COVID-19 infection. Unilateral and nodular episcleritis have been observed at the onset of symptoms.[28][29]
Necrotizing anterior scleritis can manifest as late as 3 weeks after the onset of viral symptoms.[30] Moreover, sectoral anterior scleritis has been reported up to 2 weeks after the onset of COVID-19, with noted improvement following treatment with topical and systemic steroids.[30]
Anterior Chamber
In addition to affecting the ocular surface, acute anterior uveitis has been reported independently and associated with COVID-19-related multisystem inflammatory disease.[31][32] A documented case of reactivated idiopathic anterior uveitis following COVID-19 infection has also been reported. Notably, this patient had remained quiescent for 13 years before the occurrence of this episode.[33]
Retina and Choroid
Posterior segment diseases have also been suspected in association with COVID-19 infection, exhibiting diverse etiologies, including vascular, inflammatory, and neuronal involvement. Both ACE-2 and TMPRSS2 are prominently expressed in the human retina. A recent case series involving 3 patients identified S and N COVID-19 proteins through immunofluorescence microscopy within retinal vascular endothelial cells, suggesting the presence of viral particles.[34][35]
Reports of both central retinal vein and artery occlusions have emerged in patients without typical systemic vascular risk factors. The hypothesized mechanism involves a complement-induced prothrombic and inflammatory state induced by the virus, resulting in endothelial damage and microangiopathic injury. A striking example by Walinjkar et al featured a central retinal vein occlusion in a 17-year-old female with COVID-19.[36] A similar case was seen in a previously healthy 33-year-old individual.[37] Additionally, there have been multiple instances of central retinal artery occlusions reported, potentially related to viral-induced endothelial insult and vasculitis.[38][39][40]
Acute macular neuroretinopathy (AMN) and paracentral acute middle maculopathy (PAMM), conditions characterized by ischemia in the deep retinal capillary plexus, have been observed with COVID-19. Hyperreflective changes at the level of the outer plexiform and inner nuclear layers identify these conditions.[41]
Purtscher-like retinopathy may manifest with findings of multiple bilateral cotton wool spots localized to the posterior pole. This condition typically occurs in patients with prolonged hospitalization and severe complications, including multiorgan failure and disseminated intravascular coagulation.[42][43]
Optical coherence tomography (OCT) revealed subclinical hyperreflective lesions at the inner plexiform and ganglion cell layer level in 12 adults examined after systemic disease onset. Additionally, cotton wool spots and microhemorrhages were identified on dilated fundus examinations in 4 of these patients.[44] In a study by Invernizzi and colleagues, retinal findings such as hemorrhages (9.25%), cotton wools spots (7.4%), dilated veins (27.7%), and tortuous vessels (12.9%) were observed in 54 patients with COVID-19 upon screening during fundus photography screening.[45]
These authors also found a direct correlation between retinal vein diameter and disease severity, suggesting that monitoring retinal vein diameter could be a noninvasive parameter for assessing the inflammatory response or endothelial injury in COVID-19. Lecler et al documented abnormal MRI findings in the posterior pole of 9 patients with COVID-19 consisting of 1 or several hyperintense nodules in the macular region on FLAIR-weighted images.[46] These lesions were postulated to be either direct inflammatory infiltration of the retina or microangiopathic disease caused by viral infection.
Various forms of posterior uveitis have been observed following either acute COVID-19 infection or administration of the COVID-19 vaccine. Souza et al reported a case of unilateral multifocal choroiditis, although the temporal relationship of the viral infection could be attributed to chance alone.[47] Additionally, Goyal et al published a case of bilateral multifocal choroiditis within 1 week of receiving the COVID-19 vaccine.[48] Furthermore, cases of serpiginous and ampiginous choroiditis have also been reported.[49][50]
Immune dysregulation resulting from COVID-19 infection may contribute to the reactivation of latent herpes virus, leading to acute retinal necrosis.[51] These cases highlight the importance of vigilance for potential viral reactivation and associated ocular complications in individuals with COVID-19.
Animal model studies have demonstrated the involvement of the retina in COVID-19 patients, revealing retinal vasculitis, retinal degeneration, and breakdown of the blood-retinal barrier.[52][53][54] These findings provide valuable insights into the pathophysiology of retinal complications associated with the virus.
Optic Nerve
Various neuro-ophthalmologic manifestations have been observed in association with COVID-19, primarily related to demyelinating disease. While the mechanism of these manifestations is unclear, hypotheses include direct neuronal invasion, endothelial cell dysfunction leading to ischemia and coagulopathy, or a widespread inflammatory "cytokine storm" induced by the virus.[55] Optic neuritis has been reported in several patients with the infection, alongside neuromyelitis optica spectrum disorder and anti-myelin oligodendrocyte glycoprotein (anti-MOG) antibodies.[56][57][58]
Patients have presented with subacute vision loss, a relative afferent pupillary defect, pain with eye movements, optic disc edema, and radiographic findings of acute optic neuritis following COVID-19 infection. Additionally, there have been reports of acute optic neuritis following vaccination for COVID-19.[59]
Palao et al reported a case of multiple sclerosis following COVID-19 infection in a 24-year-old patient who presented with right optic neuritis. MRI revealed right optic nerve inflammation and supratentorial periventricular demyelinating lesions.[60] These cases suggest a potential link between SARS-CoV-2 infection and the initiation or exacerbation of inflammatory and demyelinating disease.
Ophthalmologists should be consulted to assess for papilledema in patients infected with SARS-CoV-2, as cases of elevated intracranial pressure have been reported. This elevation can stem from widespread inflammation and dural venous sinus thrombosis.[61] Furthermore, MIS-C associated with COVID-19 is increasingly recognized as a distinct syndrome similar to Kawasaki disease and has been linked to both optic neuritis and elevated intracranial pressure.[62]
Another example involves a young patient who developed pseudotumor cerebri syndrome associated with MIS-C due to COVID-19. The patient presented with a new right abducens palsy, papilledema with disc hemorrhages, and a lumbar puncture revealing an opening pressure of 36 cm H2O.[63]
Extraocular Motility, Cranial Nerves
Cranial nerve III, IV, and VI palsies associated with COVID-19 have been reported in the literature, often occurring within a few days of fever and cough onset, with most cases lacking remarkable radiological features.[64][65][66] Ocular cranial neuropathies and binocular diplopia with nerve enhancement on MRI have also been observed in association with postinfectious demyelinating conditions such as Miller-Fisher and Guillain Barré syndromes. Presenting signs include one-sided mydriasis, ptosis, and limited depression and adduction alongside concurrent MRI enhancement of the corresponding oculomotor nerve.[67] Lower extremity hyporeflexia and ataxia are also consistent with Miller-Fisher syndrome.
Ocular myasthenia gravis is a postinfectious sequela of COVID-19, with researchers proposing that antibodies targeting SARS-CoV-2 proteins may cross-react with acetylcholine receptors and similar components at the neuromuscular junction.[68] The condition can be successfully managed with intravenous immunoglobulins and oral pyridostigmine.[69]
Pupils
Patients with COVID-19 may exhibit pupillary changes such as mydriasis and cholinergic super-sensitivity, indicating tonic pupils and postganglionic parasympathetic pupillary nerve fiber damage.[70][71][72] These changes underscore the diverse neurological implications of SARS-CoV-2 infection.
Nystagmus
Visual disturbances such as oscillopsia can be observed in COVID-19 patients with neurologic involvement. Viral-induced vestibular neuritis can manifest as intractable vertigo, nausea, and vomiting.[73] Central vestibular nystagmus is also associated with clinical and imaging findings consistent with rhombencephalitis.[74][75]
Visual Cortex
Perhaps the most devastating neuro-ophthalmic complication of severe COVID-19 infection is acute stroke affecting the posterior visual pathways. Studies indicate that the incidence of stroke in these patients is 7.6 times higher than that of patients with influenza, occurring in a notably younger-than-average patient population without classic vascular risk factors.[76] Patients may present with homonymous visual field deficits, prompting ophthalmologic consultation. One example is a recently published case involving a 12-year-old patient with MIS-C related to COVID-19, who presented with bilateral posterior cerebral artery ischemic strokes presenting as a homonymous visual field defect.[77]
Orbit and Ocular Adnexa
While oculoplastic and orbital manifestations of COVID-19 are rare, emerging evidence suggests a potential association between the virus and inflammatory or infectious disease. There have been 2 reported cases of sinusitis, orbital cellulitis, and intracranial abnormalities in adolescents with COVID-19.[78]
This study hypothesized that SARS-CoV-2 infection could result in congestion of the upper respiratory tract, increasing the risk of secondary bacterial infection. This theory was expanded on by Shires et al, who presented a case of bacterial orbital abscess in a patient with COVID-19. Notably, the intraoperative findings of highly avascular nasal mucosa and cultures yielded Streptococcus constellatus and Peptonipihilus indolicus, bacteria ordinarily absent in the orbit or upper respiratory mucosa.[79] The local microbiologic and immunologic environment could have been altered due to avascularity induced by thrombosis in the setting of SARS-CoV-2 infection.
A rising number of reports have documented acute invasive fungal rhino-orbital mucormycosis coinfection with COVID-19. These opportunistic pathogens thrive in the hypoxic respiratory environment induced by SARS-CoV-2 and an immunocompromised state induced by high-dose steroids and immunosuppressive therapies. The risk is further heightened in patients with poorly controlled diabetes, particularly those with diabetic ketoacidosis (DKA).[80][81][82]
Singh et al conducted a systematic review of 101 reported cases of COVID-19 patients with mucormycosis, revealing a predominantly male population (79%), with 80% having diabetes and 15% with concomitant DKA.[83] Corticosteroids had been administered in 76% of these patients, and nearly 60% of the cases reported rhino-orbital involvement.[83] Notably, orbital compartment syndrome due to concurrent COVID-19 and fulminant mucormycotic infection can also occur.[84]
There have also been reports of MRI-proven orbital myositis in 2 separate COVID-19 patients without concurrent bacterial infection.[85][86] The authors postulated that direct viral orbital invasion of the orbit or induced autoimmunity were possible mechanisms.
Patients with positive SARS-CoV-2 antibodies can develop acute dacryoadenitis. Patients respond favorably to a slow taper of steroids over 6 weeks.[87]
Lacrimal System
Epiphora has been described as an initial finding in patients with COVID-19, believed to result from inflammation of the conjunctiva.[12] No reports have indicated direct involvement of the nasolacrimal system or the lacrimal sac in COVID-19.
Manifestations in Newborn Infants
Recent data support the occurrence of frequent ocular manifestations of SARS-CoV-2 infection in newborn infants. In a study by Perez-Chimal et al conducted in Mexico, 15 infants with positive RT-PCR nasopharyngeal swabs were identified. All of these newborns exhibited ocular manifestations, periorbital edema being the most common (100%), followed by chemosis and hemorrhagic conjunctivitis (73%) and ciliary injection (53%). Unique findings included corneal edema in 6 infants (40%), 1 with rubeosis and posterior synechiae, and posterior segment manifestations, including retinopathy of prematurity in 3 infants (20%).[88] Vitreous hemorrhage was observed in 1 full-term baby, and subtle cotton wool spots in 2 other newborns.
Evaluation
A thorough history is essential, including details about the symptoms' onset, duration, and nature. Examination of the anterior segment using a slit lamp or at the bedside can confirm findings of conjunctivitis or episcleritis. Additionally, measuring visual acuity and intraocular pressure and performing a dilated fundus examination are warranted to rule out potentially more severe ocular diseases.
The examination should include a meticulous assessment of the pupils and color testing to evaluate patients for evidence of optic neuropathy. Evaluation of extraocular motility may reveal evidence of nystagmus or cranial neuropathies. Additionally, visual field testing can detect and confirm deficits associated with stroke.
SARS-CoV-2 can be detected via reverse transcription polymerase chain reaction (RT-PCR) using a virus sampling swab to sweep the lower eyelid fornices and collect tears and conjunctival secretions.[13] A conjunctival swab is the gold standard for tear collection and viral testing.[14]
Additional serum or cerebrospinal fluid testing may be helpful to evaluate for inflammatory, autoimmune, or demyelinating entities. Neuroimaging can also provide valuable information, especially in patients with optic neuritis, visual field deficits, cranial neuropathies, or other associated neurologic symptoms.
All patients should be queried about recent fever, respiratory symptoms, exposure, and travel history to determine the need for further evaluation for COVID-19. This comprehensive assessment helps identify potential cases and ensure appropriate management and precautions.
Treatment / Management
Chen et al reported gradual symptomatic improvement of COVID-19 conjunctivitis in 1 patient with the administration of ribavirin eye drops.[89] While the efficacy of targeted treatment remains unstudied, its long-term significance in a self-limited viral illness is unlikely. Nonetheless, eye care providers should be cautious and aim to reduce possible viral load and potential transmission.[90](B2)
As with other viral infections, COVID-19 conjunctivitis is presumed to be self-limited and managed with symptomatic care. In the absence of significant eye pain, decreased vision, or light sensitivity, many patients can be managed remotely with a trial of frequent preservative-free artificial tears, cold compresses, and lubricating ophthalmic ointment. A short course of topical antibiotics may be considered to prevent or treat bacterial superinfection, especially in patients with symptoms and risk factors such as contact lens wear.[91]
On March 18, 2020, the American Academy of Ophthalmology (AAO) issued guidance urging all ophthalmologists to prioritize urgent or emergent care to minimize the risk of SARS-CoV-2 transmission and conserve disposable medical supplies. They outlined specific criteria for determining the necessity of care (see Table. AAO Risk Stratification for Clinic Ophthalmic Patients).
Although preliminary studies indicate a low risk of viral transmission through ocular secretions, comprehensive large-scale research is lacking, and new data continues to emerge regularly. Therefore, healthcare providers are strongly advised to maintain proper protection of the eyes, nose, and mouth when examining patients. Given the nature and proximity of the ophthalmic examination, eye care providers and technicians may face increased susceptibility to infection.[92]
Eye care providers should utilize slit lamp breath shields and advise patients to minimize talking while seated at the slit lamp to minimize the risk of virus transmission. Implementing rigorous disinfection and sterilization protocols for shared clinic equipment such as tonometers, trial frames, pinhole occluders, B-scan probes, and contact lenses for laser procedures is essential.[2][92] Disposable barrier protection of clinic tools should be used where possible.
Table. AAO Risk Stratification for Clinic Ophthalmic Patients
| Routine ophthalmic appointments, including those for chronic conditions, annual check-ups, and follow-ups, should be postponed until the severity of COVID-19 reduces, including new patients with chronic conditions such as cataracts or ptosis. |
| The consultant surgeon reviews new patient referrals to determine urgency. If necessary, he conducts telephone interviews with the referring doctor and/or the patient. |
|
All patients scheduled for a clinic visit are assessed for the following:
|
If the patient exhibits 2 out of 3 of the symptoms noted above (see Table. AAO Risk Stratification for Clinic Ophthalmic Patients), they are referred for medical assessment. Patients with COVID-19 or symptoms such as fever, cough, or shortness of breath are examined in a separate isolation room. Ideally, only 1 person (physician, technician, etc) should be present in the room due to the small size of ophthalmic rooms, and they should wear complete personal protective equipment (PPE), including a gown, N95 mask, face shield, and gloves. Hands should be washed before and after examination for a minimum of 20 seconds with soap and water. After the ophthalmic examination, the patient is referred for further assessment by the medical team.
Protection of Medical Workers
While the 2003 SARS-CoV crisis did not lead to such widespread infection in the United States, healthcare workers accounted for about 20% of all patients with infections.[93] Recent data indicate that healthcare workers comprise 9% of COVID-10 cases in Italy.(B3)
Between March 2020 and April 7, 2021, over 3699 healthcare workers in the United States succumbed to COVID-19, with the majority being younger than 60 years. As of April 7, 2021, Italy had recorded 129,873 coronavirus cases among healthcare workers, with over 100 healthcare workers, including over 60 doctors, having died from COVID-19 infections by April 2020. Current figures are unavailable, but the numbers in other countries are expected to increase.
Ensuring that frontline medical workers wear appropriate protective gear is crucial. Additionally, monitoring these healthcare workers for signs of disease and implementing appropriate containment measures is essential.
A significant number of deaths in the United States were linked to an initial severe shortage of appropriate PPE for healthcare providers. Even with the availability of PPE, selecting the level of protective gear based on the risk of infection is crucial. Suggestions are as follows:
- Ensure the waiting room maintains minimal occupancy, with available seating at least 6 feet apart.
- For patients who do not meet any of the 3 criteria mentioned above, healthcare workers should wear a surgical mask, a face shield, and gloves and wash their hands before and after each encounter.
- If a patient meets any of the 3 criteria, healthcare workers must wear full PPE, including a gown, face shield, gloves, and N95 mask.
- Considering that droplets from sneezes can travel up to 6 meters, technicians from the Moran Eye Center have developed a slit-lamp shield using 2 plastic sheets through a laminator without paper in between, with openings cut for the eyepieces (see Image. Slit-Lamp Shield).[94] Others have improvised using old x-ray films when commercial shields are in short supply.
- During consultations, conversations are kept to a minimum to reduce potential exposure.
- Some institutions, faced with a shortage of surgical masks, have explored options such as storing used masks for potential resterilization at the end of each day.
- Up to a quarter of patients injected under sedation may experience a severe involuntary sneeze, particularly common with eyelid injections.[95][96] This is more common with eyelid injections than with retrobulbar injections. To mitigate this risk, ophthalmic surgeons must exercise caution and take appropriate precautions when administering a local anesthetic.
Surveillance of Medical Workers
In Singapore, healthcare workers reported their temperatures twice a day via an online system, which proved to be a prudent approach. Conventional "walk-by" temperature testing may offer inaccurate or comprehensive results, particularly with staff arriving early or leaving late.[97] As of April 16, 2021, Singapore had recorded 60,769 infections and only 30 deaths attributable to the virus. (B3)
All travel outside the state or country should be declared to the medical administration for review. This protocol remains relevant given the ongoing occurrence of infection in various states and countries. Additionally, all healthcare workers should promptly self-report symptoms to facilitate appropriate testing. Isolation and contact tracing measures can then be implemented as necessary.
Sterilization of Equipment
After each patient, the slit-lamp shields are disinfected with 70% ethyl alcohol, as this concentration has been demonstrated to reduce coronavirus infectivity.[94] Slitlamps, B-scan probes, and other tools are cleaned using 70% ethyl alcohol. Goldman tonometers undergo sterilization with a 10% diluted sodium hypochlorite solution, known to inactivate coronaviruses effectively.[98]
Differential Diagnosis
Ocular manifestations of COVID-19 often present with conjunctivitis, which can resemble other viral etiologies. Common ocular manifestations that should be considered in the differential diagnosis include the following:
- Viral conjunctivitis other than due to COVID-19 (eg, adenovirus)
- Bacterial conjunctivitis
- Allergic conjunctivitis
- Herpes simplex virus keratitis
- Anterior uveitis
- Corneal abrasion
- Foreign body
- Dry eye syndrome
- Exposure keratopathy in an intubated patient
- Chemosis in a critically ill patient
Prognosis
Conjunctivitis related to COVID-19 is considered self-limited, but additional research is necessary to comprehend the lasting effects of other ocular manifestations associated with the virus. Studies examining SARS-CoV-2 detection in ocular samples have suggested a potentially poorer prognosis for patients who test positive for the virus in ocular specimens.[99]
Upon further evaluation of the study cohort, it was found that patients with a higher viral load or greater severity of acute illness were more prone to testing positive for viral RNA in ocular samples. Additionally, they were more likely to have underlying comorbidities that correlated with a poorer 10-year survival rate.[99]
The authors conclude that while ocular manifestations of COVID-19 do not indicate decreased survival, they may serve as a prognostic sign and a potential indicator of disease progression from moderate to severe.[99] Further studies are required to assess this relationship more comprehensively.
Clinical data indicates the possibility of the virus spreading through tear samples of infected patients is low.[100] In a recent case series, only 1 out of 30 confirmed cases of COVID-19 illness had positive tear samples. Similarly, in another study where tear samples and nasopharyngeal swabs were tested simultaneously, nasopharyngeal swabs tested positive without any positive tear samples.[100] However, despite these findings, a review of currently available data suggests that transmission through the eyes is plausible, as evidenced by viral RNA isolation from COVID-19 patients with high-viral loads and active conjunctivitis.[101]
Complications
Most of the information regarding ocular manifestations of COVID-19 illness comes from small prospective cohorts or case studies. While most suggest self-limited disease, the long-term prognosis and complication rate remain to be determined. However, some studies indicate that mucormycosis may occur in these patients due to the immune dysregulation caused by COVID-19 infection and corticosteroid use for its treatment, especially in those with diabetes mellitus.[102]
Compromised tear film quality, which has been reported as another potential complication of COVID-19 illness, can lead to dry eye syndrome and ocular surface damage, especially in hospitalized patients.[99] Prompt recognition and management of ocular complications, including dry eye syndrome, in patients with COVID-19 are essential to prevent long-term ocular sequelae and improve overall patient comfort and well-being.
Deterrence and Patient Education
Prevention strategies remain vital in controlling disease transmission, emphasizing physical distancing, regular hand-washing, and minimizing direct touching of the eyes and face. Despite the availability of vaccines and declining infection rates in certain regions, these measures remain essential to curb the potential resurgence of COVID-19 and other infectious diseases. Other recommendations to consider include the following:
- Avoiding the use of contact lenses
- Limiting the application of cosmetics around the eye
- Opting for glasses or sunglasses
- Regularly changing sheets, pillowcases, and towels to maintain cleanliness and hygiene
Pearls and Other Issues
Key facts to keep in mind regarding ophthalmic manifestations of COVID-19 include the following:
- Ocular shedding of SARS-CoV-2 through tears is a potential concern for ophthalmologists.
- Conjunctivitis or tearing may be some patients' initial or sole presentation of COVID-19.
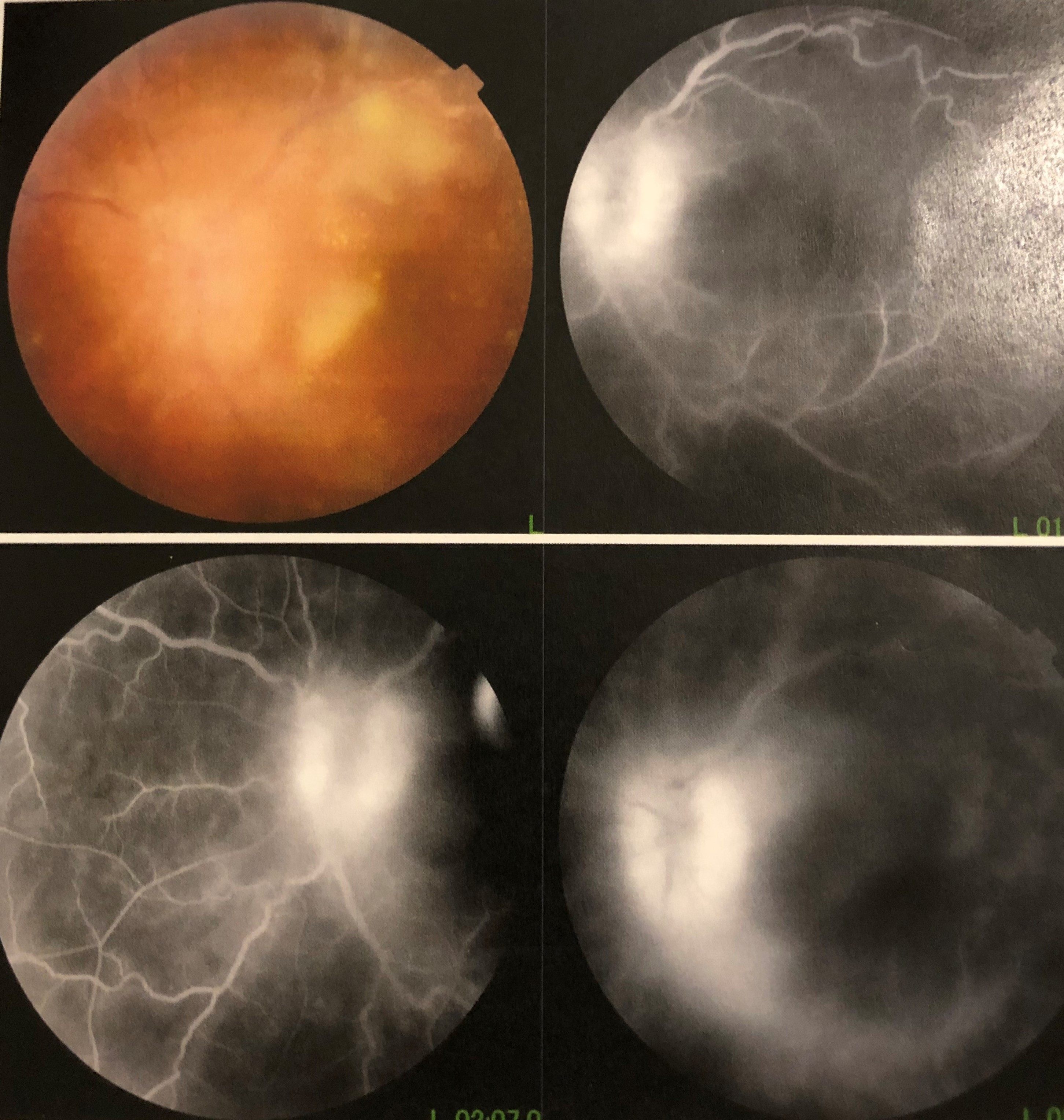
- Several ocular manifestations of COVID-19 have been observed, including retinovascular disease, uveitis, optic neuropathies, and orbital fungal coinfections (see Image. Fungal Endophthalmitis).
- SARS-CoV-2 may trigger or exacerbate inflammatory/demyelinating disease.
- In advanced cases, patients may present with chemosis (see Image. Chemosis) or follicular conjunctivitis (see Image. Follicular Conjunctivitis).
- During ocular examinations, precautions such as wearing gloves and using extension instruments (eg, cotton swabs) should be taken to minimize direct contact with secretions.
- Given that many ophthalmic clinic visitors are older and have underlying health conditions, careful screening for the necessity of in-person visits is crucial. Telemedicine is a viable alternative for non-urgent cases.
- Implementing social distancing measures within clinical settings can be challenging. Still, efforts should be made to limit the number of individuals in examination rooms, with a preference for only 1 person accompanying the patient.
- Certain medical specialties, such as ophthalmology, otolaryngology, and anesthesiology, may face increased infection risks due to close proximity to mucosal surfaces during examinations or procedures.
- Surgical protocols, including delaying entry into operating rooms after intubation or extubation, aim to reduce transmission risks regardless of the patient's COVID-19 status.
Enhancing Healthcare Team Outcomes
In outpatient, inpatient, and surgical settings, effective management of COVID-19 patients and containment of the disease require an interprofessional approach. Clear communication among hospital administration, provider teams, and clinic support staff is essential to establish expectations for patient screening, proper utilization of PPE, and integration of telemedicine technologies for consultations.
Identifying ocular manifestations of COVID-19 is crucial for early detection and effective management of patients with this disease. Therefore, all interprofessional healthcare team members (physicians, optometrists, advanced care practitioners, nurses, pharmacists) and other ancillary medical staff should be well-informed about these manifestations. Team members should feel empowered to raise concerns if they notice signs or symptoms suggestive of ocular involvement in a patient. Early recognition enables prompt intervention and contributes to improved patient outcomes. With close collaboration, healthcare professionals can enhance patient-centered care, promote patient safety, and optimize team performance in managing ophthalmic manifestations of COVID-19.
Media
(Click Image to Enlarge)
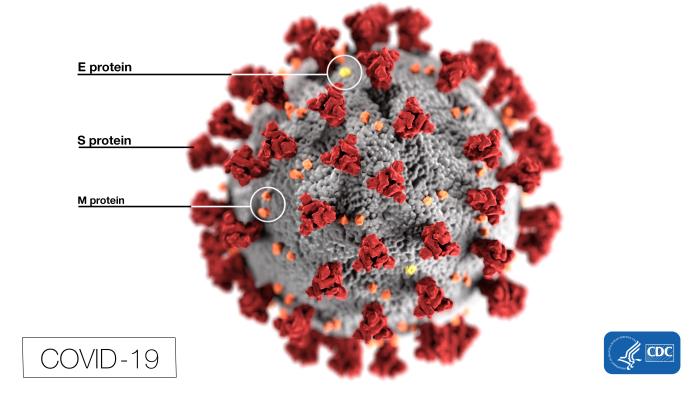
Structure of COVID-19. This illustration, created by the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by coronaviruses. Note the spikes that adorn the virus's outer surface, which impart the look of a corona surrounding the virion when viewed electron microscopically. In this view, the protein particles E, S, and M, also located on the particle's outer surface, have all been labeled as well. A novel coronavirus, SARS-CoV-2, was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China, in 2019. The infection caused by this virus has been named COVID-19.
Contributed from the CDC, Alissa Eckert, MS; Dan Higgins, MAM (Public Domain)
(Click Image to Enlarge)
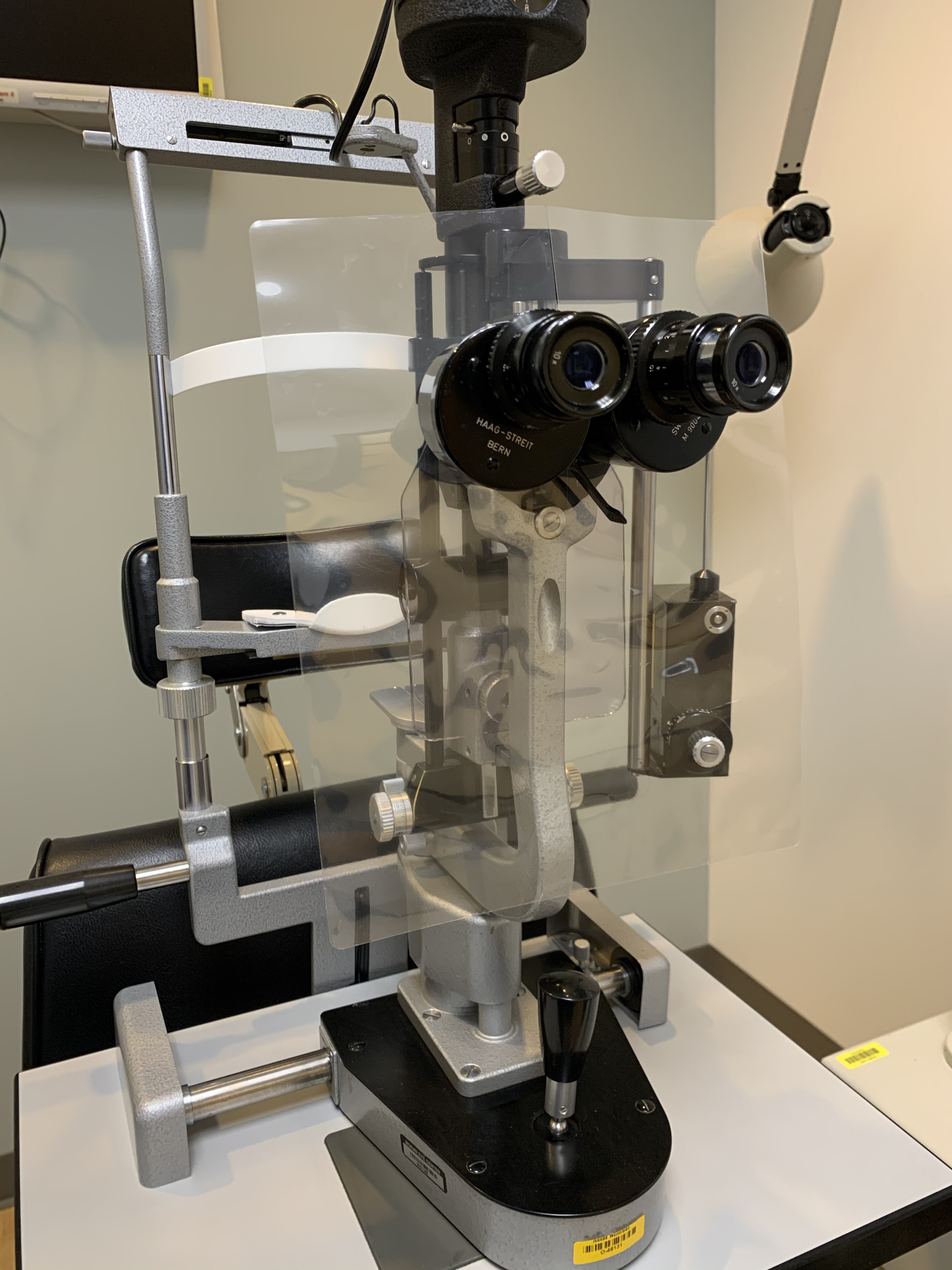
Slit-Lamp Shield. A slit-lamp shield, designed to protect the examiner, was created by Moran Technicians Stein Erickson, Emily Petersen, and Anna Reed, with assistance from others. It is constructed by passing 2 lamination sheets through a laminator without anything in between, resulting in a rigid shield that can be cleaned with alcohol and reused. Alternatively, used x-ray films may be utilized, although they lack the clarity required for optimal performance.
Contributed on behalf of the Moran Eye Center by Katherine Hu, MD and Prof. BCK Patel, MD, FRCS
(Click Image to Enlarge)
(Click Image to Enlarge)
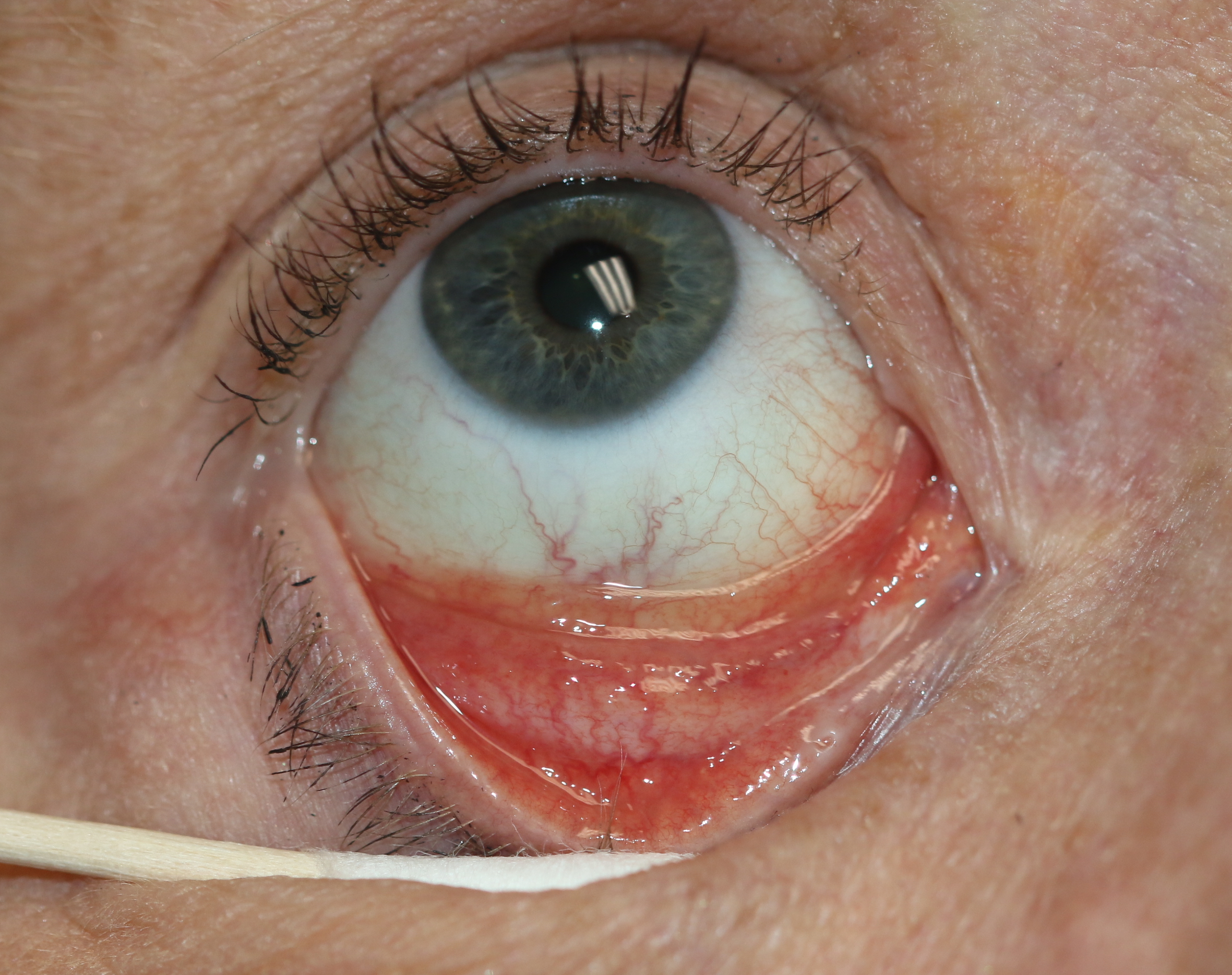
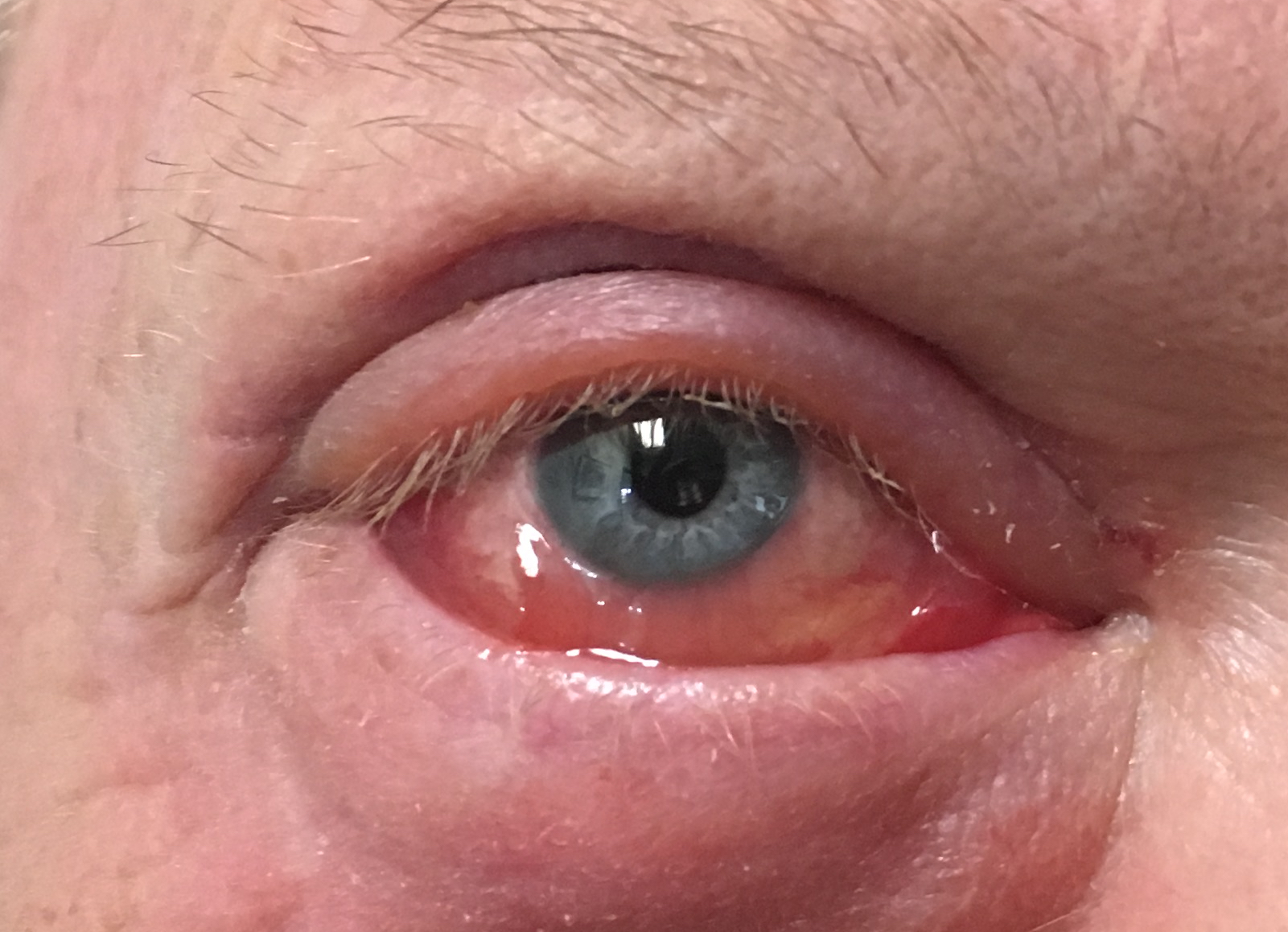
Follicular Conjunctivitis. May be seen with viral infections like herpes zoster, Epstein-Barr virus infection, infectious mononucleosis), chlamydial infections, and in reaction of topical medications and molluscum contagiosum. Follicular conjunctivitis has been described in patients with the COVID-19 infection (coronavirus infection). The inferior and superior tarsal conjunctiva and the fornices show gray-white elevated swellings, which are about 0.5 - 1 mm in diameter and have a velvety appearance.
Contributed by Prof. BCK Patel MD, FRCS
(Click Image to Enlarge)
References
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). StatPearls. 2024 Jan:(): [PubMed PMID: 32150360]
Loon SC, Teoh SC, Oon LL, Se-Thoe SY, Ling AE, Leo YS, Leong HN. The severe acute respiratory syndrome coronavirus in tears. The British journal of ophthalmology. 2004 Jul:88(7):861-3 [PubMed PMID: 15205225]
Raboud J, Shigayeva A, McGeer A, Bontovics E, Chapman M, Gravel D, Henry B, Lapinsky S, Loeb M, McDonald LC, Ofner M, Paton S, Reynolds D, Scales D, Shen S, Simor A, Stewart T, Vearncombe M, Zoutman D, Green K. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PloS one. 2010 May 19:5(5):e10717. doi: 10.1371/journal.pone.0010717. Epub 2010 May 19 [PubMed PMID: 20502660]
Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet (London, England). 2020 Feb 22:395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. Epub 2020 Feb 6 [PubMed PMID: 32035510]
Azzolini C, Donati S, Premi E, Baj A, Siracusa C, Genoni A, Grossi PA, Azzi L, Sessa F, Dentali F, Severgnini P, Minoja G, Cabrini L, Chiaravalli M, Veronesi G, Carcano G, Maffioli LS, Tagliabue A. SARS-CoV-2 on Ocular Surfaces in a Cohort of Patients With COVID-19 From the Lombardy Region, Italy. JAMA ophthalmology. 2021 Sep 1:139(9):956-963. doi: 10.1001/jamaophthalmol.2020.5464. Epub [PubMed PMID: 33662099]
Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocular immunology and inflammation. 2020 Apr 2:28(3):391-395. doi: 10.1080/09273948.2020.1738501. Epub 2020 Mar 16 [PubMed PMID: 32175797]
Level 3 (low-level) evidenceLi JO, Lam DSC, Chen Y, Ting DSW. Novel Coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear. The British journal of ophthalmology. 2020 Mar:104(3):297-298. doi: 10.1136/bjophthalmol-2020-315994. Epub [PubMed PMID: 32086236]
Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clinics and practice. 2021 Oct 21:11(4):778-784. doi: 10.3390/clinpract11040093. Epub 2021 Oct 21 [PubMed PMID: 34698149]
Ferré VM, Peiffer-Smadja N, Visseaux B, Descamps D, Ghosn J, Charpentier C. Omicron SARS-CoV-2 variant: What we know and what we don't. Anaesthesia, critical care & pain medicine. 2022 Feb:41(1):100998. doi: 10.1016/j.accpm.2021.100998. Epub 2021 Dec 10 [PubMed PMID: 34902630]
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020 Apr 30:382(18):1708-1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28 [PubMed PMID: 32109013]
Level 2 (mid-level) evidenceNasiri N, Sharifi H, Bazrafshan A, Noori A, Karamouzian M, Sharifi A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-analysis. Journal of ophthalmic & vision research. 2021 Jan-Mar:16(1):103-112. doi: 10.18502/jovr.v16i1.8256. Epub 2021 Jan 20 [PubMed PMID: 33520133]
Level 1 (high-level) evidenceWu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA ophthalmology. 2020 May 1:138(5):575-578. doi: 10.1001/jamaophthalmol.2020.1291. Epub [PubMed PMID: 32232433]
Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. Journal of medical virology. 2020 Jun:92(6):589-594. doi: 10.1002/jmv.25725. Epub 2020 Mar 12 [PubMed PMID: 32100876]
Al-Namaeh M. Ocular manifestations of COVID-19. Therapeutic advances in ophthalmology. 2022 Jan-Dec:14():25158414221083374. doi: 10.1177/25158414221083374. Epub 2022 Apr 12 [PubMed PMID: 35434520]
Level 3 (low-level) evidenceHarrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends in immunology. 2020 Dec:41(12):1100-1115. doi: 10.1016/j.it.2020.10.004. Epub 2020 Oct 14 [PubMed PMID: 33132005]
Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. bioRxiv : the preprint server for biology. 2020 May 9:():. pii: 2020.05.09.086165. doi: 10.1101/2020.05.09.086165. Epub 2020 May 9 [PubMed PMID: 32511393]
Lange C, Wolf J, Auw-Haedrich C, Schlecht A, Boneva S, Lapp T, Horres R, Agostini H, Martin G, Reinhard T, Schlunck G. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. Journal of medical virology. 2020 Oct:92(10):2081-2086. doi: 10.1002/jmv.25981. Epub 2020 Jul 11 [PubMed PMID: 32374427]
Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, Nicastri E, Bevilacqua N, Giancola ML, Corpolongo A, Ippolito G, Capobianchi MR, Castilletti C. SARS-CoV-2 Isolation From Ocular Secretions of a Patient With COVID-19 in Italy With Prolonged Viral RNA Detection. Annals of internal medicine. 2020 Aug 4:173(3):242-243. doi: 10.7326/M20-1176. Epub 2020 Apr 17 [PubMed PMID: 32302380]
Chen MJ, Chang KJ, Hsu CC, Lin PY, Jui-Ling Liu C. Precaution and prevention of coronavirus disease 2019 infection in the eye. Journal of the Chinese Medical Association : JCMA. 2020 Jul:83(7):648-650. doi: 10.1097/JCMA.0000000000000334. Epub [PubMed PMID: 32332516]
Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The Ocular Manifestations and Transmission of COVID-19: Recommendations for Prevention. The Journal of emergency medicine. 2020 Jul:59(1):137-140. doi: 10.1016/j.jemermed.2020.04.060. Epub 2020 May 8 [PubMed PMID: 32456959]
Kumar KK, Sampritha UC, Prakash AA, Adappa K, Chandraprabha S, Neeraja TG, Guru Prasad NS, Basumatary J, Gangasagara SB, Sujatha Rathod BL, Jayanthi CR. Ophthalmic manifestations in the COVID-19 clinical spectrum. Indian journal of ophthalmology. 2021 Mar:69(3):691-694. doi: 10.4103/ijo.IJO_3037_20. Epub [PubMed PMID: 33595502]
Domínguez-Varela IA, Rodríguez-Gutiérrez LA, Morales-Mancillas NR, Barrera-Sánchez M, Macías-Rodríguez Y, Valdez-García JE. COVID-19 and the eye: a review. Infectious diseases (London, England). 2021 Jun:53(6):399-403. doi: 10.1080/23744235.2021.1882697. Epub 2021 Feb 10 [PubMed PMID: 33566704]
Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020 Jun:258(6):1351-1352. doi: 10.1007/s00417-020-04695-8. Epub 2020 Apr 23 [PubMed PMID: 32328758]
Scalinci SZ, Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020:20():e00774. doi: 10.1016/j.idcr.2020.e00774. Epub 2020 Apr 24 [PubMed PMID: 32373467]
Level 3 (low-level) evidenceCheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2020 Aug:55(4):e125-e129. doi: 10.1016/j.jcjo.2020.03.003. Epub 2020 Apr 2 [PubMed PMID: 32284146]
Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. American journal of ophthalmology case reports. 2020 Sep:19():100735. doi: 10.1016/j.ajoc.2020.100735. Epub 2020 May 6 [PubMed PMID: 32377594]
Level 3 (low-level) evidenceDanthuluri V, Grant MB. Update and Recommendations for Ocular Manifestations of COVID-19 in Adults and Children: A Narrative Review. Ophthalmology and therapy. 2020 Dec:9(4):853-875. doi: 10.1007/s40123-020-00310-5. Epub 2020 Oct 15 [PubMed PMID: 33058068]
Level 3 (low-level) evidenceOtaif W, Al Somali AI, Al Habash A. Episcleritis as a possible presenting sign of the novel coronavirus disease: A case report. American journal of ophthalmology case reports. 2020 Dec:20():100917. doi: 10.1016/j.ajoc.2020.100917. Epub 2020 Sep 8 [PubMed PMID: 32923742]
Level 3 (low-level) evidenceMéndez Mangana C, Barraquer Kargacin A, Barraquer RI. Episcleritis as an ocular manifestation in a patient with COVID-19. Acta ophthalmologica. 2020 Dec:98(8):e1056-e1057. doi: 10.1111/aos.14484. Epub 2020 Jun 1 [PubMed PMID: 32483943]
Feizi S, Meshksar A, Naderi A, Esfandiari H. Anterior Scleritis Manifesting After Coronavirus Disease 2019: A Report of Two Cases. Cornea. 2021 Sep 1:40(9):1204-1206. doi: 10.1097/ICO.0000000000002795. Epub [PubMed PMID: 34351874]
Level 3 (low-level) evidenceMazzotta C, Giancipoli E. Anterior Acute Uveitis Report in a SARS-CoV-2 Patient Managed with Adjunctive Topical Antiseptic Prophylaxis Preventing 2019-nCoV Spread Through the Ocular Surface Route. International medical case reports journal. 2020:13():513-520. doi: 10.2147/IMCRJ.S260252. Epub 2020 Oct 13 [PubMed PMID: 33116943]
Level 3 (low-level) evidenceBettach E, Zadok D, Weill Y, Brosh K, Hanhart J. Bilateral anterior uveitis as a part of a multisystem inflammatory syndrome secondary to COVID-19 infection. Journal of medical virology. 2021 Jan:93(1):139-140. doi: 10.1002/jmv.26229. Epub 2020 Sep 30 [PubMed PMID: 32592496]
Sanjay S, Mutalik D, Gowda S, Mahendradas P, Kawali A, Shetty R. "Post Coronavirus Disease (COVID-19) Reactivation of a Quiescent Unilateral Anterior Uveitis". SN comprehensive clinical medicine. 2021:3(9):1843-1847. doi: 10.1007/s42399-021-00985-2. Epub 2021 Jun 7 [PubMed PMID: 34124585]
Zhou L, Xu Z, Guerra J, Rosenberg AZ, Fenaroli P, Eberhart CG, Duh EJ. Expression of the SARS-CoV-2 Receptor ACE2 in Human Retina and Diabetes-Implications for Retinopathy. Investigative ophthalmology & visual science. 2021 Jun 1:62(7):6. doi: 10.1167/iovs.62.7.6. Epub [PubMed PMID: 34086044]
Araujo-Silva CA, Marcos AAA, Marinho PM, Branco AMC, Roque A, Romano AC, Matuoka ML, Farah M, Burnier M, Moraes NF, Tierno PFGMM, Schor P, Sakamoto V, Nascimento H, de Sousa W, Belfort R Jr. Presumed SARS-CoV-2 Viral Particles in the Human Retina of Patients With COVID-19. JAMA ophthalmology. 2021 Sep 1:139(9):1015-1021. doi: 10.1001/jamaophthalmol.2021.2795. Epub [PubMed PMID: 34323931]
Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian journal of ophthalmology. 2020 Nov:68(11):2572-2574. doi: 10.4103/ijo.IJO_2575_20. Epub [PubMed PMID: 33120696]
Yahalomi T, Pikkel J, Arnon R, Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient: A case report. American journal of ophthalmology case reports. 2020 Dec:20():100992. doi: 10.1016/j.ajoc.2020.100992. Epub 2020 Nov 16 [PubMed PMID: 33225111]
Level 3 (low-level) evidenceAcharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020:21():e00867. doi: 10.1016/j.idcr.2020.e00867. Epub 2020 Jun 18 [PubMed PMID: 32572363]
Level 3 (low-level) evidenceMontesel A, Bucolo C, Mouvet V, Moret E, Eandi CM. Case Report: Central Retinal Artery Occlusion in a COVID-19 Patient. Frontiers in pharmacology. 2020:11():588384. doi: 10.3389/fphar.2020.588384. Epub 2020 Dec 23 [PubMed PMID: 33424598]
Level 3 (low-level) evidenceMurchison AP, Sweid A, Dharia R, Theofanis TN, Tjoumakaris SI, Jabbour PM, Bilyk JR. Monocular visual loss as the presenting symptom of COVID-19 infection. Clinical neurology and neurosurgery. 2021 Feb:201():106440. doi: 10.1016/j.clineuro.2020.106440. Epub 2020 Dec 15 [PubMed PMID: 33383464]
Level 3 (low-level) evidenceGascon P, Briantais A, Bertrand E, Ramtohul P, Comet A, Beylerian M, Sauvan L, Swiader L, Durand JM, Denis D. Covid-19-Associated Retinopathy: A Case Report. Ocular immunology and inflammation. 2020 Nov 16:28(8):1293-1297. doi: 10.1080/09273948.2020.1825751. Epub 2020 Oct 6 [PubMed PMID: 33021856]
Level 3 (low-level) evidenceBottini AR, Steinmetz S, Blinder KJ, Shah GK. Purtscher-Like Retinopathy in a Patient with COVID-19. Case reports in ophthalmological medicine. 2021:2021():6661541. doi: 10.1155/2021/6661541. Epub 2021 Mar 20 [PubMed PMID: 33859855]
Level 3 (low-level) evidenceRahman EZ, Shah P, Ong JE, Goldberg M, Ong SS. Purtscher-like retinopathy in a patient with COVID-19 and disseminated intravascular coagulation. American journal of ophthalmology case reports. 2021 Dec:24():101229. doi: 10.1016/j.ajoc.2021.101229. Epub 2021 Nov 11 [PubMed PMID: 34796309]
Level 3 (low-level) evidenceMarinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R Jr. Retinal findings in patients with COVID-19. Lancet (London, England). 2020 May 23:395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. Epub 2020 May 12 [PubMed PMID: 32405105]
Invernizzi A, Torre A, Parrulli S, Zicarelli F, Schiuma M, Colombo V, Giacomelli A, Cigada M, Milazzo L, Ridolfo A, Faggion I, Cordier L, Oldani M, Marini S, Villa P, Rizzardini G, Galli M, Antinori S, Staurenghi G, Meroni L. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020 Oct:27():100550. doi: 10.1016/j.eclinm.2020.100550. Epub 2020 Sep 20 [PubMed PMID: 32984785]
Lecler A, Cotton F, Lersy F, Kremer S, Héran F, SFNR's COVID Study Group. Ocular MRI Findings in Patients with Severe COVID-19: A Retrospective Multicenter Observational Study. Radiology. 2021 May:299(2):E226-E229. doi: 10.1148/radiol.2021204394. Epub 2021 Feb 16 [PubMed PMID: 33591889]
Level 2 (mid-level) evidencede Souza EC, de Campos VE, Duker JS. Atypical unilateral multifocal choroiditis in a COVID-19 positive patient. American journal of ophthalmology case reports. 2021 Jun:22():101034. doi: 10.1016/j.ajoc.2021.101034. Epub 2021 Feb 19 [PubMed PMID: 33623832]
Level 3 (low-level) evidenceGoyal M, Murthy SI, Annum S. Bilateral Multifocal Choroiditis following COVID-19 Vaccination. Ocular immunology and inflammation. 2021 May 19:29(4):753-757. doi: 10.1080/09273948.2021.1957123. Epub 2021 Aug 3 [PubMed PMID: 34344280]
Providência J, Fonseca C, Henriques F, Proença R. Serpiginous choroiditis presenting after SARS-CoV-2 infection: A new immunological trigger? European journal of ophthalmology. 2022 Jan:32(1):NP97-NP101. doi: 10.1177/1120672120977817. Epub 2020 Dec 2 [PubMed PMID: 33267645]
Tom ES, McKay KM, Saraf SS. Bilateral Ampiginous Choroiditis following Presumed SARS-CoV-2 Infection. Case reports in ophthalmological medicine. 2021:2021():1646364. doi: 10.1155/2021/1646364. Epub 2021 Aug 5 [PubMed PMID: 34367705]
Level 3 (low-level) evidenceSoni A, Narayanan R, Tyagi M, Belenje A, Basu S. Acute Retinal Necrosis as a presenting ophthalmic manifestation in COVID 19 recovered patients. Ocular immunology and inflammation. 2021 May 19:29(4):722-725. doi: 10.1080/09273948.2021.1938135. Epub 2021 Jul 6 [PubMed PMID: 34228583]
Chin MS, Hooper LC, Hooks JJ, Detrick B. Identification of α-fodrin as an autoantigen in experimental coronavirus retinopathy (ECOR). Journal of neuroimmunology. 2014 Jul 15:272(1-2):42-50. doi: 10.1016/j.jneuroim.2014.05.002. Epub 2014 May 10 [PubMed PMID: 24864013]
Level 3 (low-level) evidenceWang Y, Detrick B, Yu ZX, Zhang J, Chesky L, Hooks JJ. The role of apoptosis within the retina of coronavirus-infected mice. Investigative ophthalmology & visual science. 2000 Sep:41(10):3011-8 [PubMed PMID: 10967058]
Level 3 (low-level) evidenceVinores SA, Wang Y, Vinores MA, Derevjanik NL, Shi A, Klein DA, Detrick B, Hooks JJ. Blood-retinal barrier breakdown in experimental coronavirus retinopathy: association with viral antigen, inflammation, and VEGF in sensitive and resistant strains. Journal of neuroimmunology. 2001 Oct 1:119(2):175-82 [PubMed PMID: 11585619]
Level 3 (low-level) evidenceLuís ME, Hipólito-Fernandes D, Mota C, Maleita D, Xavier C, Maio T, Cunha JP, Tavares Ferreira J. A Review of Neuro-Ophthalmological Manifestations of Human Coronavirus Infection. Eye and brain. 2020:12():129-137. doi: 10.2147/EB.S268828. Epub 2020 Oct 30 [PubMed PMID: 33154692]
Sawalha K, Adeodokun S, Kamoga GR. COVID-19-Induced Acute Bilateral Optic Neuritis. Journal of investigative medicine high impact case reports. 2020 Jan-Dec:8():2324709620976018. doi: 10.1177/2324709620976018. Epub [PubMed PMID: 33238757]
Level 3 (low-level) evidenceZhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2020 Sep:40(3):398-402. doi: 10.1097/WNO.0000000000001049. Epub [PubMed PMID: 32604245]
de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: A case report. Multiple sclerosis and related disorders. 2020 Nov:46():102474. doi: 10.1016/j.msard.2020.102474. Epub 2020 Sep 1 [PubMed PMID: 32892062]
Level 3 (low-level) evidenceLeber HM, Sant'Ana L, Konichi da Silva NR, Raio MC, Mazzeo TJMM, Endo CM, Nascimento H, de Souza CE. Acute Thyroiditis and Bilateral Optic Neuritis following SARS-CoV-2 Vaccination with CoronaVac: A Case Report. Ocular immunology and inflammation. 2021 Aug 18:29(6):1200-1206. doi: 10.1080/09273948.2021.1961815. Epub 2021 Aug 17 [PubMed PMID: 34402726]
Level 3 (low-level) evidencePalao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Multiple sclerosis and related disorders. 2020 Oct:45():102377. doi: 10.1016/j.msard.2020.102377. Epub 2020 Jul 7 [PubMed PMID: 32698095]
Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R, Riina HA, Radmanesh A, Nelson PK. Cerebral Venous Thrombosis Associated with COVID-19. AJNR. American journal of neuroradiology. 2020 Aug:41(8):1370-1376. doi: 10.3174/ajnr.A6644. Epub 2020 Jun 18 [PubMed PMID: 32554424]
Baccarella A, Linder A, Spencer R, Jonokuchi AJ, King PB, Maldonado-Soto A, Boneparth A, Hooe BS, Schweickert AJ, Carlin RF, Kingery F, Vargas WS, Sewell TB, Silver WG. Increased Intracranial Pressure in the Setting of Multisystem Inflammatory Syndrome in Children, Associated With COVID-19. Pediatric neurology. 2021 Feb:115():48-49. doi: 10.1016/j.pediatrneurol.2020.11.008. Epub 2020 Nov 22 [PubMed PMID: 33333460]
Verkuil LD, Liu GT, Brahma VL, Avery RA. Pseudotumor cerebri syndrome associated with MIS-C: a case report. Lancet (London, England). 2020 Aug 22:396(10250):532. doi: 10.1016/S0140-6736(20)31725-6. Epub 2020 Aug 11 [PubMed PMID: 32795406]
Level 3 (low-level) evidenceBelghmaidi S, Nassih H, Boutgayout S, El Fakiri K, El Qadiry R, Hajji I, Bourrahouate A, Moutaouakil A. Third Cranial Nerve Palsy Presenting with Unilateral Diplopia and Strabismus in a 24-Year-Old Woman with COVID-19. The American journal of case reports. 2020 Oct 15:21():e925897. doi: 10.12659/AJCR.925897. Epub 2020 Oct 15 [PubMed PMID: 33056942]
Level 3 (low-level) evidenceOliveira RMC, Santos DH, Olivetti BC, Takahashi JT. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection. Arquivos de neuro-psiquiatria. 2020 Jun:78(6):385-386. doi: 10.1590/0004-282X20200052. Epub [PubMed PMID: 32609196]
Greer CE, Bhatt JM, Oliveira CA, Dinkin MJ. Isolated Cranial Nerve 6 Palsy in 6 Patients With COVID-19 Infection. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2020 Dec:40(4):520-522. doi: 10.1097/WNO.0000000000001146. Epub [PubMed PMID: 32941331]
Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, Harpe J, Greer C, Mints G, Salama G, Tsiouris AJ, Leifer D. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 Aug 4:95(5):221-223. doi: 10.1212/WNL.0000000000009700. Epub 2020 May 1 [PubMed PMID: 32358218]
Restivo DA, Centonze D, Alesina A, Marchese-Ragona R. Myasthenia Gravis Associated With SARS-CoV-2 Infection. Annals of internal medicine. 2020 Dec 15:173(12):1027-1028. doi: 10.7326/L20-0845. Epub 2020 Aug 10 [PubMed PMID: 32776781]
Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G, Hufendiek K, Wegner F. Postinfectious Onset of Myasthenia Gravis in a COVID-19 Patient. Frontiers in neurology. 2020:11():576153. doi: 10.3389/fneur.2020.576153. Epub 2020 Oct 6 [PubMed PMID: 33123081]
Ortiz-Seller A, Martínez Costa L, Hernández-Pons A, Valls Pascual E, Solves Alemany A, Albert-Fort M. Ophthalmic and Neuro-ophthalmic Manifestations of Coronavirus Disease 2019 (COVID-19). Ocular immunology and inflammation. 2020 Nov 16:28(8):1285-1289. doi: 10.1080/09273948.2020.1817497. Epub 2020 Oct 6 [PubMed PMID: 33021422]
Ordás CM, Villacieros-Álvarez J, Pastor-Vivas AI, Corrales-Benítez Á. Concurrent tonic pupil and trochlear nerve palsy in COVID-19. Journal of neurovirology. 2020 Dec:26(6):970-972. doi: 10.1007/s13365-020-00909-1. Epub 2020 Sep 10 [PubMed PMID: 32910433]
Kaya Tutar N, Kale N, Tugcu B. Adie-Holmes syndrome associated with COVID-19 infection: A case report. Indian journal of ophthalmology. 2021 Mar:69(3):773-774. doi: 10.4103/ijo.IJO_3589_20. Epub [PubMed PMID: 33595525]
Level 3 (low-level) evidenceMalayala SV, Raza A. A Case of COVID-19-Induced Vestibular Neuritis. Cureus. 2020 Jun 30:12(6):e8918. doi: 10.7759/cureus.8918. Epub 2020 Jun 30 [PubMed PMID: 32760619]
Level 3 (low-level) evidenceWong PF, Craik S, Newman P, Makan A, Srinivasan K, Crawford E, Dev D, Moudgil H, Ahmad N. Lessons of the month 1: A case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clinical medicine (London, England). 2020 May 15:20(3):293-294. doi: 10.7861/clinmed.2020-0182. Epub 2020 May 15 [PubMed PMID: 32371417]
Level 3 (low-level) evidenceLlorente Ayuso L, Torres Rubio P, Beijinho do Rosário RF, Giganto Arroyo ML, Sierra-Hidalgo F. Bickerstaff encephalitis after COVID-19. Journal of neurology. 2021 Jun:268(6):2035-2037. doi: 10.1007/s00415-020-10201-1. Epub 2020 Sep 3 [PubMed PMID: 32880723]
Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, Kahan J, Lansdale KN, LeMoss NM, Murthy SB, Stieg PE, Fink ME, Iadecola C, Segal AZ, Cusick M, Campion TR Jr, Diaz I, Zhang C, Navi BB. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA neurology. 2020 Jul 2:77(11):1-7. doi: 10.1001/jamaneurol.2020.2730. Epub 2020 Jul 2 [PubMed PMID: 32614385]
Wilkinson SW, Etheridge T, Swiston CJ, Vegunta S, Wiggins RH, Warner JEA. Bilateral Posterior Cerebral Artery Stroke from COVID-Related Multisystem Inflammatory Syndrome in a Child. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2022 Sep 1:42(3):e548-e550. doi: 10.1097/WNO.0000000000001468. Epub 2021 Nov 11 [PubMed PMID: 34812758]
Turbin RE, Wawrzusin PJ, Sakla NM, Traba CM, Wong KG, Mirani N, Eloy JA, Nimchinsky EA. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit (Amsterdam, Netherlands). 2020 Aug:39(4):305-310. doi: 10.1080/01676830.2020.1768560. Epub 2020 May 18 [PubMed PMID: 32419568]
Shires CB, Klug T, Dryden S, Ford J. Unusual cause of acute sinusitis and orbital abscess in COVID-19 positive patient: Case report. International journal of surgery case reports. 2021 Feb:79():164-168. doi: 10.1016/j.ijscr.2021.01.043. Epub 2021 Jan 15 [PubMed PMID: 33477076]
Level 3 (low-level) evidenceMekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, Simko JP, Winn BJ. Acute Invasive Rhino-Orbital Mucormycosis in a Patient With COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic plastic and reconstructive surgery. 2021 Mar-Apr 01:37(2):e40-e80. doi: 10.1097/IOP.0000000000001889. Epub [PubMed PMID: 33229953]
Mehta S, Pandey A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus. 2020 Sep 30:12(9):e10726. doi: 10.7759/cureus.10726. Epub 2020 Sep 30 [PubMed PMID: 33145132]
Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a Viral Land: A Tale of Two Pathogens. Indian journal of ophthalmology. 2021 Feb:69(2):244-252. doi: 10.4103/ijo.IJO_3774_20. Epub [PubMed PMID: 33463566]
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes & metabolic syndrome. 2021 Jul-Aug:15(4):102146. doi: 10.1016/j.dsx.2021.05.019. Epub 2021 May 21 [PubMed PMID: 34192610]
Level 3 (low-level) evidenceWerthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. The American journal of emergency medicine. 2021 Apr:42():264.e5-264.e8. doi: 10.1016/j.ajem.2020.09.032. Epub 2020 Sep 16 [PubMed PMID: 32972795]
Level 3 (low-level) evidenceArmstrong BK, Murchison AP, Bilyk JR. Suspected orbital myositis associated with COVID-19. Orbit (Amsterdam, Netherlands). 2021 Dec:40(6):532-535. doi: 10.1080/01676830.2021.1962366. Epub 2021 Aug 17 [PubMed PMID: 34402364]
Eleiwa T, Abdelrahman SN, ElSheikh RH, Elhusseiny AM. Orbital inflammatory disease associated with COVID-19 infection. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2021 Aug:25(4):232-234. doi: 10.1016/j.jaapos.2021.04.002. Epub 2021 May 6 [PubMed PMID: 33965589]
Martínez Díaz M, Copete Piqueras S, Blanco Marchite C, Vahdani K. Acute dacryoadenitis in a patient with SARS-CoV-2 infection. Orbit (Amsterdam, Netherlands). 2022 Jun:41(3):374-377. doi: 10.1080/01676830.2020.1867193. Epub 2021 Jan 5 [PubMed PMID: 33402004]
Pérez-Chimal LG, Cuevas GG, Di-Luciano A, Chamartín P, Amadeo G, Martínez-Castellanos MA. Ophthalmic manifestations associated with SARS-CoV-2 in newborn infants: a preliminary report. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2021 Apr:25(2):102-104. doi: 10.1016/j.jaapos.2020.11.007. Epub 2021 Feb 16 [PubMed PMID: 33601042]
Level 3 (low-level) evidenceChen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, Xin N, Huang Z, Liu L, Zhang G, Wang J. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. The British journal of ophthalmology. 2020 Jun:104(6):748-751. doi: 10.1136/bjophthalmol-2020-316304. Epub 2020 Apr 7 [PubMed PMID: 32265202]
Level 2 (mid-level) evidenceAmesty MA, Alió Del Barrio JL, Alió JL. COVID-19 Disease and Ophthalmology: An Update. Ophthalmology and therapy. 2020 Sep:9(3):1-12. doi: 10.1007/s40123-020-00260-y. Epub 2020 May 22 [PubMed PMID: 32445134]
Varu DM, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Musch DC, Mah FS, Dunn SP, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Conjunctivitis Preferred Practice Pattern®. Ophthalmology. 2019 Jan:126(1):P94-P169. doi: 10.1016/j.ophtha.2018.10.020. Epub 2018 Oct 23 [PubMed PMID: 30366797]
Seah I, Su X, Lingam G. Revisiting the dangers of the coronavirus in the ophthalmology practice. Eye (London, England). 2020 Jul:34(7):1155-1157. doi: 10.1038/s41433-020-0790-7. Epub 2020 Feb 6 [PubMed PMID: 32029919]
Loon SC, Lun K. SARS: a timely reminder. The British journal of ophthalmology. 2013 Sep:97(9):1217-8. doi: 10.1136/bjophthalmol-2013-303596. Epub 2013 Jun 22 [PubMed PMID: 23793905]
Level 3 (low-level) evidenceXie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor air. 2007 Jun:17(3):211-25 [PubMed PMID: 17542834]
Wessels IF, Wessels DA, Zimmerman GJ. The photic sneeze reflex and ocular anesthesia. Ophthalmic surgery and lasers. 1999 Mar:30(3):208-11 [PubMed PMID: 10100255]
Morley AM, Jazayeri F, Ali S, Malhotra R. Factors prompting sneezing in intravenously sedated patients receiving local anesthetic injections to the eyelids. Ophthalmology. 2010 May:117(5):1032-6. doi: 10.1016/j.ophtha.2009.09.007. Epub 2010 Jan 15 [PubMed PMID: 20079540]
Jun ISY, Hui KKO, Songbo PZ. Perspectives on Coronavirus Disease 2019 Control Measures for Ophthalmology Clinics Based on a Singapore Center Experience. JAMA ophthalmology. 2020 May 1:138(5):435-436. doi: 10.1001/jamaophthalmol.2020.1288. Epub [PubMed PMID: 32232466]
Level 3 (low-level) evidenceKampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. The Journal of hospital infection. 2020 Mar:104(3):246-251. doi: 10.1016/j.jhin.2020.01.022. Epub 2020 Feb 6 [PubMed PMID: 32035997]
Sabage LE, Sun YJ, Wolf J, Sabage J, Mazzo A, Santos CF, Mahajan VB, Manzoni Lourençone LF. Conjunctival Swabs Reveal Higher Detection Rate Compared to Schirmer Strips for SARS-CoV-2 RNA Detection in Tears of Hospitalized COVID-19 Patients. Journal of clinical medicine. 2022 Nov 24:11(23):. doi: 10.3390/jcm11236929. Epub 2022 Nov 24 [PubMed PMID: 36498504]
Dong J, Chen R, Zhao H, Zhu Y. COVID-19 and ocular complications: A review of ocular manifestations, diagnostic tools, and prevention strategies. Advances in ophthalmology practice and research. 2023 Feb-Mar:3(1):33-38. doi: 10.1016/j.aopr.2022.11.001. Epub 2022 Dec 1 [PubMed PMID: 36471811]
Level 3 (low-level) evidenceDavis G, Li K, Thankam FG, Wilson DR, Agrawal DK. Ocular transmissibility of COVID-19: possibilities and perspectives. Molecular and cellular biochemistry. 2022 Mar:477(3):849-864. doi: 10.1007/s11010-021-04336-6. Epub 2022 Jan 23 [PubMed PMID: 35066705]
Level 3 (low-level) evidenceDesai EJ, Pandya A, Upadhya I, Patel T, Banerjee S, Jain V. Epidemiology, Clinical Features and Management of Rhino Orbital Mucormycosis in Post COVID 19 Patients. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2022 Mar:74(1):103-107. doi: 10.1007/s12070-021-02807-2. Epub 2021 Aug 15 [PubMed PMID: 34414101]