Introduction
Aortoiliac occlusive disease (AIOD), is a variant of peripheral artery disease (PAD) that affects the infrarenal aorta and iliac arteries, obstructing blood flow to distal organs through narrowed arterial lumens or embolization of plaques. Like other arterial diseases, this condition can present a wide range of symptoms, from asymptomatic cases to limb-threatening emergencies. For many patients, collateral circulation can be sufficient to manage symptoms nonoperatively. However, obstructive lesions are commonly found in the infrarenal aorta, common iliac artery, internal iliac (hypogastric) artery, external iliac artery, or combinations of these vessels.
Recognizing the numerous risk factors for AIOD allows clinicians to implement medical therapies that alleviate symptoms and extend patient survival. The advent of prosthetic graft materials in the 1960s revolutionized the surgical treatment of AIOD, offering a viable solution for patients with significant aortic and iliac occlusions. This review will examine the pathophysiology, clinical manifestations, diagnostic approaches, and contemporary management options, focusing on surgical and endovascular interventions to improve outcomes in patients with AIOD.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
AIOD is a PAD variant affecting the lower aorta and iliac arteries. Similar to PAD, AIOD is typically caused by progressive atherosclerosis, characterized by the buildup of plaques composed of lipids, cholesterol, calcium, and other substances within the arterial walls. These plaques can obstruct blood flow to the lower extremities, pelvis, and abdominal organs, leading to stenosis and eventual occlusion of the infrarenal aorta, common iliac, internal iliac (hypogastric), or external iliac arteries.
Several inciting factors contribute to the development of atherosclerosis and AIOD, including diabetes, hyperhomocysteinemia, hypertension, hyperlipidemia, and tobacco use.[1] Smoking is a significant risk factor that accelerates plaque formation by promoting inflammation and endothelial dysfunction. Other critical risk factors include age, family history, ethnicity, and sex. Diabetes and hypertension exacerbate vascular stress and increase the likelihood of atherosclerotic plaque formation. At the same time, hyperlipidemia—especially elevated levels of low-density lipoprotein (LDL) cholesterol—directly contributes to arterial plaque buildup.
In rare cases, large-vessel vasculitis, such as Takayasu arteritis, can also lead to AIOD.[2][3] This vasculitis causes inflammation and narrowing of the aorta and its major branches, mimicking the effects of atherosclerosis. Recognizing these diverse etiologies is essential for providing targeted management strategies that address the underlying disease process and the risk factors contributing to AIOD.
Epidemiology
AIOD primarily affects older adults, with prevalence increasing significantly after age 50. The exact prevalence of AIOD and PAD is challenging to pinpoint, as many patients are asymptomatic. Estimates for the general population range from 3.56% to over 14%, with studies indicating that prevalence rises with age—affecting 14% to 20% of individuals over 70 and up to 23% of those over 80.[4][5][6][7] AIOD is more common in men, non-Hispanic Black individuals, and those with known risk factors, including diabetes, hypertension, hyperlipidemia, and smoking. Younger patients with localized AIOD often have a history of heavy smoking and elevated cholesterol. In contrast, older males with widespread multilevel disease frequently have diabetes and are at greater risk for coexisting coronary and cerebrovascular conditions.
AIOD, as a variant of PAD, shares similar risk factors with atherosclerosis, such as hypertension, hyperlipidemia, and tobacco usage. Additionally, nonatherosclerotic causes, though rare, such as large vessel vasculitis (eg, Takayasu arteritis), are significant in certain populations, particularly younger women. Geographic and racial disparities are noted, with Black individuals at higher risk due an increased prevalence of hypertension and diabetes.[8] Despite the variability in prevalence estimates, the consistent rise in AIOD with age highlights the importance of early detection and risk factor management, especially in high-risk populations, to mitigate the disease’s impact.
Pathophysiology
AIOD gradually obstructs blood flow in the infrarenal aorta and iliac arteries, primarily due to the development of atherosclerosis. The pathophysiology of AIOD is rooted in endothelial injury, which initiates a cascade of events promoting atherosclerotic plaque formation.[9] Various risk factors such as diabetes, hypertension, hyperlipidemia, and smoking expose endothelial cells to injury through several mechanisms. These factors contribute to endothelial dysfunction by enhancing the expression of leukocyte adhesion molecules, increasing inflammatory cytokines, and reducing nitric oxide production.
As a result of these processes, atherosclerosis forms as lipids, macrophages, and smooth muscle cells accumulate within the arterial wall. Over time, these deposits coalesce into fibrous plaques that can calcify, further narrowing the arterial lumen and restricting blood flow to distal organs. Additionally, increased production of endothelial vasoconstrictors, superoxide anion formation, and prothrombotic factors exacerbate vascular dysfunction. As the plaques progress, the underlying vascular smooth muscle loses integrity, and increased serum-free fatty acids and insulin levels further hinder cell function.
In AIOD, this narrowing typically occurs in the infrarenal aorta, common iliac arteries, or external iliac arteries. Initially, collateral circulation may compensate for reduced blood flow, allowing some patients to remain asymptomatic. However, as atherosclerosis advances and these compensatory mechanisms fail, symptoms like claudication, rest pain, and critical limb ischemia emerge. Furthermore, the plaques may rupture or dislodge, leading to distal embolization and exacerbating ischemic symptoms. If untreated, this progressive ischemia can result in tissue loss and potentially limb amputation. Nonatherosclerotic causes, such as large vessel vasculitis like Takayasu arteritis, can also contribute to similar vascular occlusion and ischemic consequences.
History and Physical
Obtaining a medical history and performing a physical examination are critical in assessing disease severity and guiding management. Patients commonly present with cramping pain in the lower extremities that occurs during and after physical activity and is relieved by rest. This classic symptom, claudication, is caused by reduced muscle blood flow due to arterial stenosis. The location of the muscle cramping can help determine the severity and extent of the stenosis. More proximal symptoms, such as buttock or thigh claudication, typically suggest more significant or higher-level occlusions, often involving the aortoiliac region.
A subset of AIOD patients may present with Leriche syndrome, a triad of symptoms including buttock claudication, erectile dysfunction, and absent femoral pulses.[10] This syndrome, named after Dr René Leriche, a French surgeon, indicates severe aortoiliac occlusion.[11] In more advanced cases, patients may seek emergent care due to complications from severe stenosis or acute embolism, leading to chronic limb-threatening ischemia (CLTI).[12] CLTI is defined by rest pain, gangrene, or lower limb ulceration lasting more than 2 weeks, indicating critical arterial insufficiency in the affected limb.[13]
A thorough medical history helps distinguish AIOD from other vascular or musculoskeletal conditions. At the same time, a detailed physical examination, including pulse assessments and inspection of the lower extremities for ulcerations or gangrene, is crucial for determining the presence of ischemia and planning treatment.
Evaluation
Evaluating AIOD involves a comprehensive approach, integrating clinical history, physical examination, and diagnostic testing to confirm the presence and severity of arterial obstruction. In patients with multiple vascular risk factors, such as claudication and absent femoral pulses, the diagnosis is often straightforward. Still, some may have palpable pedal pulses at rest due to strong collateral circulation, making thorough history-taking crucial.
Laboratory Evaluation
Laboratory evaluations help to identify underlying risk factors contributing to AIOD.[1] Serum lipid profiles, hemoglobin A1c, lipoprotein A, and homocysteine levels can reveal conditions such as hyperlipidemia and diabetes, which are primary contributors to atherosclerosis. Prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet counts are essential in suspected thrombosis cases. For further clarification, additional tests, such as anticardiolipin antibodies, antithrombin III, factor II (prothrombin) C-20210a, factor V Leiden, and proteins C and S may be necessary. Given the association with coronary artery disease, an electrocardiogram (ECG) should also be part of the evaluation.[5]
Ankle-Brachial Index
The ankle-brachial index (ABI) is a first-line screening test recommended by the American Heart Association (AHA) and the United States Preventive Services Task Force for diagnosing arterial disease because it is affordable, reliable, and noninvasive.[14][15][16] These characteristics make it ideal for initial screening. An ABI score of less than 0.9 confirms arterial occlusion.[17] However, in some cases, a normal resting ABI can be misleading due to collateral circulation. Repeating the ABI after graded exercise treadmill testing can help detect occlusive disease, especially in patients with AIOD symptoms.
Imaging Studies
- Pulse volume recordings
- Pulse volume recordings are recommended alongside ABI as part of the initial evaluation. They measure changes in blood volume within the limb, offering insight into the extent of arterial blockages.
- Doppler ultrasonography
- A Duplex ultrasound is a commonly used noninvasive tool for detecting blood flow disturbances and pinpointing stenotic or occlusive areas in the aorta and iliac arteries. This study helps guide the need for further imaging.[18]
- Computed tomography angiography (CTA)
- Magnetic resonance angiography (MRA)
- While effective in imaging vascular structures, MRA can overestimate the degree of stenosis and carries the risk of unnecessary contrast exposure, limiting its use in certain patients.[21]
- Digital subtraction angiography (DSA)
- Often regarded as the gold standard for detailed vascular imaging, DSA is typically reserved for cases where endovascular intervention is planned, as it provides real-time visualization and the opportunity for simultaneous treatment.
Additional Studies
In patients with a history of embolic events, CLTI, or recurrent claudication despite a negative ABI or Doppler result, graded exercise testing, segmental limb pressures, or more invasive imaging, such as intravascular ultrasound (IVUS), may be necessary. These tests provide better clarity on the location and severity of the occlusion and help guide definitive treatment strategies.
These diagnostic modalities offer a comprehensive understanding of AIOD, enabling personalized treatment plans that address the underlying risk factors and vascular obstruction. This approach helps improve outcomes, including symptom relief and long-term cardiovascular health.
Treatment / Management
There has been a significant shift in the treatment of PAD, with angioplasty and stenting now serving as first-line therapies for most patients with aortoiliac, renal, subclavian, and coronary occlusive disease. The onset and severity of symptoms guide the management of AIOD. In cases of CLTI, prompt intervention is critical to prevent further tissue necrosis and gangrene. The "PLAN" approach—patient risk, limb staging, and anatomic pattern—can be used to stage the disease effectively and guide treatment.[13](A1)
Medical Management
Medical management is available for nonacute cases, especially in poor surgical candidates. Primary measures include the outpatient optimization and management of diabetes, hyperlipidemia, hypertension, prothrombotic states, and tobacco use.[14][22][23] Appropriate healthy diet and exercise are also advised.[24][25] Supervised exercise programs may increase the walking distance from 180% to 340%.[26](A1)
Claudication symptoms are treatable with cilostazol or pentoxifylline. Cilostazol is a phosphodiesterase III inhibitor that may benefit graft patency and prevent stenosis after surgical intervention.[27] Pentoxifylline, a methylxanthine derivative, also provides relief but is less effective than cilostazol.
Antithrombotic Agents
The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial exhibited better antiplatelet management with clopidogrel than aspirin by lowering death rates from ischemic stroke, myocardial infarctions, or other vascular-related causes. Other antiplatelet agents have not been studied head-to-head, but dual antiplatelet therapy is not indicated for primary treatment. Vorapaxar, an antagonist of the protease-activated receptor (PAR-1), has improved acute limb ischemia events when administered with antiplatelet agents.[28][29] Vitamin K antagonists have not improved outcomes alone or in combination with aspirin.[30][31] Subset studies of patients with peripheral artery disease have shown reduced major adverse limb events when taking rivaroxaban with aspirin.[32][33](A1)
Surgical and Endovascular Treatment
Surgical and endovascular revascularization techniques include open and minimally invasive techniques, each with distinct advantages based on patient conditions.[1][34][35] They play a pivotal role in treating AIOD, offering options to restore blood flow and alleviate symptoms like claudication and critical limb ischemia.(B2)
Surgical revascularization techniques
Open surgical revascularization involves bypassing areas of stenosis or occlusion using vascular conduits. The type of bypass used depends on the degree and location of the blockage, the patient's comorbidities, and surgical risk. There are several surgical options:
- Aortoiliac bypass graft: This bypass requires greater exposure and aortic clamping, making it more invasive but effective. The surgeon exposes the infrarenal and iliac arteries through an abdominal incision.[36] A Y-shaped polytetrafluoroethylene (PTFE) graft anastomoses the aorta and iliac arteries, bypassing the blockage. This approach has shown excellent long-term results, with 5-year patency rates above 90%.[37]
- Aortobifemoral bypass (AFB): A commonly used surgical intervention, AFB uses a PTFE graft to bypass the aorta to the femoral arteries. This procedure is highly effective for extensive occlusive disease, with 5- and 10-year patency rates of 85% to 90% and 75% to 80%, respectively.[38]
- Axillofemoral bypass: This extraanatomic bypass is used for patients with contraindications to aortoiliac or AFB bypass due to severe comorbidities. The procedure involves tunneling a PTFE graft from the axillary artery to the femoral arteries, providing an alternative route for blood flow. While less durable than an AFB bypass, it offers acceptable patency and can be performed in higher-risk patients.[38]
- Femorofemoral and axillopopliteal bypass: These are variations of the axillofemoral bypass, often used in select cases with unilateral disease or patients with challenging anatomy.[39] (B3)
Endovascular revascularization techniques
Endovascular procedures are less invasive than open surgery and involve the use of catheter-based techniques to restore patency:
- Percutaneous transluminal angioplasty (PTA): This technique uses an inflatable balloon catheter passed through a guidewire across the stenotic or occluded vessel. The balloon is inflated to compress the atherosclerotic plaque against the arterial wall, thereby widening the lumen. Primary patency rates after PTA are nearly 90% at 1 year, and primary assisted and secondary patency is 92.3%.[40][41][42] PTA can be performed with or without stent placement. Research has shown that covered stents provide better outcomes than bare-metal stents.[43] Endovascular treatments have also proven beneficial in challenging calcified lesions.[44]
- Thromboendarterectomy (TEA): TEA involves surgically removing atherosclerotic plaque directly from the arterial wall, typically performed with PTA. This procedure is more invasive than PTA but can be highly effective for patients with localized occlusive disease.[34] (A1)
Choosing the Best Therapeutic Approach
The choice between open surgery and endovascular revascularization depends on several factors, including the extent of disease, patient comorbidities, and overall surgical risk. Open surgical approaches, like AFB, are often preferred for younger, healthier patients with long-segment or heavily calcified disease. In comparison, endovascular techniques are favored for older or high-risk patients due to their minimally invasive nature and lower in-hospital mortality rates. Both approaches have shown excellent long-term patency and limb salvage rates, and advances in endovascular techniques have further expanded treatment options for AIOD.
Differential Diagnosis
The following differentials should always be considered in patients presenting with symptoms and signs of AIOD:
- Vascular
- Arterial aneurysm
- Arterial dissection
- Embolism
- Arteritis
- Takayasu
- Giant cell
- Venous claudication
- Nonvascular
- Musculoskeletal pain
- Neurogenic claudication
Prognosis
The prognosis of AIOD varies based on disease severity, treatment, and patient comorbidities. Without intervention, the prognosis is poor, as AIOD can lead to progressive ischemia, especially in cases involving CLTI. However, the development of self-compensating collateral circulation may improve outcomes, particularly in patients with milder disease.[45] In contrast, more distal atherosclerotic lesions are associated with worse outcomes.[46] Medical management provides significant benefits, potentially delaying or eliminating the need for surgical intervention by addressing risk factors like smoking, hypertension, and hyperlipidemia.
After surgical intervention, the prognosis improves significantly. AFB surgery, a common procedure for severe AIOD, demonstrates excellent long-term outcomes with primary patency rates of 86.2% at 5 years and 77.6% at 10 years. The 10-year limb salvage and overall survival rates are 97.7% and 91.7%, respectively.[47] Although the 30-day mortality following AFB surgery is 2% to 3%, the long-term benefits make this an effective option for many patients.
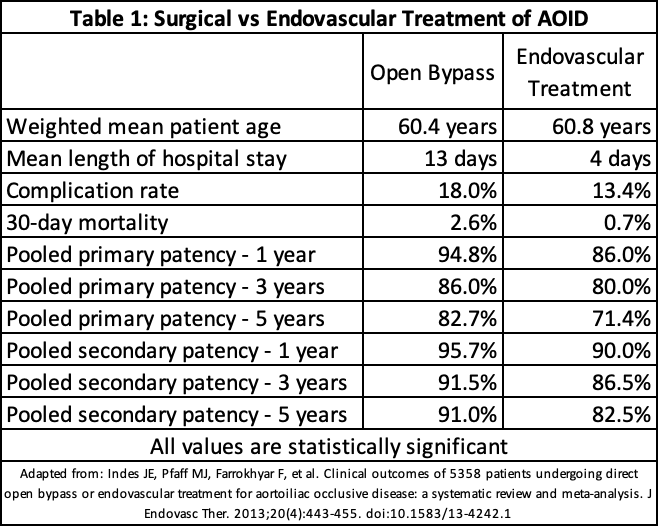
Endovascular interventions, such as PTA, offer another treatment option with even lower in-hospital mortality of 0.6%. Although the long-term patency rates are slightly lower than those seen with open surgery, they remain high—96% at 1 year and 94% at 2 years—making endovascular treatment a less invasive alternative with a favorable safety profile.[44] Meta-analytic comparisons between open bypass and endovascular procedures show similar efficacy in limb salvage and survival, providing clinicians with multiple options tailored to individual patient needs (see Image. Aortoiliac Occlusive Disease Treatment Comparisons).[48]
Complications
Complications of untreated AOID include weakness, fatigue, impotence, and sexual dysfunction as a result of decreased blood flow.[1] Heart failure, myocardial infarction, gangrene, and amputation are also increased in unmanaged AOID.[49][50][51][52] Surgical and endovascular treatment risks include thrombosis of the graft, wound infection, bleeding, and complications from anesthesia.
Deterrence and Patient Education
The patients are educated on lifestyle modifications and risk-reduction techniques to prevent disease progression. This frequently includes smoking cessation, increased physical activity, and dietary modifications. Patients should get regular follow up after every 3 to 6 months.
Pearls and Other Issues
Because patients with AIOD often have other comorbidities such as chronic obstructive pulmonary disease, diabetes, or hypertension, the preoperative workup is essential to prevent postoperative complications. In many cases, a cardiology referral is needed to assess the heart. Exercise stress testing, or nuclear myocardial perfusion scan in patients who cannot ambulate, is recommended. Smoking should be discontinued for at least 4 weeks to prevent pulmonary complications of the surgery, and ongoing abstinence should be strongly encouraged.
Enhancing Healthcare Team Outcomes
Effective management of aortoiliac occlusive disease (AIOD) requires a multidisciplinary approach to ensure optimal patient outcomes, enhance safety, and deliver patient-centered care. Physicians, including vascular surgeons and interventional radiologists, must utilize specialized skills in diagnostic imaging, decision-making regarding medical versus surgical intervention, and performing procedures like aortofemoral bypass or endovascular stenting. Advanced practitioners are key in preoperative evaluations, postoperative care, and patient education, ensuring adherence to lifestyle modifications and medical therapy. Nurses who monitor patients for signs of ischemia or complications facilitate patient education and contribute to ongoing assessments to ensure timely interventions, thus improving patient safety and outcomes.
Interprofessional communication and care coordination are essential in treating AIOD, as each healthcare professional brings unique expertise to the care plan. Pharmacists help optimize medication regimens by managing anticoagulation and other pharmacotherapies for risk factors like hyperlipidemia and hypertension. Effective communication between all team members, including physical therapists and dietitians, ensures a coordinated effort to manage patient risk factors and prevent disease progression. Regular interdisciplinary team meetings and using electronic health records to share up-to-date patient information improve team performance, reduce medical errors, and foster a comprehensive approach to managing AIOD, ultimately enhancing patient-centered care and improving long-term outcomes.
Media
(Click Image to Enlarge)
References
Frederick M, Newman J, Kohlwes J. Leriche syndrome. Journal of general internal medicine. 2010 Oct:25(10):1102-4. doi: 10.1007/s11606-010-1412-z. Epub 2010 Jun 22 [PubMed PMID: 20568019]
Level 3 (low-level) evidenceQi Y, Yang L, Zhang H, Liang E, Song L, Cai J, Jiang X, Zou Y, Qian H, Wu H, Zhou X, Hui R, Zheng D. The presentation and management of hypertension in a large cohort of Takayasu arteritis. Clinical rheumatology. 2018 Oct:37(10):2781-2788. doi: 10.1007/s10067-017-3947-4. Epub 2017 Dec 14 [PubMed PMID: 29238882]
Level 2 (mid-level) evidenceChacko S, Joseph G, Thomson V, George P, George O, Danda D. Carbon dioxide Angiography-Guided Renal-Related Interventions in Patients with Takayasu Arteritis and Renal Insufficiency. Cardiovascular and interventional radiology. 2018 Jul:41(7):998-1007. doi: 10.1007/s00270-018-1936-x. Epub 2018 Mar 16 [PubMed PMID: 29549415]
Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. Journal of vascular surgery. 2013 Sep:58(3):673-81.e1. doi: 10.1016/j.jvs.2013.01.053. Epub 2013 May 2 [PubMed PMID: 23642926]
Level 2 (mid-level) evidenceDiehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, von Stritzky B, Tepohl G, Trampisch HJ. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004 Jan:172(1):95-105 [PubMed PMID: 14709362]
Level 2 (mid-level) evidenceCriqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005 Oct 25:112(17):2703-7 [PubMed PMID: 16246968]
Allison MA, Cushman M, Solomon C, Aboyans V, McDermott MM, Goff DC Jr, Criqui MH. Ethnicity and risk factors for change in the ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis. Journal of vascular surgery. 2009 Nov:50(5):1049-56. doi: 10.1016/j.jvs.2009.05.061. Epub 2009 Jul 22 [PubMed PMID: 19628357]
Hackler EL 3rd, Hamburg NM, White Solaru KT. Racial and Ethnic Disparities in Peripheral Artery Disease. Circulation research. 2021 Jun 11:128(12):1913-1926. doi: 10.1161/CIRCRESAHA.121.318243. Epub 2021 Jun 10 [PubMed PMID: 34110901]
Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003 Sep 23:108(12):1527-32 [PubMed PMID: 14504252]
Brown KN, Muco E, Gonzalez L. Leriche Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 30855836]
Wooten C, Hayat M, du Plessis M, Cesmebasi A, Koesterer M, Daly KP, Matusz P, Tubbs RS, Loukas M. Anatomical significance in aortoiliac occlusive disease. Clinical anatomy (New York, N.Y.). 2014 Nov:27(8):1264-74. doi: 10.1002/ca.22444. Epub 2014 Jul 25 [PubMed PMID: 25065617]
Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G, Min J, Torii S, Poojary P, Anand SS, Bax JJ, Yusuf S, Virmani R, Narula J. Pathology of Peripheral Artery Disease in Patients With Critical Limb Ischemia. Journal of the American College of Cardiology. 2018 Oct 30:72(18):2152-2163. doi: 10.1016/j.jacc.2018.08.002. Epub 2018 Aug 27 [PubMed PMID: 30166084]
Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, GVG Writing Group. Global vascular guidelines on the management of chronic limb-threatening ischemia. Journal of vascular surgery. 2019 Jun:69(6S):3S-125S.e40. doi: 10.1016/j.jvs.2019.02.016. Epub 2019 May 28 [PubMed PMID: 31159978]
Level 1 (high-level) evidenceSociety for Vascular Surgery Lower Extremity Guidelines Writing Group, Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, Moneta GL, Murad MH, Powell RJ, Reed AB, Schanzer A, Sidawy AN, Society for Vascular Surgery. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. Journal of vascular surgery. 2015 Mar:61(3 Suppl):2S-41S. doi: 10.1016/j.jvs.2014.12.009. Epub 2015 Jan 28 [PubMed PMID: 25638515]
Level 1 (high-level) evidenceWriting Committee Members, Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME, ACC/AHA Task Force Members, Halperin JL, Levine GN, Al-Khatib SM, Birtcher KK, Bozkurt B, Brindis RG, Cigarroa JE, Curtis LH, Fleisher LA, Gentile F, Gidding S, Hlatky MA, Ikonomidis J, Joglar J, Pressler SJ, Wijeysundera DN. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary. Vascular medicine (London, England). 2017 Jun:22(3):NP1-NP43. doi: 10.1177/1358863X17701592. Epub [PubMed PMID: 28494710]
Guirguis-Blake JM, Evans CV, Redmond N, Lin JS. Screening for Peripheral Artery Disease Using the Ankle-Brachial Index: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018 Jul 10:320(2):184-196. doi: 10.1001/jama.2018.4250. Epub [PubMed PMID: 29998343]
Level 1 (high-level) evidencede Groote P, Millaire A, Deklunder G, Marache P, Decoulx E, Ducloux G. Comparative diagnostic value of ankle-to-brachial index and transcutaneous oxygen tension at rest and after exercise in patients with intermittent claudication. Angiology. 1995 Feb:46(2):115-22 [PubMed PMID: 7702195]
Level 2 (mid-level) evidenceKoelemay MJ, den Hartog D, Prins MH, Kromhout JG, Legemate DA, Jacobs MJ. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. The British journal of surgery. 1996 Mar:83(3):404-9 [PubMed PMID: 8665208]
Ahmed S, Raman SP, Fishman EK. CT angiography and 3D imaging in aortoiliac occlusive disease: collateral pathways in Leriche syndrome. Abdominal radiology (New York). 2017 Sep:42(9):2346-2357. doi: 10.1007/s00261-017-1137-0. Epub [PubMed PMID: 28401281]
Romano M, Mainenti PP, Imbriaco M, Amato B, Markabaoui K, Tamburrini O, Salvatore M. Multidetector row CT angiography of the abdominal aorta and lower extremities in patients with peripheral arterial occlusive disease: diagnostic accuracy and interobserver agreement. European journal of radiology. 2004 Jun:50(3):303-8 [PubMed PMID: 15145492]
Menke J, Larsen J. Meta-analysis: Accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease. Annals of internal medicine. 2010 Sep 7:153(5):325-34. doi: 10.7326/0003-4819-153-5-201009070-00007. Epub [PubMed PMID: 20820041]
Level 1 (high-level) evidenceGerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017 Mar 21:135(12):e726-e779. doi: 10.1161/CIR.0000000000000471. Epub 2016 Nov 13 [PubMed PMID: 27840333]
Level 1 (high-level) evidenceEckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24:129(25 Suppl 2):S76-99. doi: 10.1161/01.cir.0000437740.48606.d1. Epub 2013 Nov 12 [PubMed PMID: 24222015]
Level 1 (high-level) evidenceWeitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE Jr, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996 Dec 1:94(11):3026-49 [PubMed PMID: 8941154]
Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995 Sep 27:274(12):975-80 [PubMed PMID: 7674529]
Level 1 (high-level) evidenceBouwens E, Klaphake S, Weststrate KJ, Teijink JA, Verhagen HJ, Hoeks SE, Rouwet EV. Supervised exercise therapy and revascularization: Single-center experience of intermittent claudication management. Vascular medicine (London, England). 2019 Jun:24(3):208-215. doi: 10.1177/1358863X18821175. Epub 2019 Feb 22 [PubMed PMID: 30795714]
Tara S, Kurobe H, de Dios Ruiz Rosado J, Best CA, Shoji T, Mahler N, Yi T, Lee YU, Sugiura T, Hibino N, Partida-Sanchez S, Breuer CK, Shinoka T. Cilostazol, Not Aspirin, Prevents Stenosis of Bioresorbable Vascular Grafts in a Venous Model. Arteriosclerosis, thrombosis, and vascular biology. 2015 Sep:35(9):2003-10. doi: 10.1161/ATVBAHA.115.306027. Epub 2015 Jul 16 [PubMed PMID: 26183618]
Bonaca MP, Creager MA, Olin J, Scirica BM, Gilchrist IC Jr, Murphy SA, Goodrich EL, Braunwald E, Morrow DA. Peripheral Revascularization in Patients With Peripheral Artery Disease With Vorapaxar: Insights From the TRA 2°P-TIMI 50 Trial. JACC. Cardiovascular interventions. 2016 Oct 24:9(20):2157-2164. doi: 10.1016/j.jcin.2016.07.034. Epub [PubMed PMID: 27765312]
Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA, TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. The New England journal of medicine. 2012 Apr 12:366(15):1404-13. doi: 10.1056/NEJMoa1200933. Epub 2012 Mar 24 [PubMed PMID: 22443427]
Level 1 (high-level) evidenceWAVE Investigators. The effects of oral anticoagulants in patients with peripheral arterial disease: rationale, design, and baseline characteristics of the Warfarin and Antiplatelet Vascular Evaluation (WAVE) trial, including a meta-analysis of trials. American heart journal. 2006 Jan:151(1):1-9 [PubMed PMID: 16368284]
Level 1 (high-level) evidenceWarfarin Antiplatelet Vascular Evaluation Trial Investigators, Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, Budaj A, Sussex B, Liu L, Guzman R, Cina C, Crowell R, Keltai M, Gosselin G. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. The New England journal of medicine. 2007 Jul 19:357(3):217-27 [PubMed PMID: 17634457]
Level 1 (high-level) evidenceEikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S, COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. The New England journal of medicine. 2017 Oct 5:377(14):1319-1330. doi: 10.1056/NEJMoa1709118. Epub 2017 Aug 27 [PubMed PMID: 28844192]
Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O'Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S, COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2018 Jan 20:391(10117):219-229. doi: 10.1016/S0140-6736(17)32409-1. Epub 2017 Nov 10 [PubMed PMID: 29132880]
Level 1 (high-level) evidenceMetcalfe MJ, Natarajan R, Selvakumar S. Use of extraperitoneal iliac artery endarterectomy in the endovascular era. Vascular. 2008 Nov-Dec:16(6):310-5 [PubMed PMID: 19344587]
Level 2 (mid-level) evidenceKrankenberg H, Schlüter M, Schwencke C, Walter D, Pascotto A, Sandstede J, Tübler T. Endovascular reconstruction of the aortic bifurcation in patients with Leriche syndrome. Clinical research in cardiology : official journal of the German Cardiac Society. 2009 Oct:98(10):657-64. doi: 10.1007/s00392-009-0052-y. Epub 2009 Aug 14 [PubMed PMID: 19685001]
Rutherford RB. Options in the surgical management of aorto-iliac occlusive disease: a changing perspective. Cardiovascular surgery (London, England). 1999 Jan:7(1):5-12 [PubMed PMID: 10073753]
Level 3 (low-level) evidenceKang N, Kenel-Pierre S, DeAmorim H, Karwowski J, Bornak A. Endovascular Aortoiliac Revascularization in a Patient with Spinal Cord Injury and Hip Contracture. Annals of vascular surgery. 2018 Feb:47():281.e1-281.e4. doi: 10.1016/j.avsg.2017.08.039. Epub 2017 Sep 8 [PubMed PMID: 28893707]
Shah SS, Pillai GS, Greif BA, Wang S, Lewis AJ, Ryer EJ, Elmore JR, Salzler GG. Outcomes of Aortobifemoral Bypass Based on Configuration of the Proximal Anastomosis. Annals of vascular surgery. 2023 Nov:97():66-73. doi: 10.1016/j.avsg.2023.05.007. Epub 2023 May 25 [PubMed PMID: 37244482]
Pursell R, Sideso E, Magee TR, Galland RB. Critical appraisal of femorofemoral crossover grafts. The British journal of surgery. 2005 May:92(5):565-9 [PubMed PMID: 15810055]
Ichihashi S, Higashiura W, Itoh H, Sakaguchi S, Nishimine K, Kichikawa K. Long-term outcomes for systematic primary stent placement in complex iliac artery occlusive disease classified according to Trans-Atlantic Inter-Society Consensus (TASC)-II. Journal of vascular surgery. 2011 Apr:53(4):992-9. doi: 10.1016/j.jvs.2010.10.069. Epub 2011 Jan 7 [PubMed PMID: 21215582]
Level 2 (mid-level) evidenceVan Haren RM, Goldstein LJ, Velazquez OC, Karmacharya J, Bornak A. Endovascular treatment of TransAtlantic Inter-Society Consensus D aortoiliac occlusive disease using unibody bifurcated endografts. Journal of vascular surgery. 2017 Feb:65(2):398-405. doi: 10.1016/j.jvs.2016.08.084. Epub 2016 Oct 17 [PubMed PMID: 27765483]
Level 3 (low-level) evidenceGroot Jebbink E, Holewijn S, Slump CH, Lardenoije JW, Reijnen MMPJ. Systematic Review of Results of Kissing Stents in the Treatment of Aortoiliac Occlusive Disease. Annals of vascular surgery. 2017 Jul:42():328-336. doi: 10.1016/j.avsg.2017.01.009. Epub 2017 Apr 6 [PubMed PMID: 28390920]
Level 1 (high-level) evidenceMwipatayi BP, Thomas S, Wong J, Temple SE, Vijayan V, Jackson M, Burrows SA, Covered Versus Balloon Expandable Stent Trial (COBEST) Co-investigators. A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease. Journal of vascular surgery. 2011 Dec:54(6):1561-70. doi: 10.1016/j.jvs.2011.06.097. Epub 2011 Sep 9 [PubMed PMID: 21906903]
Level 2 (mid-level) evidencePiffaretti G, Fargion AT, Dorigo W, Pulli R, Gattuso A, Bush RL, Pratesi C, ILIACS Registry Group. Outcomes From the Multicenter Italian Registry on Primary Endovascular Treatment of Aortoiliac Occlusive Disease. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2019 Oct:26(5):623-632. doi: 10.1177/1526602819863081. Epub 2019 Jul 22 [PubMed PMID: 31331235]
Morotti A, Busso M, Cinardo P, Bonomo K, Angelino V, Cardinale L, Veltri A, Guerrasio A. When collateral vessels matter: asymptomatic Leriche syndrome. Clinical case reports. 2015 Nov:3(11):960-1. doi: 10.1002/ccr3.390. Epub 2015 Sep 10 [PubMed PMID: 26576282]
Level 3 (low-level) evidenceAboyans V, Desormais I, Lacroix P, Salazar J, Criqui MH, Laskar M. The general prognosis of patients with peripheral arterial disease differs according to the disease localization. Journal of the American College of Cardiology. 2010 Mar 2:55(9):898-903. doi: 10.1016/j.jacc.2009.09.055. Epub [PubMed PMID: 20185041]
Level 2 (mid-level) evidenceLee GC, Yang SS, Park KM, Park Y, Kim YW, Park KB, Park HS, Do YS, Kim DI. Ten year outcomes after bypass surgery in aortoiliac occlusive disease. Journal of the Korean Surgical Society. 2012 Jun:82(6):365-9. doi: 10.4174/jkss.2012.82.6.365. Epub 2012 May 29 [PubMed PMID: 22708098]
Indes JE, Pfaff MJ, Farrokhyar F, Brown H, Hashim P, Cheung K, Sosa JA. Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysis. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2013 Aug:20(4):443-55. doi: 10.1583/13-4242.1. Epub [PubMed PMID: 23914850]
Level 1 (high-level) evidenceKeller K, Beule J, Oliver Balzer J, Coldewey M, Munzel T, Dippold W, Wild P. A 56-year-old man with co-prevalence of Leriche syndrome and dilated cardiomyopathy: case report and review. Wiener klinische Wochenschrift. 2014 Mar:126(5-6):163-8. doi: 10.1007/s00508-013-0476-2. Epub 2013 Dec 17 [PubMed PMID: 24343041]
Level 3 (low-level) evidenceKashou AH, Braiteh N, Zgheib A, Kashou HE. Acute aortoiliac occlusive disease during percutaneous transluminal angioplasty in the setting of ST-elevation myocardial infarction: a case report. Journal of medical case reports. 2018 Jan 11:12(1):6. doi: 10.1186/s13256-017-1544-4. Epub 2018 Jan 11 [PubMed PMID: 29321037]
Level 3 (low-level) evidenceJOHNSON JK. Ascending thrombosis of abdominal aorta as fatal complication of Leriche's syndrome. A.M.A. archives of surgery. 1954 Nov:69(5):663-8 [PubMed PMID: 13206556]
Bredahl K, Jensen LP, Schroeder TV, Sillesen H, Nielsen H, Eiberg JP. Mortality and complications after aortic bifurcated bypass procedures for chronic aortoiliac occlusive disease. Journal of vascular surgery. 2015 Jul:62(1):75-82. doi: 10.1016/j.jvs.2015.02.025. Epub [PubMed PMID: 26115920]