Neuroanatomy, Autonomic Nervous System Visceral Afferent Fibers and Pain
Neuroanatomy, Autonomic Nervous System Visceral Afferent Fibers and Pain
Introduction
The autonomic nervous system (ANS) consists of general visceral efferent (GVE) fibers that create a motor response due to general visceral afferent (GVA) fiber stimulation. Although general visceral afferent fibers are part of the ANS, they are not classified as part of the sympathetic or parasympathetic system. However, these visceral sensory nerves often colocalize within sympathetic and parasympathetic nerves.[1]
GVA fibers carry sensory impulses from internal organs to the central nervous system (CNS). Stimuli that activate GVA fibers include hunger, blood pressure, organ distention, and visceral inflammation.[2] These afferent fibers allow the body to monitor the internal environment and adjust effector organs to maintain homeostasis.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
GVA fibers are primarily pseudounipolar neurons; they initially develop as bipolar neurons before altering their morphology to resemble unipolar neurons.[3] During development, the two processes emerging from the cell body fuse to form a single continuous process with a distal and proximal point, between which lies the cell body. The cell body of a GVA fiber can be found somewhere along a cranial nerve or in the dorsal root of the spinal cord. These GVA cell bodies are, for the most part, extracranial, except for the GVA fibers of the trigeminal nerve, whose nucleus lies within the brainstem.[4] The cell body controls all neuronal functions and allows information to pass between the receptive distal part and the centrally projecting part.
Visceral afferent fibers are supported by Schwann cells, a type of glial cell that supplies nutrients to peripheral nerves and has a role in nerve regeneration.[5] Schwann cells also insulate (myelinate) the axons of neurons from the peripheral nervous system by forming the myelin sheath.[6] This sheath is not continuous, leaving unmyelinated gaps along the axon called nodes of Ranvier. Together, the myelin sheath and nodes of Ranvier improve the axonal conduction velocity resulting in a faster response time.
Three types of sensory receptors can activate GVA fibers: mechanoreceptors, nociceptors, and chemoreceptors. Each of these receptors detects a specific stimulus associated with a different set of nerve fibers. Mechanoreceptors respond to mechanical pressure or physical deformation.[7] They receive their innervation through type II or III A-delta sensory fibers. Nociceptors detect pain and are innervated by type III and IV C fibers.[8] Chemoreceptors detect chemical changes (ie, pH, carbon dioxide, and oxygen levels).[9][10] Cranial nerves innervate this latter receptor type.
Embryology
The nervous system initially arises from neural plate development.[11] An indentation called the neural groove appears along the neural plate in the midline. As neurulation progresses, the neural groove deepens while the neural folds (the borders of the neural plate) converge on the dorsal midline to create the neural tube.
This neural tube is the future site of the CNS. The neural crest develops on the roof plate of the neural tube. Neural crest cells transition from epithelial to mesenchymal cells, dividing from the neuroepithelium and migrating through the periphery. These neural crest cells differentiate into varied cell types, including cells of the PNS.[12] Further differentiation of PNS cells leads to the development of GVA fibers.
Blood Supply and Lymphatics
Vasa nervorum vessels are the main blood supply for GVA fibers. These small arteries branch off from adjacent blood vessels and supply nutrients to each peripheral nerve.[13]
Nerves
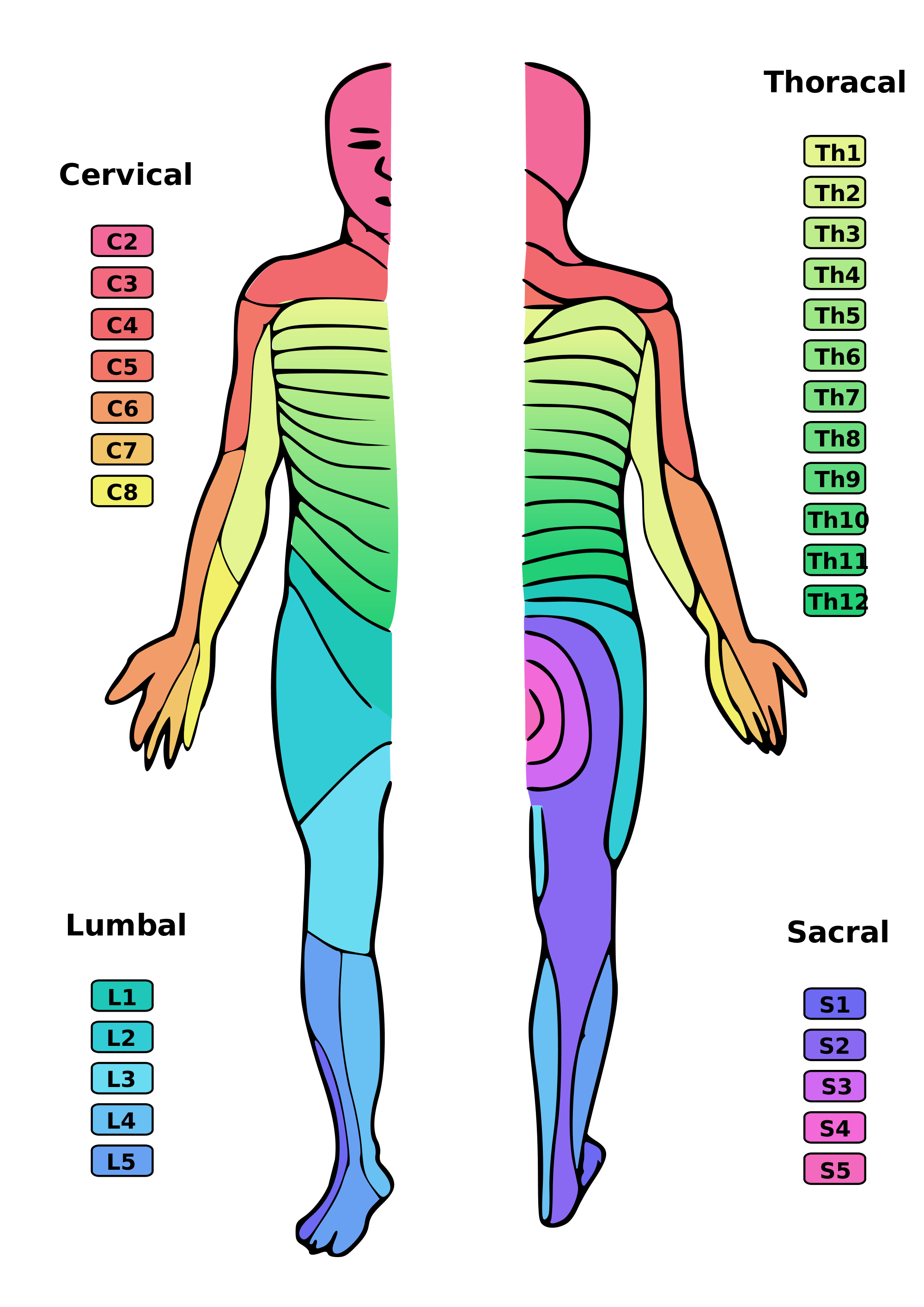
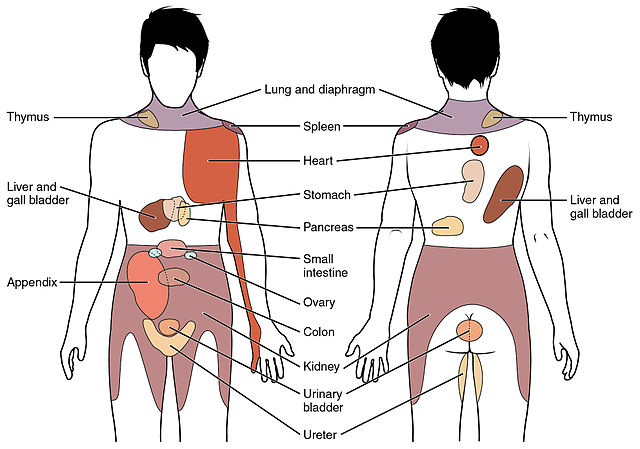
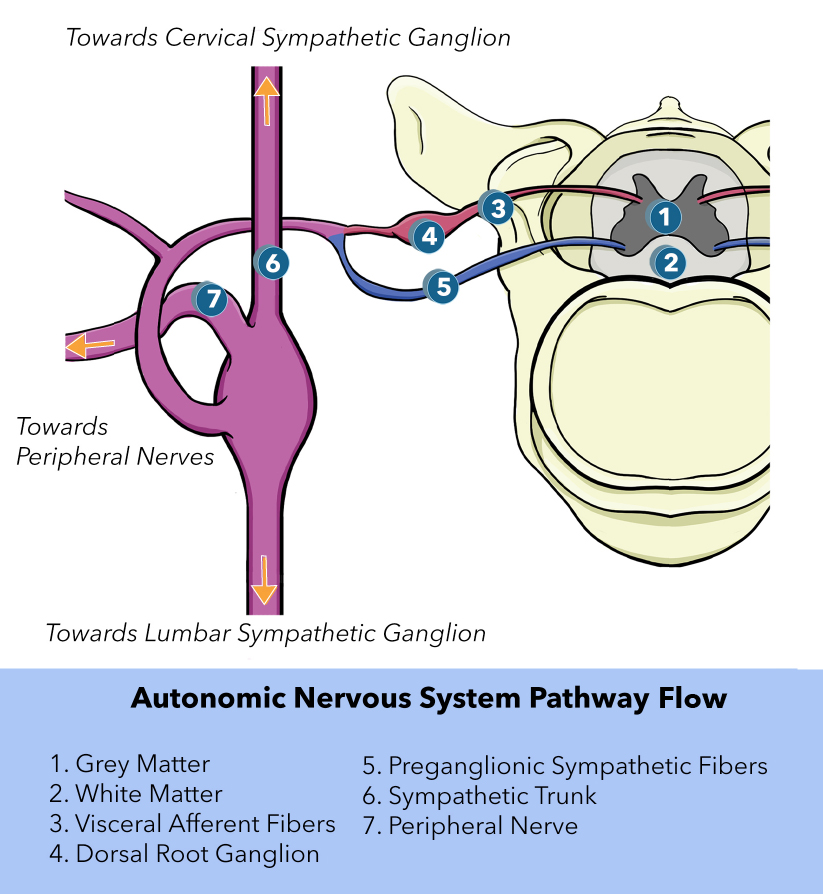
Once the sensory receptor is activated, the GVA impulse travels through the pseudounipolar neurons to reach the CNS. When the GVA fiber reaches the dorsal horn of the spinal cord, it terminates on a second-order neuron. [14] These neurons then ascend upwards into the brain for further processing. Under normal circumstances, visceral afferent activity does not reach the level of consciousness. However, if the visceral afferent activity is pain-related, it can reach the level of consciousness. Visceral pain is frequently felt in an area remote from the location of the affected organ; this is known as referred pain. The chart at the end of this article provides more information about where referred pain appears for specific organs.
The cranial nerves with GVA fibers are the facial nerve (CN VII), the glossopharyngeal nerve (CN IX), and the vagus nerve (CN X). The facial nerve has a small number of GVA fibers and primarily uses sensory neurons in the geniculate ganglion.[15] The glossopharyngeal nerve provides GVA fibers to the carotid sinus, carotid body, and other structures.[16]
The carotid sinus is located proximally to the internal carotid artery and contains baroreceptors sensitive to blood pressure. The carotid body is located at the bifurcation of the carotid artery with chemoreceptors that measure partial pressures of oxygen. Fibers from both the carotid sinus and the carotid body terminate on the solitary nucleus. Other anatomical structures innervated by GVA fibers of the glossopharyngeal nerve include the mucosal membranes of the inner surface of the middle ear, the Eustachian tube, the tonsils, the posterior third of the tongue, and the posterior/upper surfaces of the pharynx. Fibers from these areas contain cell bodies in the inferior ganglion of CN IX and terminate in the nucleus of the solitary tract.
The vagus nerve has GVA fibers in the tongue, larynx, pharynx, trachea, esophagus, lungs, bronchi, stomach, heart, and intestines.[17] Lung GVA fibers help regulate the depth of breathing. Cardiac vagal fibers innervate the aortic arch baroreceptors. Vagal GVA fibers innervate chemoreceptors of the abdomen, heart, bronchi, and carotid body.[18] All these vagal fiber cell bodies can be found within the inferior ganglia. The central processes of these neurons enter the medulla and terminate on the nucleus of the solitary tract.
Muscles
Stimulation of GVA fibers influences the motor response of their respective GVE fibers. These visceromotor fibers innervate either smooth muscle, cardiac muscle, or glands.
Physiologic Variants
Receptors associated with GVA fibers will vary in sensitivity and affect the body's ability to maintain homeostasis. Upregulation or downregulation of these receptors will change the intensity of the signal propagated through the afferent fiber. This intensity is of particular importance in the perception of referred pain.
Accurate interpretation of nerve conduction studies requires understanding variations in the anatomy of nerves.[19] This knowledge becomes increasingly important for patients that need to undergo surgery. Correctly mapping a patient's nerve variants can reduce surgical risk.
Surgical Considerations
Denervation of GVA fibers can be the desired outcome of surgical treatment or the problematic sequelae of invasive intervention. A disorder with a denervation treatment is carotid sinus syndrome caused by overactive carotid sinus baroreceptor stimulation.[20] This syndrome can cause temporary loss of consciousness and recurrent dizziness. Surgical denervation of the carotid sinus leads to a decrease in sympathetic and vagal baroreflex sensitivity. However, it increases blood pressure variability without leading to chronic hypertension.[21]
Clinical Significance
Diabetic neuropathy is a pathologic process that may occur in those with diabetes. Hyperglycemia interferes with nerve conduction and weakens the walls of the vasa nervorum, depriving nerves of oxygen and nutrients. Diabetic neuropathy can affect the nerves of the autonomic system, especially GVA fibers, potentially contributing to dysfunctional blood pressure regulation and gastroparesis.[22] GVA fibers affected by diabetic neuropathy have a reduced GVE fiber response.
Current knowledge indicates that referred pain occurs because multiple primary sensory neurons converge on a single ascending tract in the spinal cord. When painful stimuli activate visceral receptors, the brain is unable to distinguish between the visceral signals and somatic signals; the brain interprets that the pain is coming from somatic regions (e, skin, skeletal musculature, and bones) of the body rather than the visceral regions (ie, spleen, kidney, and heart). For example, patients with angina pectoris, a type of cardiac pain, experience referred pain in the chest and upper left arm.[23]
Another example is patients with temporomandibular disorder (TMD), who may experience referred pain in the teeth or other sections in the orofacial area.[24] Areas of referred pain can be cross-referenced against dermatome charts to help identify the visceral organ sending pain signals.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Mazzone SB, Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiological reviews. 2016 Jul:96(3):975-1024. doi: 10.1152/physrev.00039.2015. Epub [PubMed PMID: 27279650]
Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016 Jun 30:166(1):209-21. doi: 10.1016/j.cell.2016.05.011. Epub 2016 May 26 [PubMed PMID: 27238020]
de Moraes ER, Kushmerick C, Naves LA. Morphological and functional diversity of first-order somatosensory neurons. Biophysical reviews. 2017 Oct:9(5):847-856. doi: 10.1007/s12551-017-0321-3. Epub 2017 Sep 9 [PubMed PMID: 28889335]
Connor M, Naves LA, McCleskey EW. Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Molecular pain. 2005 Oct 24:1():31 [PubMed PMID: 16242047]
Level 3 (low-level) evidenceJessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harbor perspectives in biology. 2015 May 8:7(7):a020487. doi: 10.1101/cshperspect.a020487. Epub 2015 May 8 [PubMed PMID: 25957303]
Level 3 (low-level) evidenceSalzer JL. Schwann cell myelination. Cold Spring Harbor perspectives in biology. 2015 Jun 8:7(8):a020529. doi: 10.1101/cshperspect.a020529. Epub 2015 Jun 8 [PubMed PMID: 26054742]
Level 3 (low-level) evidenceIheanacho F, Vellipuram AR. Physiology, Mechanoreceptors. StatPearls. 2023 Jan:(): [PubMed PMID: 31082112]
Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. The Journal of clinical investigation. 2010 Nov:120(11):3760-72. doi: 10.1172/JCI42843. Epub 2010 Nov 1 [PubMed PMID: 21041958]
Level 3 (low-level) evidenceLópez-Barneo J, Ortega-Sáenz P, González-Rodríguez P, Fernández-Agüera MC, Macías D, Pardal R, Gao L. Oxygen-sensing by arterial chemoreceptors: Mechanisms and medical translation. Molecular aspects of medicine. 2016 Feb-Mar:47-48():90-108. doi: 10.1016/j.mam.2015.12.002. Epub 2015 Dec 18 [PubMed PMID: 26709054]
Nattie E, Li A. Central chemoreceptors: locations and functions. Comprehensive Physiology. 2012 Jan:2(1):221-54. doi: 10.1002/cphy.c100083. Epub [PubMed PMID: 23728974]
Level 3 (low-level) evidenceButler SJ, Bronner ME. From classical to current: analyzing peripheral nervous system and spinal cord lineage and fate. Developmental biology. 2015 Feb 15:398(2):135-46. doi: 10.1016/j.ydbio.2014.09.033. Epub 2014 Oct 24 [PubMed PMID: 25446276]
Level 3 (low-level) evidenceNewbern JM. Molecular control of the neural crest and peripheral nervous system development. Current topics in developmental biology. 2015:111():201-31. doi: 10.1016/bs.ctdb.2014.11.007. Epub 2015 Jan 22 [PubMed PMID: 25662262]
Level 3 (low-level) evidenceZochodne DW. Local blood flow in peripheral nerves and their ganglia: Resurrecting key ideas around its measurement and significance. Muscle & nerve. 2018 Jun:57(6):884-895. doi: 10.1002/mus.26031. Epub 2018 Jan 11 [PubMed PMID: 29211922]
Jänig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biological psychology. 1996 Jan 5:42(1-2):29-51 [PubMed PMID: 8770369]
Dulak D, Naqvi IA. Neuroanatomy, Cranial Nerve 7 (Facial). StatPearls. 2023 Jan:(): [PubMed PMID: 30252375]
Thomas K, Minutello K, M Das J. Neuroanatomy, Cranial Nerve 9 (Glossopharyngeal). StatPearls. 2023 Jan:(): [PubMed PMID: 30969699]
Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Frontiers in psychiatry. 2018:9():44. doi: 10.3389/fpsyt.2018.00044. Epub 2018 Mar 13 [PubMed PMID: 29593576]
Kenny BJ, Bordoni B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). StatPearls. 2023 Jan:(): [PubMed PMID: 30725856]
Jeon SK, Paik DJ, Hwang YI. Variations in sural nerve formation pattern and distribution on the dorsum of the foot. Clinical anatomy (New York, N.Y.). 2017 May:30(4):525-532. doi: 10.1002/ca.22873. Epub 2017 Apr 8 [PubMed PMID: 28281304]
McConnell OJ, Longley R, Gunasekera M. Isometachromin, a new cytotoxic sesquiterpenoid from a deep water sponge of the family Spongiidae. Experientia. 1992 Sep 15:48(9):891-2 [PubMed PMID: 1397187]
Level 3 (low-level) evidenceTimmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. The Journal of physiology. 2003 Nov 15:553(Pt 1):3-11 [PubMed PMID: 14528027]
Level 3 (low-level) evidenceRathmann W, Enck P, Frieling T, Gries FA. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes care. 1991 Nov:14(11):1086-9 [PubMed PMID: 1797493]
Foreman RD, Garrett KM, Blair RW. Mechanisms of cardiac pain. Comprehensive Physiology. 2015 Apr:5(2):929-60. doi: 10.1002/cphy.c140032. Epub [PubMed PMID: 25880519]
Murray GM. Guest Editorial: referred pain. Journal of applied oral science : revista FOB. 2009 Nov-Dec:17(6):i [PubMed PMID: 20027423]
Level 3 (low-level) evidence