Introduction
The internal capsule (IC) is a subcortical white matter structure situated in the inferomedial portion of each cerebral hemisphere. It is composed of myelinated ascending and descending fiber tracts that course past the basal ganglia to connect the cerebral hemispheres with subcortical structures, the brainstem, and the spinal cord. As it traverses the basal ganglia structures, it divides the caudate nucleus and thalamus from the putamen and globus pallidus. Anatomically, the internal capsule can be divided into the anterior limb, genu, posterior limb, retrolenticular segment, and sublenticular segment.[1][2][3]
The various portions of the internal capsule primarily receive their vascular supply from perforating arteries that arise from the anterior cerebral artery, middle cerebral artery, anterior choroidal artery, and the internal carotid artery. These perforating arteries are prone to lipohyalinosis leading to ischemic damage of areas of the internal capsule resulting in clinically significant motor and sensory deficits.[4]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
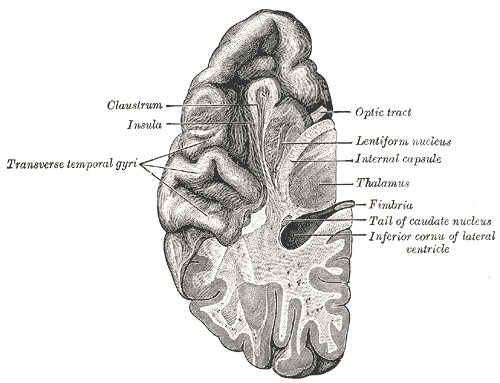
The internal capsule is a two-way tract for the transmission of information to and from the cerebral cortex. It lies in the inferomedial portion of each cerebral hemisphere. On transverse sections of the brain, the internal capsule is a V-shaped structure with the apex pointing medially. The lentiform nucleus forms the lateral bounds of the internal capsule, while the thalamus and caudate form the medial bounds. Above the superior border of the lentiform nucleus, the fibers of the internal capsule arrange in a radiating pattern known as the corona radiata.[2]
Fibers of the corona radiata travel caudally and become densely packed to form the internal capsule. The fibers become even more densely packed as they continue past the basal ganglia, forming the basis pedunculi at the midbrain. As the axons from the internal capsule travel down the brain, their numbers decrease as many descending axonal tracts terminate in the thalamus and various other nuclei in the brainstem. The internal capsule subdivides into the anterior limb, genu, posterior limb, retrolenticular segment, and sublenticular segment. Each portion of the internal capsule carries distinct ascending and descending axonal tracts that each have critical functions.[1][2]
The internal capsule's anterior limb is bounded by the head of the caudate nucleus medially and the lentiform nucleus laterally. The anterior limb contains fibers of the anterior thalamic radiation and frontopontine fibers.[1] Anterior thalamic radiation fibers connect the anterior and medial thalamus with the prefrontal cortex and the cingulate gyrus.[5] Frontopontine fibers originate from the frontal lobe and terminate in pontine nuclei.[6] The anterior limb also contains fiber tracts that travel transversely between the caudate nucleus and the putamen. Fiber tracts in the anterior limb are associated with processing emotion, cognition, decision making, and motivation.[7] Abnormalities in the white matter of the anterior limb are seen to be abnormal in psychiatric illnesses such as schizophrenia, bipolar disorder, and obsessive-compulsive disorder.[7]
In a study utilizing diffusion MRI, researchers segmented the anterior limb into five regions.[7] These regions separate into two mediolateral areas and three dorsoventral areas. The regions were further categorized based on the cortical origins of the fibers tracts, which included the ventromedial prefrontal cortex (vmPFC), ventrolateral prefrontal cortex (vlPFC), orbitofrontal cortex (OFC), dorsomedial prefrontal cortex (dmPFC), dorsolateral prefrontal cortex (dlPFC), and the dorsal anterior cingulate cortex (dACC). The ventralmost region of the anterior limb contained fibers from the vmPFC and OFC. Dorsal to this region is the main area of the anterior limb, which contains fibers from vlPFC, dACC, dlPFC, and the dmPFC.[7]
The internal capsule's posterior limb is bounded by the thalamus medially and the lentiform nucleus laterally. The posterior limb contains fibers of the posterior thalamic radiation, corticospinal tract, corticorubral tract, and corticopontine tract.[1] The anterior half of the posterior limb contains the corticospinal tract, corticorubral tract, and corticopontine tract. The corticospinal tract originates from the primary motor cortex and premotor areas. Fibers from the premotor areas are situated rostrally to fibers from the primary motor cortex.[8]
The corticospinal tract is the primary motor pathway that innervates lower motor neurons. Corticopontine fibers originate from all areas of the cerebral cortex, course to the cerebellum, and terminate in deeper pontine nuclei. These fibers are crucial for the coordination of voluntary motor functions.[9] Corticorubral fibers originate from the primary motor area and sensory areas and terminate in the red nucleus.[10] The posterior third of the posterior limb contains major somatosensory relays from the thalamus to the primary somatosensory cortex. The third-order sensory fibers from the posterolateral nucleus of the thalamus course through this region of the posterior limb and terminate in the somesthetic area in the postcentral gyrus of the cerebral cortex. At this level, the contralateral half of the body is represented as inverted, with the hand and mouth situated inferiorly and the leg situated superiorly, and with the foot and anogenital region on the medial surface of the hemisphere.[11]
The dentatothalamic tract from the dentate nucleus end by synapsing with cells in the contralateral ventrolateral nucleus of the thalamus. The axons of the thalamic neurons ascend through the internal capsule to terminate in the primary motor area of the cerebral cortex. Through this pathway, the dentate influences motor activity on the same side of the body by acting on the motor neurons of the opposite cerebral cortex.[2] Furthermore, these descending fibers from the cerebral cortex converge in the corona radiata and pass through the posterior limb of the internal capsule. At this level, the fibers closest to the genu correlate with the cervical portions of the body, while those situated more posteriorly correlate with the lower extremity. As the tract courses through the midbrain, it constitutes the middle three-fifths of the basis pedunculi. Here, the fibers are arranged with the cervical portions of the body situated medially, and fibers representing the lower extremity are placed laterally.[2][3]
The location of the genu of the internal capsule is at the apex of the pallidal part of the lentiform nucleus. The anterior and posterior limbs join at a right angle in this region to form the genu.[1]. Tracts that course through the genu include superior thalamic radiation fibers and corticobulbar tract fibers. Corticobulbar tract fibers originate from the primary motor cortex, premotor cortex, and supplementary motor areas. They course through the genu and terminate at the appropriate cranial nerve nuclei within the brainstem. The corticobulbar tract controls the muscles of the face and neck. This tract is necessary for the movement of facial musculature, mastication, and swallowing. The superior thalamic radiation fibers connect ventral nuclear group thalamic nuclei with the postcentral gyrus and appear to carry somaesthetic sensations that pass through the thalamus.[12]
The location of the sublenticular segment of the internal capsule is below the lentiform nucleus. This area contains auditory radiation fibers which course from the medial geniculate body and terminate in the transverse temporal gyri of Heschl. The retrolenticular segment of the internal capsule is around the posterior edge of the lentiform nucleus. It contains fibers of the optic radiation which connect the lateral geniculate nucleus to the calcarine fissure (a.k.a geniculocalcarine radiations). Parts of the optic radiation also course through the sublenticular segment of the internal capsule. The optic radiation transmits visual information from the retina to the visual cortex.[2]
Embryology
The internal capsule is a part of the telencephalon during embryologic development. The telencephalon consists of the two cerebral hemispheres of the brain. Each hemisphere is composed of the cerebral cortex, underlying white matter structures, and basal nuclei. Just like most structures of the brain, the origin of the telencephalon traces to the ectoderm, one of three germinal layers that form in the developing embryo. During embryogenesis, the notochord induces a strip of the ectoderm to form the neural plate. Further signaling from the notochord induces neurulation of the neural plate forming the neural groove and subsequently the neural tube. From the neural tube, the prosencephalon, mesencephalon, and rhombencephalon form, which give rise to all regions of the brain. The prosencephalon splits and develops into the telencephalon and diencephalon.[13]
Blood Supply and Lymphatics
Arterial Blood Supply
Each section of the internal capsule receives vascular supply from perforating branches of the main cerebral arteries. These include the anterior cerebral artery, middle cerebral artery, anterior choroidal artery, and internal carotid artery. The superior levels of the anterior limb, genu, and posterior limb get their supply from perforating arteries of the middle cerebral artery. The inferior levels of the anterior limb obtain their blood supply from the Heubner artery and perforating arteries of the anterior cerebral artery. The inferior levels of the genu get supplied by perforating arteries of the internal carotid artery and proximal perforating arteries of the anterior choroidal artery. Perforating arteries of the anterior choroidal artery supply the inferior levels of the posterior limb. The retrolenticular limb and sublenticular limb of the internal capsule are vascularized primarily by distal perforating arteries of the anterior choroidal artery.[4][14]
Venous Blood Supply
The striate veins drain the corpus striatum and internal capsule. The superiorstriate veins run dorsally and drain into the tributaries of the internal cerebra veins. The putamen, caudate nucleus, and internal capsule are all drained by the inferior striate veins. These inferior striate veins converge to exit through the anterior-perforated substance and join the deep middle cerebral and basal veins.[3][15]
Surgical Considerations
The internal capsule and the other white fiber structures can be affected by various pathologies, such as degeneration, demyelination, deficiencies (vitamin deficiency), vascular (infarction, hemorrhage, AVM, angioma), hypoxia, intrinsic neoplastic (glioma, oligodendroglioma, ganglioglioma, primitive neuroectodermal tumor), infection (tuberculosis, pyogenic abscess), parasitic involvement (neurocysticercosis, hydatid cyst), trauma, iatrogenic (postsurgical), epilepsy, psychiatric illness, etc. Commonly, intraventricular lesions such as ependymoma, glioma, meningioma, colloid cysts, etc., can extend into the internal capsule and other white fiber tracts. Appropriate neurosurgical anatomy and orientation are essential, along with physiological, pathological, and microsurgical skills for dealing with these pathologies.[1]
One paper described the concept of the central core within each cerebral hemisphere as an obvious anatomical delimitation with important clinical and surgical implications.[16] The central core is a solid block between the brainstem and the cerebral lobes composed of the insular surface, basal ganglia, and thalamus with connection to the rest of the supratentorial compartment via the cerebral isthmus. This also involves the continuation of the internal and external capsule towards the cerebral lobes. The subdivision of the central core into quadrants allows for a better understanding of the complex anatomy of the central core structures.[3]
Furthermore, another paper used a similar concept of “central core” to describe a vertical plane positioned at the anterior limit of the pyramidal tract allowing the surgeon to identify the posteriorly placed genu and the posterior limb of the internal capsule. Secondly, due to the vascularization of the internal capsule being a surgical barrier, the central core can be divided into medial and lateral portions along the parasagittal plane. For lesions present medially to the vascular supply of the internal carotid artery, a supracarotid-infrafrontal approach or an anterior transcallosal approach extending through the floor of the lateral ventricle has been described. Alternatively, for lesions on the lateral side, an anterior transinsular route has been suggested.[17]
Clinical Significance
Ischemic Strokes Involving the Internal Capsule
The internal capsule is prone to cerebrovascular accidents because the perforating arteries that supply the region are predisposed to occlusion or rupture due to their small diameter. Ischemic strokes secondary to blockage of the perforating arteries are known as lacunar strokes. The mechanisms of lacunar strokes include lipohyalinosis of perforating blood vessels, atherosclerosis of the large trunk vessels that supply perforators, and embolic occlusion of the perforating arteries.[18] Lipohyalinosis of perforating arteries is the most common cause of lacunar strokes and correlates with chronic hypertension states. It also has associations with diabetes and hyperlipidemia. The hallmark of lipohyalinosis is vessel wall thickening leading to a reduction of luminal diameter.[18][19]
Lacunar strokes primarily affect the deep structures of the brain, such as the putamen, caudate nucleus, thalamus, and internal capsule. Depending on the location of a lesion, the symptoms of lacunar strokes will require differentiation from cortical strokes. These deep strokes usually have an absence of cortical deficits such as seizures, aphasia, agnosia, and dysgraphia. Other cortical deficits include apraxia, alexia, and amnesia. The progression of symptoms of lacunar strokes are abrupt in onset and evolve within minutes.[20] In some cases, the symptoms may develop over several hours. Many variations of lacunar stroke syndromes exist. Classic lacunar stroke syndrome that arises from internal capsule lesions are pure motor strokes, ataxic hemiparesis, and clumsy hand-dysarthria.[21]
Pure motor strokes have a characteristic presentation of contralateral hemiparesis that affects the face, arm, and leg in equal parts. Ataxic hemiparesis presents with a combination of ataxia and weakness on the same side of the body. These stroke syndromes can include secondary lesions of the posterior limb of the internal capsule. Clumsy-hand dysarthria presents with difficulty articulating speech and weakness in the hands and results from damage to the anterior limb of the internal capsule or genu.[22]
Due to the blood supply of the internal capsule, infarctions of different portions of the internal capsule have varied clinical presentations. The deficits secondary to infarctions of the internal capsule correlate with the fiber tracts found in each limb. The anterior limb of the internal capsule contains frontopontine fibers and anterior thalamic radiation fibers. Lesions of the anterior limb can manifest as confusion, impaired attention, agitation, and dysarthria. The genu contains corticobulbar tract fibers. Lesions of the genu can cause face and tongue weakness in addition to dysarthria. The posterior limb contains fibers of the pyramidal and extrapyramidal tracts. It also contains fibers of posterior thalamic radiations.[23]
As previously stated, lesions of the anterior half of the posterior limb can cause pure motor hemiparesis contralateral to the location of the lesion. Damage to the posterior third of the posterior limb can lead to contralateral hemisensory deficits. The retrolenticular segment of the internal capsule largely contains fibers of the optic radiations. Infarctions of this portion can lead to homonymous hemianopia, superior quadrantanopia, or inferior quadrantanopia. Finally, the sublenticular segment contains fibers of the auditory radiations. Lesions of the sublenticular limb can create auditory deficits. In general, the internal capsule is an important white matter structure that contains fiber tracts coordinating major cognitive, motor, and sensory pathways. Understanding the diverse blood supply of the internal capsule and the function of each limb is important for characterizing internal capsule lesions.[14]
Hemorrhagic Strokes Involving the Internal Capsule
Severe hemorrhage involving the internal capsule, subcortical connections between the cerebral cortex, and other subcortical nuclei may be damaged. Moreover, some of the nuclei themselves may be destroyed. The interruption of other descending tracts from these subcortical centers would produce the following clinical signs as mentioned above: (a) severe paralysis on the opposite side of the body; (b) spasticity of the paralyzed muscles; (c) exaggerated deep muscle reflexes on the opposite side of the body to the lesion (clonus may be demonstrated); and (d) clasp knife reaction, which may be felt In the paralyzed muscles.[24]
Media
(Click Image to Enlarge)
References
Chowdhury F, Haque M, Sarkar M, Ara S, Islam M. White fiber dissection of brain; the internal capsule: a cadaveric study. Turkish neurosurgery. 2010 Jul:20(3):314-22. doi: 10.5137/1019-5149.JTN.3052-10.2. Epub [PubMed PMID: 20669103]
Costa M, Braga VL, Yağmurlu K, Centeno RS, Cavalheiro S, Chaddad-Neto F. A Technical Guide for Fiber Tract Dissection of the Internal Capsule. Turkish neurosurgery. 2018:28(6):934-939. doi: 10.5137/1019-5149.JTN.20884-17.1. Epub [PubMed PMID: 29465740]
Ribas EC, Yağmurlu K, de Oliveira E, Ribas GC, Rhoton A. Microsurgical anatomy of the central core of the brain. Journal of neurosurgery. 2018 Sep:129(3):752-769. doi: 10.3171/2017.5.JNS162897. Epub 2017 Dec 22 [PubMed PMID: 29271710]
Vitosević Z, Cetković M, Vitosević B, Jović D, Rajković N, Millisavljević M. [Blood supply of the internal capsule and basal nuclei]. Srpski arhiv za celokupno lekarstvo. 2005 Jan-Feb:133(1-2):41-5 [PubMed PMID: 16053174]
Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, Gado MH, Barch DM, Csernansky JG. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry research. 2010 Aug 30:183(2):144-50. doi: 10.1016/j.pscychresns.2010.04.013. Epub 2010 Jul 8 [PubMed PMID: 20619618]
Jang SH, Chang PH, Kim YK, Seo JP. Anatomical location of the frontopontine fibers in the internal capsule in the human brain: a diffusion tensor tractography study. Neuroreport. 2014 Jan 22:25(2):117-21. doi: 10.1097/WNR.0000000000000076. Epub [PubMed PMID: 24366326]
Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner SR, McLaughlin NCR, Mandeville J, Versace A, Phillips ML, Lehman JF, Yendiki A, Haber SN. Functional Segmentation of the Anterior Limb of the Internal Capsule: Linking White Matter Abnormalities to Specific Connections. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018 Feb 21:38(8):2106-2117. doi: 10.1523/JNEUROSCI.2335-17.2017. Epub 2018 Jan 22 [PubMed PMID: 29358360]
Qian C, Tan F. Internal capsule: The homunculus distribution in the posterior limb. Brain and behavior. 2017 Mar:7(3):e00629. doi: 10.1002/brb3.629. Epub 2017 Feb 6 [PubMed PMID: 28293471]
Level 2 (mid-level) evidenceTredici G, Barajon I, Pizzini G, Sanguineti I. The organization of corticopontine fibres in man. Acta anatomica. 1990:137(4):320-3 [PubMed PMID: 2368586]
Rüber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012 Aug 7:79(6):515-22. doi: 10.1212/WNL.0b013e31826356e8. Epub 2012 Jul 25 [PubMed PMID: 22843266]
Hoon AH Jr, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Developmental medicine and child neurology. 2009 Sep:51(9):697-704. doi: 10.1111/j.1469-8749.2009.03306.x. Epub 2009 Mar 30 [PubMed PMID: 19416315]
Level 2 (mid-level) evidenceYounes K,Hasan KM,Kamali A,McGough CE,Keser Z,Hasan O,Melicher T,Kramer LA,Schulz PE, Diffusion Tensor Imaging of the Superior Thalamic Radiation and Cerebrospinal Fluid Distribution in Idiopathic Normal Pressure Hydrocephalus. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2019 Mar; [PubMed PMID: 30461106]
Müller F, O'Rahilly R. The first appearance of the future cerebral hemispheres in the human embryo at stage 14. Anatomy and embryology. 1988:177(6):495-511 [PubMed PMID: 3377191]
Djulejić V,Marinković S,Georgievski B,Stijak L,Aksić M,Puškaš L,Milić I, Clinical significance of blood supply to the internal capsule and basal ganglia. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016 Mar; [PubMed PMID: 26596401]
Rhoton AL Jr, The cerebral veins. Neurosurgery. 2002 Oct [PubMed PMID: 12234449]
Ribas GC, Oliveira Ed. [The insula and the central core concept]. Arquivos de neuro-psiquiatria. 2007 Mar:65(1):92-100 [PubMed PMID: 17420835]
Potts MB, Chang EF, Young WL, Lawton MT, UCSF Brain AVM Study Project. Transsylvian-transinsular approaches to the insula and basal ganglia: operative techniques and results with vascular lesions. Neurosurgery. 2012 Apr:70(4):824-34; discussion 834. doi: 10.1227/NEU.0b013e318236760d. Epub [PubMed PMID: 21937930]
Arboix A,Martí-Vilalta JL, Lacunar stroke. Expert review of neurotherapeutics. 2009 Feb; [PubMed PMID: 19210194]
Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. Journal of stroke. 2015 Jan:17(1):2-6. doi: 10.5853/jos.2015.17.1.2. Epub 2015 Jan 30 [PubMed PMID: 25692102]
Gore M,Bansal K,Asuncion RMD, Lacunar Stroke StatPearls. 2022 Jan; [PubMed PMID: 33085363]
Tanaka K, Yamada T, Torii T, Yoshimura T, Takase K, Togao O, Wakata Y, Hiwatashi A, Nakashima N, Kira J, Murai H. Pure dysarthria and dysarthria-facial paresis syndrome due to internal capsule and/or corona radiata infarction. BMC neurology. 2015 Oct 7:15():184. doi: 10.1186/s12883-015-0439-5. Epub 2015 Oct 7 [PubMed PMID: 26445963]
Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982 Aug:32(8):871-6 [PubMed PMID: 7048128]
Yaghi S, Raz E, Yang D, Cutting S, Mac Grory B, Elkind MS, de Havenon A. Lacunar stroke: mechanisms and therapeutic implications. Journal of neurology, neurosurgery, and psychiatry. 2021 May 26:():. pii: jnnp-2021-326308. doi: 10.1136/jnnp-2021-326308. Epub 2021 May 26 [PubMed PMID: 34039632]
Arboix A, Rodríguez-Aguilar R, Oliveres M, Comes E, García-Eroles L, Massons J. Thalamic haemorrhage vs internal capsule-basal ganglia haemorrhage: clinical profile and predictors of in-hospital mortality. BMC neurology. 2007 Oct 5:7():32 [PubMed PMID: 17919332]
Level 2 (mid-level) evidence