Introduction
Permissive hypotension, commonly known as hypotensive resuscitation, is the method of deliberately allowing the patient's mean arterial pressure (MAP) to remain lower than normal physiological levels. This technique is commonly employed to address acute hemorrhagic volume depletion arising from severe trauma. Hemorrhagic shock is one of the most common causes of mortality in trauma patients. Permissive hypotension serves only as a temporizing measure before hemorrhage control or surgery can be performed. Hemodynamic parameters should be corrected to normal ranges when blood products and improved hemorrhage control measures are available.
Hypotensive resuscitation's role in patients with penetrating trauma was initially investigated by Bickell WH et al, though the concept dates back to World War I. Various studies have described a mortality reduction when blood pressures are adjusted below the normal physiologic range. Postoperative recovery time is also shorter when permissive hypotension is put into practice, as opposed to immediately normalizing an individual's blood pressure.[1][2]
However, data do not support any specific algorithmic pressure stabilization approaches in patients with different confounding factors, such as age, preexisting health conditions, and injury mechanisms. Given the methods of performing this intervention, the challenge of hypotensive resuscitation lies in how low one should set the limiting threshold.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
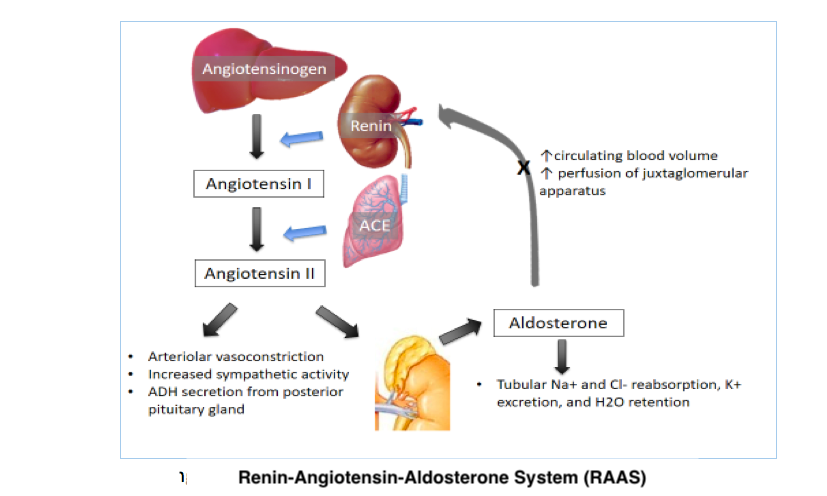
The body normally maintains appropriate vital organ perfusion by regulating blood volume and pressure. The body tightly regulates blood volume to maintain adequate organ perfusion while preventing excessive fluid overload. Homeostasis is achieved through renal and hormonal control of water and electrolyte balance. The most vital hormones in maintaining blood volume are the antidiuretic hormone and the renin-angiotensin-aldosterone system's (RAAS) components (see Image. Renin-Angiotensin-Aldosterone System). Fluid shifts between the intracellular and extracellular compartments also contribute to blood volume stability.
Blood pressure regulation involves complex neurologic, hormonal, cardiac, and renal controls (see Image. Blood Pressure Regulation). The autonomic nervous system heavily influences cardiac output and vascular tone and can stimulate the RAAS when blood pressure decreases. Normal blood pressure typically ranges from 90/60 mmHg to 120/80 mmHg, necessary to ensure adequate tissue perfusion.
The human body responds to significant blood loss by activating the sympathetic nervous system to maintain blood pressure and perfusion to vital organs. Initial sympathetic stimulation results in vasoconstriction, increased cardiac contractility and rate, catecholamine secretion, and RAAS activation. Peripheral vasoconstriction reduces blood loss but can compromise tissue perfusion when excessive.
MAP is a measure of the average arterial pressure during a cardiac cycle. MAP is calculated using the following formula:
MAP = DBP + 1/3(SBP - DBP)
Where "DBP" stands for "diastolic blood pressure and "SBP" stands for "systolic blood pressure." The MAP is an important cardiovascular parameter because it reflects the perfusion pressure, which drives blood flow to tissues and organs throughout the body. Adequate MAP is necessary to ensure that organs receive sufficient oxygen and nutrients to function properly.
Introducing excessive fluids in the trauma setting can cause abrupt cardiac preload and output increase. MAP increase reduces peripheral vasoconstriction and produces further blood loss.[3] Excessive fluid repletion may also disrupt the body's natural coagulation response, potentially leading to dilutional coagulopathy.[4] Managing the overhydration-induced coagulopathy requires blood product supplementation to restore hemostatic balance.[5][6]
Thus, excessive crystalloid resuscitation may result in the following:
- A rise in cardiac output, thus increasing the MAP, reducing peripheral vasoconstriction, and causing more bleeding
- Dilution coagulopathy (see Image. Trauma-Induced Coagulopathy)
- "Popping the clot” phenomenon or potential clot dislodgement when attempting to normalize blood pressure in a patient with severe blood loss
- Hypothermia
- Edema causing abdominal compartment syndrome
- Organ failure
Permissive hypotension allows minimal peripheral vasodilation, helping maintain sufficient organ perfusion and reducing the risk of multisystem organ failure. Limiting fluid resuscitation can likewise prevent coagulopathy and further damage to injured blood vessels. Permissive hypotension is a deliberate strategy to maintain blood pressure at lower-than-normal levels in patients with trauma or hemorrhage. The goal of this treatment is to maintain organ perfusion without exacerbating bleeding, which can occur with aggressive fluid resuscitation.
Indications
Hypotensive resuscitation may be used to rapidly stabilize people with hypovolemic shock, particularly in traumatic hemorrhage cases, before definitive hemorrhage control procedures can be performed. However, this intervention is only a temporizing measure. Promptly identifying and addressing the bleeding source are still crucial to patient outcomes. Permissive hypotension should not be employed after controlling bleeding in the operating room.
The practice of hypotensive resuscitation has mainly been studied in hemodynamically unstable patients, particularly individuals with severe traumatic hemorrhage. However, the intervention's outcomes in blunt trauma remain unclear. Patients should be evaluated for any prior history that may put them at risk during this procedure. Individuals without a history of chronic hypertension are appropriate candidates for this practice.[7] This technique can worsen preexisting cardiovascular conditions and endocrine abnormalities involving fluid balance, such as syndrome of inappropriate antidiuretic hormone and diabetes insipidus.
Nontraumatic conditions that may be managed by permissive hypotension include leaking abdominal aortic aneurysm, dissecting aneurysm, pulmonary contusion, and bleeding duodenal ulcer. The procedure may also be employed in patients undergoing neurovascular surgery.
Contraindications
Permissive hypotension may worsen outcomes in blunt trauma cases as the intervention may lead to tissue hypoperfusion. Data do not support hypotensive resuscitation's appropriateness in the prehospital stabilization of patients with blunt trauma. On the contrary, high mortality rates have been reported in individuals with blunt trauma in whom this strategy has been attempted.
The permissive hypotensive strategy is contraindicated in patients presenting with traumatic brain (TBI) and spine injury. Such cases require that the MAP be greater than 80 mm Hg—translating to a cerebral perfusion pressure of approximately 60 mm Hg—to maintain the normal central nervous system perfusion pressure. The Brain Trauma Foundation has recommended against maintaining SBP below 90 mm Hg in patients with TBI.[8] Permissive hypotension has been reported to result in higher mortality when systolic pressures drop below normal physiologic levels. Low systolic pressures decrease cerebral perfusion pressure.[9][10]
Incremental fluid infusions are recommended to raise the MAP above 80 mm Hg in cases of significant cerebral hemorrhagic trauma leading to increased intracranial pressure. This intervention allows the maintenance of cerebral perfusion pressure at 60 mm Hg and avoids a potential ischemic event.[11] Recently, recommendations have emerged advocating for the management of SBP based on patient age among patients with TBI. As part of this approach, new thresholds for permissive hypotension were established following a retrospective data review. The Brain Trauma Foundation has set the age-based guidelines well above the permissive hypotension limits:
- 110 mm Hg in individuals 15 to 49 years of age
- 100 mm Hg in people 50 to 69 years of age
- 110 mm Hg in patients 70 years and older [12][13]
Permissive hypotension may be contraindicated in older patients with chronic arterial hypertension.[14]
Preparation
Vincent and De Baker divided fluid resuscitative methods into 4 phases. The 1st resuscitation phase is the rescue or salvage phase, which aims to reestablish the minimum perfusion necessary to sustain life in the presence of life-threatening hypovolemic shock. The 2nd is the optimization phase, which aims to prevent decompensation after the patient has been rescued from life-threatening shock. This phase maintains the optimal cardiac output for appropriate tissue perfusion.
The 3rd is the stabilization phase, which maintains a patient's normal physiologic SBP and MAP. The 4th is the de-escalation phase, which slows down fluid administration and allows the healthcare team to determine if stability has been achieved following supportive therapy and fluids. Permissive hypotension is generally used only during the initial rescue and optimization phases.[15]
Technique or Treatment
Different guidelines are applied when determining the initial infusion volume and rate during permissive hypotension. Animal models can help guide hypotensive resuscitation methods in humans.[16][17] Research favors resuscitation at a rate of 60 to 80 mL/kg/h to maintain an SBP of 80 to 90 mm Hg or a MAP of 40 to 60 mm Hg.[18] However, standardized, conclusive data regarding the optimum pressures or algorithmic approaches to adjusting pressures are lacking. Many sources agree that permissive hypotension can be achieved in patients with either a MAP of around 50 mm Hg or SBP of 80 to 90 mm Hg. Fluid infusions of 100 to 200 mL may be given to ensure the patient remains within the permissible pressure ranges while under evaluation.
The injury mechanism may also impact resuscitative efforts. Different injury types require different SBP control approaches. Retrospective data suggest that permissive hypotension may be achieved if the MAP is near 50 mm Hg or the SBP is between 80 and 90 mm Hg.[19][20][21]
Penetrating traumas may require SBP levels ranging from 60 to 70 mm Hg. People with penetrating trauma and TBI and individuals with blunt trauma without TBI may need to maintain SBP levels between 80 and 90 mm Hg during resuscitation. Patients with both TBI and blunt trauma should be managed in nonhypotensive conditions, with SBP ranging between 100 and 110 mm Hg and adjusted based on age.[22]
The choice between crystalloid and colloid solutions during initial resuscitation is highly debated. Studies indicate that crystalloids may either positively influence or do not affect coagulation promotion during the initial resuscitation of patients with significant blood loss.[23][24][25] In contrast, reports show that early colloid administration may have either a positive, neutral, or negative effect on early coagulation.[26][27][28]
Moderate crystalloid 0.9% saline amounts have demonstrated some procoagulatory effects.[29] However, caution is advised with certain solutions during resuscitation. Administering colloid dextrans or hydroxyethyl starch solutions may impair coagulation, particularly in quantities exceeding 1.5 liters.[30] Colloids may be considered for patients unable to tolerate large crystalloid volumes, especially when concerns about fluid overload exist.[31] Notably, albumin is contraindicated in patients with TBI.
Serial laboratory tests should be obtained to assess for coagulopathy and anemia. Mild to moderate hypovolemic episodes may not necessitate blood product administration, but severe blood loss with rapid decompensation may require transfusions. A hemoglobin level below 7 g/dL warrants a packed red blood cell (RBC) transfusion. The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Randomized Clinical Trial supports the use of a 1:1:1 ratio of plasma, RBC, and platelets to prevent early coagulopathy and address anemia during early resuscitation.[32] Prothrombin time, partial thromboplastin time, bleeding time, and international normalized ratio should be monitored to assess transfusion adequacy.
Hydration management should include urine output checks to determine fluid adequacy following initial rescue and resuscitation efforts. Physical examination indicators of hydration status, such as capillary refill, peripheral pulses, heart rate, and blood pressure, are essential. Although less precise than serial urine output assessments, physical exams provide a quick assessment of fluid status.
Blood pressure targets are based on the 6th edition of the European guidelines on managing major bleeding and coagulopathy following trauma. For patients with trauma without clinical signs of brain injury, a restricted volume replacement strategy targeting an SBP of 80 to 90 mm Hg or MAP of 50 to 60 mm Hg is recommended until major bleeding is controlled. However, for patients with severe TBI (GCS ≤ 8), a MAP of at least 80 mm Hg is recommended.
Clinical Significance
Severe trauma is time-sensitive. Prehospital fluid administration is an effective strategy for stabilizing patients with major hemorrhagic shock before intraoperative procedures. This intervention is associated with significant mortality reduction and increased survival in patients with trauma.[33] However, the benefits of prehospital fluid administration may not apply to all patients with volume loss. Prehospital fluid crystalloid administration of more than 500 mL in patients without prehospital hypotension has been shown to have poor outcomes.[34] Adopting a goal-directed resuscitation approach, depending on the presence or absence of prehospital hypotension, is essential for patients experiencing severe, life-threatening hemorrhage. This approach facilitates early venous access, enabling timely administration of fluids and essential resuscitation agents in-hospital.[35][36]
Hypotensive resuscitation helps prevent blood loss by minimizing overhydration's vasodilatory and coagulopathic effects. This technique decreases the need to administer blood products, such as fresh frozen plasma, to maintain clotting function. Permissive hypotension can be a cost-effective resuscitation method if approached carefully. This intervention can improve patient recovery rates and expedite discharge, thus reducing post-hospitalization complications.
Enhancing Healthcare Team Outcomes
Seamless coordination among medical teams is crucial in managing severe trauma and hemorrhage. In modern healthcare, permissive hypotension requires a collaborative effort from a diverse team of healthcare professionals.
During the prehospital phase, emergency medical services assess the patient's vital signs, initiate intravenous access, and administer fluids to stabilize the patient's condition. In cases where permissive hypotension is indicated, emergency medical services providers may adjust their approach to fluid resuscitation by maintaining a lower-than-normal blood pressure to minimize ongoing bleeding.
In the hospital setting, physicians, including surgeons, emergency medicine physicians, and intensivists, make clinical decisions and provide medical oversight. Nurses monitor vital signs, administer fluids and medications, and provide direct patient care. Pharmacists ensure appropriate medication management. Respiratory therapists assist with ventilator management.
Physical therapists aid in mobility and rehabilitation. Occupational therapists help improve functional autonomy. Speech therapists address swallowing or communication difficulties. Social workers address psychosocial concerns. This collaborative approach ensures comprehensive, patient-centered care, optimizing outcomes for patients undergoing permissive hypotension.[33][34][37]
Nursing, Allied Health, and Interprofessional Team Monitoring
Evidence-based guidelines have yet to clearly elucidate systolic and mean arterial pressure adjustment based on other aspects of the patient's background, such as age, weight, height, and gender. Clinicians should closely monitor the patient's mentation and vitals after initiating fluids. Following the initial rescue phase, blood pressure may be gradually raised, and urine output may be monitored to determine the patient's homeostasis.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
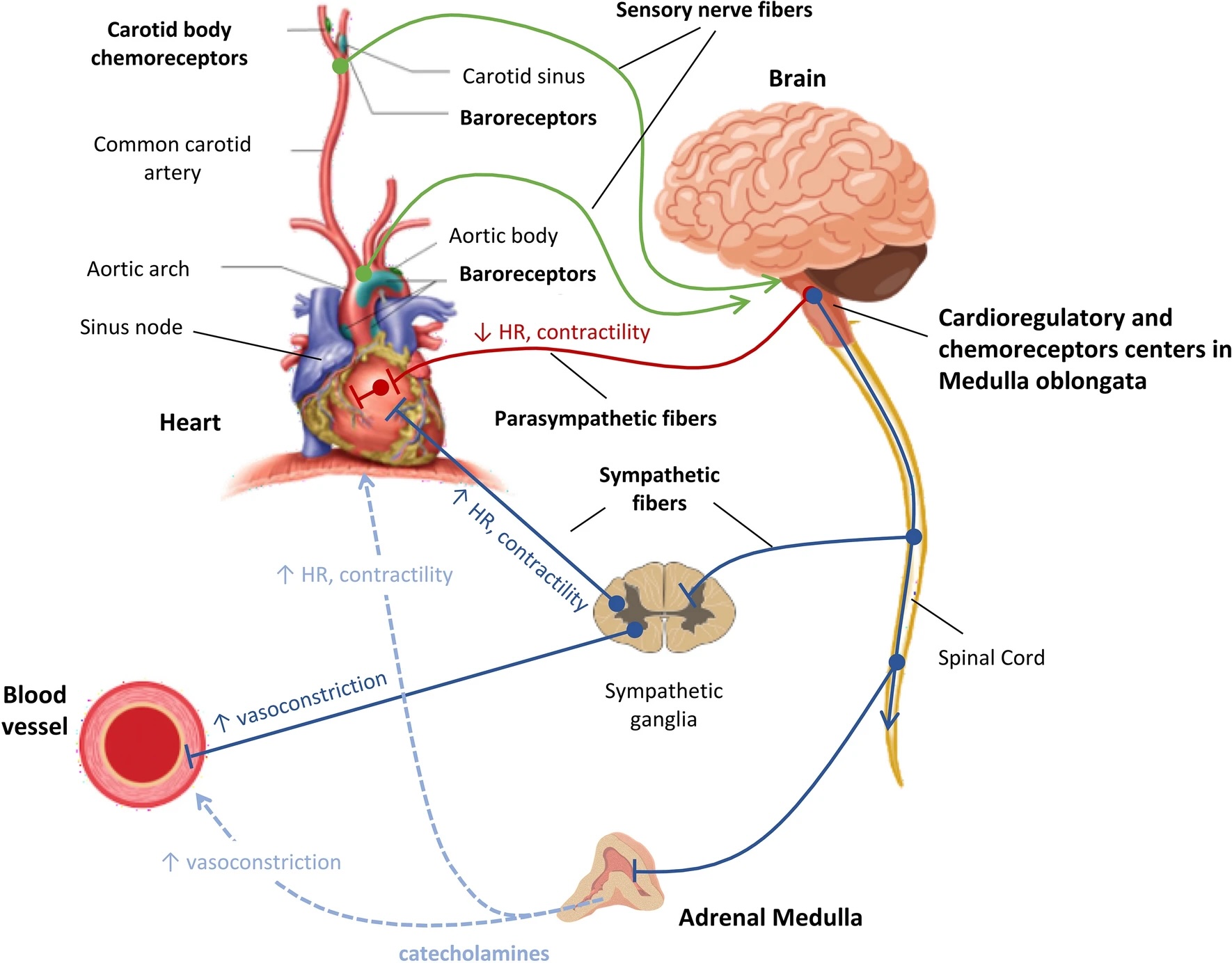
Blood Pressure Regulation. The arterial baroreceptors in the aortic arch and carotid sinuses monitor blood pressure, while cardiopulmonary baroreceptors in the heart and vessels detect changes in blood volume. Chemoreceptors in the carotid bodies and aortic arch respond to oxygen and carbon dioxide level changes. The vasomotor center in the brainstem regulates circulation, receiving sensory input from the glossopharyngeal and vagus nerves. Sympathetic nervous system fibers from the medulla oblongata innervate target organs via ganglia near the spinal cord, releasing epinephrine and norepinephrine. Parasympathetic nervous system fibers originate from the brainstem, regulating cardiac function through the vagus nerve. During septic shock, vagal inhibition and sympathetic stimulation create an imbalance, favoring SNS dominance.
Carrara M, Ferrario M, Bollen Pinto B, Herpain A. The autonomic nervous system in septic shock and its role as a future therapeutic target: a narrative review. Ann Intensive Care. 2021;11(1):80. doi: 10.1186/s13613-021-00869-7.
(https://creativecommons.org/licenses/by/4.0)
(Click Image to Enlarge)
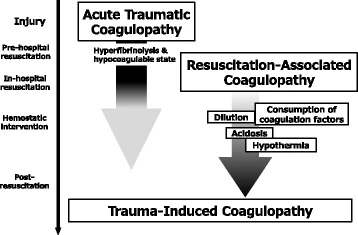
Trauma-Induced Coagulopathy. Two phases of trauma-induced coagulopathy are acute traumatic coagulopathy (ATC) and resuscitation-associated coagulopathy. ATC, triggered by trauma and shock, emerges immediately after injury and persists into the resuscitation phase. Resuscitation-associated coagulopathy exacerbates ATC with factors like hypothermia, metabolic acidosis, and dilutional coagulopathy during therapeutic interventions, persisting into the post-resuscitation phase.
Kushimoto S, Kudo D, Kawazoe Y. Acute traumatic coagulopathy and trauma-induced coagulopathy: an overview. J Intensive Care. 5, 6 (2017;5(1):6. doi: 10.1186/s40560-016-0196-6.
(http://creativecommons.org/licenses/by/4.0/)
References
Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. The New England journal of medicine. 1994 Oct 27:331(17):1105-9 [PubMed PMID: 7935634]
Level 1 (high-level) evidenceMorrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, Liscum KR, Wall MJ Jr, Mattox KL. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. The Journal of trauma. 2011 Mar:70(3):652-63. doi: 10.1097/TA.0b013e31820e77ea. Epub [PubMed PMID: 21610356]
Level 1 (high-level) evidenceSmith JB, Pittet JF, Pierce A. Hypotensive Resuscitation. Current anesthesiology reports. 2014 Sep 1:4(3):209-215 [PubMed PMID: 25294973]
Barak M, Rudin M, Vofsi O, Droyan A, Katz Y. Fluid administration during abdominal surgery influences on coagulation in the postoperative period. Current surgery. 2004 Sep-Oct:61(5):459-62 [PubMed PMID: 15475095]
Level 2 (mid-level) evidenceTorres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. The journal of trauma and acute care surgery. 2013 Nov:75(5):759-66. doi: 10.1097/TA.0b013e3182a92514. Epub [PubMed PMID: 24158192]
Level 3 (low-level) evidenceGeeraedts LM Jr, Kaasjager HA, van Vugt AB, Frölke JP. Exsanguination in trauma: A review of diagnostics and treatment options. Injury. 2009 Jan:40(1):11-20. doi: 10.1016/j.injury.2008.10.007. Epub 2009 Jan 8 [PubMed PMID: 19135193]
Brenner M, Stein DM, Hu PF, Aarabi B, Sheth K, Scalea TM. Traditional systolic blood pressure targets underestimate hypotension-induced secondary brain injury. The journal of trauma and acute care surgery. 2012 May:72(5):1135-9. doi: 10.1097/TA.0b013e31824af90b. Epub [PubMed PMID: 22673237]
. The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Hypotension. Journal of neurotrauma. 2000 Jun-Jul:17(6-7):591-5 [PubMed PMID: 10937905]
Level 1 (high-level) evidenceTremblay LN, Rizoli SB, Brenneman FD. Advances in fluid resuscitation of hemorrhagic shock. Canadian journal of surgery. Journal canadien de chirurgie. 2001 Jun:44(3):172-9 [PubMed PMID: 11407826]
Level 3 (low-level) evidenceRosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. Journal of neurosurgery. 1995 Dec:83(6):949-62 [PubMed PMID: 7490638]
Wise R, Faurie M, Malbrain MLNG, Hodgson E. Strategies for Intravenous Fluid Resuscitation in Trauma Patients. World journal of surgery. 2017 May:41(5):1170-1183. doi: 10.1007/s00268-016-3865-7. Epub [PubMed PMID: 28058475]
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1:80(1):6-15. doi: 10.1227/NEU.0000000000001432. Epub [PubMed PMID: 27654000]
Dash HH, Chavali S. Management of traumatic brain injury patients. Korean journal of anesthesiology. 2018 Feb:71(1):12-21. doi: 10.4097/kjae.2018.71.1.12. Epub 2018 Feb 1 [PubMed PMID: 29441170]
Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, Duranteau J, Filipescu D, Grottke O, Grønlykke L, Harrois A, Hunt BJ, Kaserer A, Komadina R, Madsen MH, Maegele M, Mora L, Riddez L, Romero CS, Samama CM, Vincent JL, Wiberg S, Spahn DR. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Critical care (London, England). 2023 Mar 1:27(1):80. doi: 10.1186/s13054-023-04327-7. Epub 2023 Mar 1 [PubMed PMID: 36859355]
Vincent JL, De Backer D. Circulatory shock. The New England journal of medicine. 2013 Oct 31:369(18):1726-34. doi: 10.1056/NEJMra1208943. Epub [PubMed PMID: 24171518]
Rezende-Neto JB, Rizoli SB, Andrade MV, Lisboa TA, Cunha-Melo JR. Rabbit model of uncontrolled hemorrhagic shock and hypotensive resuscitation. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2010 Dec:43(12):1153-9 [PubMed PMID: 21085888]
Level 3 (low-level) evidenceZhang YM, Gao B, Wang JJ, Sun XD, Liu XW. Effect of Hypotensive Resuscitation with a Novel Combination of Fluids in a Rabbit Model of Uncontrolled Hemorrhagic Shock. PloS one. 2013:8(6):e66916. doi: 10.1371/journal.pone.0066916. Epub 2013 Jun 21 [PubMed PMID: 23805284]
Level 3 (low-level) evidenceSantry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock (Augusta, Ga.). 2010 Mar:33(3):229-41. doi: 10.1097/SHK.0b013e3181c30f0c. Epub [PubMed PMID: 20160609]
Level 3 (low-level) evidencePaul JE, Ling E, Lalonde C, Thabane L. Deliberate hypotension in orthopedic surgery reduces blood loss and transfusion requirements: a meta-analysis of randomized controlled trials. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2007 Oct:54(10):799-810 [PubMed PMID: 17934161]
Level 1 (high-level) evidenceDutton RP. Controlled hypotension for spinal surgery. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2004 Oct:13 Suppl 1(Suppl 1):S66-71 [PubMed PMID: 15197633]
Tran A, Yates J, Lau A, Lampron J, Matar M. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: A systematic review and meta-analysis of randomized controlled trials. The journal of trauma and acute care surgery. 2018 May:84(5):802-808. doi: 10.1097/TA.0000000000001816. Epub [PubMed PMID: 29370058]
Level 1 (high-level) evidenceCoppola S, Froio S, Chiumello D. Fluid resuscitation in trauma patients: what should we know? Current opinion in critical care. 2014 Aug:20(4):444-50. doi: 10.1097/MCC.0000000000000115. Epub [PubMed PMID: 24927043]
Level 3 (low-level) evidenceRuttmann TG, James MF, Viljoen JF. Haemodilution induces a hypercoagulable state. British journal of anaesthesia. 1996 Mar:76(3):412-4 [PubMed PMID: 8785143]
Jamnicki M, Zollinger A, Seifert B, Popovic D, Pasch T, Spahn DR. The effect of potato starch derived and corn starch derived hydroxyethyl starch on in vitro blood coagulation. Anaesthesia. 1998 Jul:53(7):638-44 [PubMed PMID: 9771171]
Konrad CJ, Markl TJ, Schuepfer GK, Schmeck J, Gerber HR. In vitro effects of different medium molecular hydroxyethyl starch solutions and lactated Ringer's solution on coagulation using SONOCLOT. Anesthesia and analgesia. 2000 Feb:90(2):274-9 [PubMed PMID: 10648306]
Fries D, Innerhofer P, Klingler A, Berresheim U, Mittermayr M, Calatzis A, Schobersberger W. The effect of the combined administration of colloids and lactated Ringer's solution on the coagulation system: an in vitro study using thrombelastograph coagulation analysis (ROTEG. Anesthesia and analgesia. 2002 May:94(5):1280-7, table of contents [PubMed PMID: 11973205]
Gorton H, Lyons G, Manraj P. Preparation for regional anaesthesia induces changes in thrombelastography. British journal of anaesthesia. 2000 Mar:84(3):403-4 [PubMed PMID: 10793606]
Level 1 (high-level) evidenceNagy KK, Davis J, Duda J, Fildes J, Roberts R, Barrett J. A comparison of pentastarch and lactated Ringer's solution in the resuscitation of patients with hemorrhagic shock. Circulatory shock. 1993 Aug:40(4):289-94 [PubMed PMID: 7690689]
Level 1 (high-level) evidenceBrazil EV, Coats TJ. Sonoclot coagulation analysis of in-vitro haemodilution with resuscitation solutions. Journal of the Royal Society of Medicine. 2000 Oct:93(10):507-10 [PubMed PMID: 11064686]
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SA, CHEST Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. The New England journal of medicine. 2012 Nov 15:367(20):1901-11. doi: 10.1056/NEJMoa1209759. Epub 2012 Oct 17 [PubMed PMID: 23075127]
Level 1 (high-level) evidenceFinfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. The New England journal of medicine. 2004 May 27:350(22):2247-56 [PubMed PMID: 15163774]
Level 1 (high-level) evidenceHolcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G, PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015 Feb 3:313(5):471-82. doi: 10.1001/jama.2015.12. Epub [PubMed PMID: 25647203]
Level 2 (mid-level) evidenceHampton DA, Fabricant LJ, Differding J, Diggs B, Underwood S, De La Cruz D, Holcomb JB, Brasel KJ, Cohen MJ, Fox EE, Alarcon LH, Rahbar MH, Phelan HA, Bulger EM, Muskat P, Myers JG, del Junco DJ, Wade CE, Cotton BA, Schreiber MA, PROMMTT Study Group. Prehospital intravenous fluid is associated with increased survival in trauma patients. The journal of trauma and acute care surgery. 2013 Jul:75(1 Suppl 1):S9-15. doi: 10.1097/TA.0b013e318290cd52. Epub [PubMed PMID: 23778518]
Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cuschieri J, Sperry JL, Inflammation and the Host Response to Injury Investigators. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. The journal of trauma and acute care surgery. 2013 May:74(5):1207-12; discussion 1212-4. doi: 10.1097/TA.0b013e31828c44fd. Epub [PubMed PMID: 23609269]
Revell M, Porter K, Greaves I. Fluid resuscitation in prehospital trauma care: a consensus view. Emergency medicine journal : EMJ. 2002 Nov:19(6):494-8 [PubMed PMID: 12421770]
Level 3 (low-level) evidenceGreaves I, Porter KM, Revell MP. Fluid resuscitation in pre-hospital trauma care: a consensus view. Journal of the Royal College of Surgeons of Edinburgh. 2002 Apr:47(2):451-7 [PubMed PMID: 12018688]
Level 3 (low-level) evidenceHussmann B, Lefering R, Waydhas C, Touma A, Kauther MD, Ruchholtz S, Lendemans S, Trauma Registry of the German Society for Trauma Surgery. Does increased prehospital replacement volume lead to a poor clinical course and an increased mortality? A matched-pair analysis of 1896 patients of the Trauma Registry of the German Society for Trauma Surgery who were managed by an emergency doctor at the accident site. Injury. 2013 May:44(5):611-7. doi: 10.1016/j.injury.2012.02.004. Epub 2012 Feb 28 [PubMed PMID: 22377276]
Level 2 (mid-level) evidence