Introduction
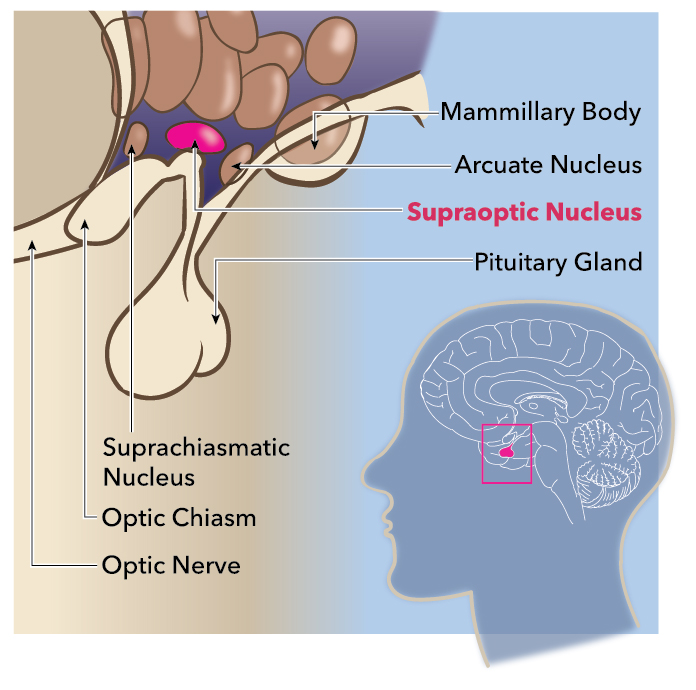
The hypothalamus is a structure of the human brain that governs a wide range of physiological processes, including circadian rhythm, homeostasis, and stress response, as well as growth and development. Regulation of these activities is achieved, in part, via the activities of the hypothalamic-pituitary-adrenal axis (HPA). The supraoptic nucleus is a collection of magnocellular neurosecretory cells (MNCs) located within the anterior hypothalamus that participate in the HPA axis. The primary function of these cells is to produce and secrete the peptide hormone vasopressin, also known as antidiuretic hormone (ADH) and oxytocin. Vasopressin serves to maintain the body's osmotic balance and regulate the body's blood pressure by instigating aquaporin expression in the kidney's distal tubule and collecting duct to increase water absorption. Oxytocin is a neuropeptide that is well known for its importance in lactation and parturition.[1][2][3][4][5]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The supraoptic nucleus is located bilaterally to the optic chiasm and receives projections from the medial preoptic, arcuate, and parabrachial nuclei. The supraoptic nucleus subdivides into the pars principalis, the pars intraoptica, and the pars tuberalis. The pars principalis possesses neurons connected to the lateral borders of the optic chiasm and optic tracts while the pars intraoptica contains neurons along the axons of the optic chiasm, and the pars tuberalis consists of neurons that are medial to the optic tracts. The cell bodies of each neuron contained within the cluster produce vasopressin which is then transported through a series of long axons. Each of these axons ends within the posterior lobe of the pituitary gland. Here, the vasopressin is stored as secretory granules within structures known as Herring bodies. The firing of these magnocellular cells is under the control of both the intrinsic membrane properties of the cells themselves in addition to input by other osmosensitive neurons. The measurement of blood osmolarity is only achievable via the circumventricular organs (CVOs) of the brain. These structures provide extensive networks of highly permeable capillaries, allowing for areas of permeability to the blood-brain-barrier. Specifically, the CVOs that provide access to the blood for the sensation of osmolarity are the subfornical organ and the organum vasculosum. The sensation of increased blood osmolarity triggers activation of the MNCs of the supraoptic nuclei and thus the release of vasopressin. The released vasopressin then enters the bloodstream and is sensed by receptors in the kidney’s collecting duct, leading to increased water absorption in addition to systemic vasoconstriction. Under normal conditions, supraoptic MNCs release enough vasopressin to maintain plasma concentrations at 1 to 3 pg/mL, which causes the reabsorption of approximately 30 liters of water each day.[6][7][8][9][10][11]
Embryology
Neurons of the supraoptic nuclei begin their formation early in development, and adult numbers of vasopressin producing cells are typically present by the second trimester. The postulate is that the formation of the Herring bodies occurs not within the posterior pituitary, where they ultimately reside, but within the axons of the hypothalamic magnocellular cells during the vasopressin secretory cycle. Once vasopressin granules are contained within mature Herring bodies, they are no longer able to travel retrograde through the axon and must either be released or degraded.[12][13][7]
Blood Supply and Lymphatics
All parts of the hypothalamus receive vascular supply from arteries derived from the circle of Willis. Specifically, the anterior hypothalamus, including the supraoptic nuclei, is supplied by branches of the anterior cerebral and anterior communicating arteries. This system provides a dense assortment of collateral blood supplies, which contributes to the stroke-resistant nature of the hypothalamus.[14]
Nerves
Neurons of the supraoptic nucleus project to the neural lobe of the hypophysis. Studies involving hypophysectomy have shown that this procedure results in the loss of most of the neurons of the supraoptic nucleus.[15]
Surgical Considerations
Previous studies have suggested that axonal injury to the hypothalamo-neurohypophyseal tract can cause the MCNs in the hypothalamus to degenerate and the development of central diabetes insipidus (DI). Injury to the vasopressin and oxytocin neurons results in morphological changes to the neurohypophysis.[15] These injuries can include compression of the pituitary stalk,[16][17] hypophysectomy,[18] neurohypophysectomy,[19][20][21] and transection of the pituitary stalk.[22][23][24] Absolute resection of the pituitary gland can result in an inability to store and secrete vasopressin in the posterior pituitary. Research has shown plasma vasopressin concentration to reduce after axotomy. Dohanics et al. demonstrated, through a stereotaxic method, compression of the pituitary stalk led to significant DI along with significant degeneration of the vasopressin neuron population in the supraoptic nucleus and paraventricular nucleus. Their study indicated, 21 days after surgery, only 35% and 27% of vasopressin neurons in the supraoptic nucleus and paraventricular nucleus survived, respectively.[16] In another study, Alonso et al. demonstrated, after transecting the neurohypophyseal axons through the median eminence, the number of oxytocin-producing neurons of the supraoptic nucleus slightly decreased by 20% compared to controls whereas the number of vasopressin-producing neurons reduced significantly by 60%. Many of the axons that regenerate in the median eminence are oxytocin neurohypophyseal neurons.[24] However, when the experimental animals had a median eminence transection and a unilateral cut in the lateral hypothalamus just above the supraoptic nucleus, survival of oxytocin-producing and vasopressin-producing neurons dropped significantly in the ipsilateral supraoptic nucleus versus the contralateral supraoptic nucleus. These results suggest that oxytocin-producing neurons (and on a smaller scale, vasopressin-producing neurons) of the supraoptic nucleus possess collateral projections in the lateral hypothalamus that shield them from retrograde degeneration during injury.[24]
Clinical Significance
The two major disease states characterized by pathologies related to the MNCs of the supraoptic nucleus and/or their product vasopressin are the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and DI.
SIADH results from an inappropriately high activity of vasopressin, which causes excessive renal water absorption leading to hyponatremia and hypervolemia, among other complications. Central SIADH has several etiologies, all of which function to dysregulate hypothalamic vasopressin secretion. Examples of CNS-related causes include meningitis, tumor mass effect, hemorrhage, hydrocephalus, and multiple sclerosis.
DI results from an inappropriately low activity of vasopressin, causing a lack of renal water absorption leading to severe dehydration, polyuria, hypernatremia, and hypovolemia. Central DI results from either by a lack of vasopressin production by the hypothalamus or by a lack of vasopressin release by the posterior pituitary. The main variants of central DI are idiopathic, acquired, and genetic. Acquired central DI can be the result of tumor mass effect, infection, trauma, and autoimmune damage. Genetic central DI is the rarest form resulting from an autosomal dominant mutation to the arginine vasopressin-neurophysin II gene.[25][26]
Media
References
Aguilera G. HPA axis responsiveness to stress: implications for healthy aging. Experimental gerontology. 2011 Feb-Mar:46(2-3):90-5. doi: 10.1016/j.exger.2010.08.023. Epub 2010 Sep 9 [PubMed PMID: 20833240]
Level 3 (low-level) evidenceBrown CH. Magnocellular Neurons and Posterior Pituitary Function. Comprehensive Physiology. 2016 Sep 15:6(4):1701-1741. doi: 10.1002/cphy.c150053. Epub 2016 Sep 15 [PubMed PMID: 27783857]
Wilkin LD, Mitchell LD, Ganten D, Johnson AK. The supraoptic nucleus: afferents from areas involved in control of body fluid homeostasis. Neuroscience. 1989:28(3):573-84 [PubMed PMID: 2710332]
Level 3 (low-level) evidenceCuzzo B, Padala SA, Lappin SL. Physiology, Vasopressin. StatPearls. 2023 Jan:(): [PubMed PMID: 30252325]
Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd. Oxytocin: the great facilitator of life. Progress in neurobiology. 2009 Jun:88(2):127-51. doi: 10.1016/j.pneurobio.2009.04.001. Epub 2009 Apr 10 [PubMed PMID: 19482229]
Level 3 (low-level) evidenceFlament-Durand J. The hypothalamus: anatomy and functions. Acta psychiatrica Belgica. 1980 Jul-Aug:80(4):364-75 [PubMed PMID: 7025580]
Level 3 (low-level) evidenceKrsulovic J, Peruzzo B, Alvial G, Yulis CR, Rodríguez EM. The destination of the aged, nonreleasable neurohypophyseal peptides stored in the neural lobe is associated to the remodeling of the neurosecretory axon. Microscopy research and technique. 2005 Dec 15:68(6):347-59 [PubMed PMID: 16358285]
Level 3 (low-level) evidenceSilva MP, Cedraz-Mercez PL, Varanda WA. Effects of nitric oxide on magnocellular neurons of the supraoptic nucleus involve multiple mechanisms. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2014 Feb:47(2):90-100. doi: 10.1590/1414-431X20133326. Epub 2014 Jan 17 [PubMed PMID: 24519124]
Level 3 (low-level) evidenceBear MH, Reddy V, Bollu PC. Neuroanatomy, Hypothalamus. StatPearls. 2023 Jan:(): [PubMed PMID: 30252249]
Brown CH, Bains JS, Ludwig M, Stern JE. Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. Journal of neuroendocrinology. 2013 Aug:25(8):678-710. doi: 10.1111/jne.12051. Epub [PubMed PMID: 23701531]
Level 3 (low-level) evidenceCrespo D, Ramos J, Gonzalez C, Fernandez-Viadero C. The supraoptic nucleus: a morphological and quantitative study in control and hypophysectomised rats. Journal of anatomy. 1990 Apr:169():115-23 [PubMed PMID: 2384330]
Level 3 (low-level) evidenceSwaab DF, Hofman MA, Lucassen PJ, Purba JS, Raadsheer FC, Van de Nes JA. Functional neuroanatomy and neuropathology of the human hypothalamus. Anatomy and embryology. 1993 Apr:187(4):317-30 [PubMed PMID: 8512084]
Level 3 (low-level) evidenceYukitake Y, Taniguchi Y, Kurosumi K. Ultrastructural studies on the secretory cycle of the neurosecretory cells and the formation of Herring bodies in the paraventricular nucleus of the rat. Cell and tissue research. 1977 Feb 2:177(1):1-8 [PubMed PMID: 65228]
Level 3 (low-level) evidenceDaniel PM. The blood supply of the hypothalamus and pituitary gland. British medical bulletin. 1966 Sep:22(3):202-8 [PubMed PMID: 5329958]
Level 3 (low-level) evidenceWang Y, Zhao C, Wang Z, Wang C, Feng W, Huang L, Zhang J, Qi S. Apoptosis of supraoptic AVP neurons is involved in the development of central diabetes insipidus after hypophysectomy in rats. BMC neuroscience. 2008 Jun 25:9():54. doi: 10.1186/1471-2202-9-54. Epub 2008 Jun 25 [PubMed PMID: 18578860]
Level 3 (low-level) evidenceDohanics J, Hoffman GE, Smith MS, Verbalis JG. Functional neurolobectomy induced by controlled compression of the pituitary stalk. Brain research. 1992 Mar 20:575(2):215-22 [PubMed PMID: 1571781]
Level 3 (low-level) evidenceElias PC, Elias LL, Castro M, Antunes-Rodrigues J, Moreira AC. Hypothalamic-pituitary-adrenal axis up-regulation in rats submitted to pituitary stalk compression. The Journal of endocrinology. 2004 Feb:180(2):297-302 [PubMed PMID: 14765982]
Level 3 (low-level) evidenceRaisman G. An ultrastructural study of the effects of hypophysectomy on the supraoptic nucleus of the rat. The Journal of comparative neurology. 1973 Jan 15:147(2):181-207 [PubMed PMID: 4682774]
Level 3 (low-level) evidenceBODIAN D, MAREN TH. The effect of neuro- and adenohypophysectomy on retrograde degeneration in hypothalamic nuclei of the rat. The Journal of comparative neurology. 1951 Jun:94(3):485-511 [PubMed PMID: 14850590]
Level 3 (low-level) evidenceHerman JP, Marciano FF, Gash DM. Vasopressin administration prevents functional recovery of the vasopressinergic neurosecretory system following neurohypophysectomy. Neuroscience letters. 1986 Dec 23:72(3):239-46 [PubMed PMID: 3822229]
Level 3 (low-level) evidenceMiller JH, Grattan DR, Averill RL. Effect of alcohol, neurohypophysectomy, and vasopressin antagonists on hemorrhage-induced bradycardia in the rat. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.). 1993 Mar:202(3):320-30 [PubMed PMID: 8437988]
Level 3 (low-level) evidenceCAMPAGNA MJ, DODGE HW Jr, CLARK EC. Water exchange in dogs following partial section of the pituitary stalk. The American journal of physiology. 1957 Oct:191(1):59-63 [PubMed PMID: 13478685]
Level 3 (low-level) evidenceHICKMAN JA. The effect of section of the pituitary stalk on the supraoptic and paraventricular nuclei of the ferret. The Journal of endocrinology. 1961 Jul:22():371-6 [PubMed PMID: 13714132]
Level 3 (low-level) evidenceAlonso G, Bribes E, Chauvet N. Survival and regeneration of neurons of the supraoptic nucleus following surgical transection of neurohypophysial axons depend on the existence of collateral projections of these neurons to the dorsolateral hypothalamus. Brain research. 1996 Mar 4:711(1-2):34-43 [PubMed PMID: 8680872]
Level 3 (low-level) evidenceShepshelovich D, Leibovitch C, Klein A, Zoldan S, Milo G, Shochat T, Rozen-zvi B, Gafter-Gvili A, Lahav M. The syndrome of inappropriate antidiuretic hormone secretion: Distribution and characterization according to etiologies. European journal of internal medicine. 2015 Dec:26(10):819-24. doi: 10.1016/j.ejim.2015.10.020. Epub 2015 Nov 10 [PubMed PMID: 26563934]
Lu HA. Diabetes Insipidus. Advances in experimental medicine and biology. 2017:969():213-225. doi: 10.1007/978-94-024-1057-0_14. Epub [PubMed PMID: 28258576]
Level 3 (low-level) evidence