Introduction
Primary Progressive Aphasia Overview
Aphasia is a language disorder arising from damage to a specific brain area controlling language comprehension, formulation, and expression. Primary progressive aphasia (PPA) refers to a group of neurodegenerative diseases in which gradual speech and language impairment are the primary presenting symptoms, unaccompanied by marked cognitive, physical, or behavioral changes.[1] Classic aphasia occurring after conditions such as stroke develops abruptly, but PPA has a gradual onset.
PPA characterization and differentiation based on linguistic and nonlinguistic profiles are now easier to accomplish due to extensive research. Advanced neuroimaging techniques like diffusion-weighted imaging (DWI) and resting-state functional magnetic resonance imaging (fMRI) allow healthcare providers to go beyond distinct focal gray matter atrophy patterns to a deeper understanding of PPA's effects on structural and functional connectivity changes. However, despite significant diagnostic advances, a clear consensus on its pathology remains elusive. Nevertheless, improved PPA characterization may positively impact patient management.
Fluent aphasia refers to deficits related to comprehension and is usually associated with Wernicke brain area pathologies. In contrast, nonfluent aphasias produce a failure in written or verbal language expression and are often associated with Broca brain area lesions. The general concept of a primary progressive language impairment disease process without cognitive effects was first described in the 1890s by Pick and Serieux. However, Mesulam is presently credited for exploring "slowly progressive aphasia" and coining its name in the 1980s.[2] "Slowly progressive aphasia" was later renamed in the literature as "primary progressive aphasia."[3]
PPA is closely related to Alzheimer and Pick diseases. Thus, PPA classification and diagnosis can sometimes be controversial.[4] PPA's 3 main variants are nonfluent/agrammatic (nfvPPA), semantic (svPPA), and logopenic (lvPPA).[5] PPA presents similarly to primary progressive apraxia, a gradual, degenerative disorder affecting the planning, programming, and execution of sensorimotor commands required for speech production.[6] Pathologically, nfvPPA is grouped under the frontotemporal dementia (FTD) syndromes. In contrast, the logopenic variant is frequently classified as an atypical Alzheimer disease form.
Anatomical Considerations for PPA
In PPA, neuroanatomical changes primarily affect brain regions associated with language processing. The specific areas vary depending on the PPA subtype, but commonly involved brain regions affect left-hemisphere language functions. The Broca area within the left inferior frontal gyrus' posterior portion is responsible for language production and grammar. The Wernicke area within the left superior temporal gyrus' posterior aspect is involved in language comprehension. The angular gyrus at the parietal, temporal, and occipital lobe junction is implicated in semantic processing and reading. The left temporal lobe is crucial in language processing, including semantic memory and word retrieval. Damage to these areas can lead to word-finding and semantic knowledge deficits.
The frontal lobes, especially the left inferior frontal gyrus, are involved in language production, grammar, and articulation. Injury to these areas results in nonfluent or agrammatic speech. The parietal lobes, including the angular gyrus, contribute to language processing, particularly in reading and semantic comprehension. Damage to these regions can impair reading comprehension and semantic processing. Some PPA cases may also affect posterior language areas beyond the traditional language cortex. These areas include regions involved in visual processing and multisensory integration, impacting reading and comprehension abilities. Understanding PPA's neuroanatomical basis is crucial for accurate diagnosis, prognosis estimation, and developing targeted interventions tailored to individual patients' specific language deficits and affected brain regions.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
PPA is a group of neurodegenerative disorders primarily affecting the language (dominant) areas of the brain. The disorder is usually considered an FTD subtype. Since 2011, FTD has been classified clinically and pathologically into the more common behavioral variant (bvFTD) and primary progressive aphasia (PPA).[7] The 3 PPA variants arise from neurodegenerative changes in different brain areas. The semantic and nonfluent variants are related to FTD, while the logopenic type resembles Alzheimer disease more closely. The semantic variant mainly affects the anterior temporal region. The nonfluent variant impacts the inferior frontal gyrus and precentral cortex regions. The logopenic variant involves the left posterior superior and middle temporal gyri and inferior parietal lobule.[8] The etiology in each type is detailed below.
Nonfluent or Agrammatic PPA Variant
NfvPPA is most commonly associated with an FTD-4R tau protein form.[9][10][11] Other reports indicate TDP-43-A pathology in nfvPPA. Some nfvPPA cases are associated with progranulin (PGRN) or chromosome 9 open reading frame 72 (C9orf72) gene mutations.[12] Less frequently, Alzheimer disease pathology has also been reported in nfvPPA. The 3 genes primarily associated with FTD are microtubule-associated protein tau (MAPT), granulin (GRN), and C9orf72. Frontotemporal lobar degeneration is frequently associated with nfvPPA. Interestingly, prospectively recruited cohorts of PPA suggest mutations are rarely the cause of nfvPPA.[13]
NfvPPA is the most variable of the PPAs and lacks clinicopathologic associations. GRN gene mutations on chromosome 17q21.31, which encodes for a protein called "progranulin," have been identified in 2 families with PPA.[14] These mutations are associated with bvFTD.
Semantic PPA Variant
SvPPA is nearly always associated with underlying TDP-43-C pathological aggregates found in 75–100% of cases in clinicopathological correlation series. This variant is most often associated with FTD tau in the remainder of patients. SvPPA is most consistently associated with left (dominant) temporal lobe pathology.[15]
Logopenic PPA Variant
LvPPA is most often caused by Alzheimer disease pathology in as many as 95% of cases.[16] This condition is recognized as one of the potential focal and early-onset presentations of Alzheimer disease, although other pathological profiles such as Lewy body dementia, TDP-43, and tau have been less commonly reported.[17]
Epidemiology
PPA's prevalence is estimated to be 3 to 4 per 100,000 population.[18] A recent registry study revealed that this condition's incidence was around 1.14 per 100,000 person-years, compared with 35.7 per 100,000 person-years for Alzheimer disease.[19] The nfvPPA's prevalence is approximately 0.5 to 3.9 per 100,000 people. Men and women are equally affected, with an average age of about 60. No demographic, environmental, or socioeconomic risk factors have been identified. Approximately 56% to 86% of individuals with PPA have coexisting speech apraxia. A recent study showed that bvFTD was more common in men, whereas PPA was more common in women.[20]
Pathophysiology
The pathology underlying svPPA mainly involves the atrophy and hypometabolism of the anterior temporal lobes, particularly the temporal poles, with a notable left-sided predominance. In 80% of cases, svPPA exhibits aggregates of TAR DNA-binding protein 43kDa (TDP-43), similar to the pathological features observed in FTD and amyotrophic lateral sclerosis.
The pathophysiology of nfvPPA remains under investigation. This condition exhibits more pathology and disease progression variability than other PPA types. However, involvement of the dominant inferior frontal lobe (Broca area) is consistently observed, with atrophy of the insular cortex in the dominant hemisphere also common.[21] This condition's variability coincides with brain regions associated with language output, sentence processing, and motor speech programming. The pronounced variability of nfvPPA compared to other variants persists histologically and remains a subject of investigation. Many patients exhibit abnormal tau deposition upon postmortem examination, while a minority may show signs of TDP-43 or Alzheimer pathology.
Meanwhile, lvPPA is most commonly an aphasic Alzheimer disease variant. Patients most frequently develop the typical clinical Alzheimer disease features. The pathology is similar to Alzheimer disease but with more left-sided perisylvian or parietal atrophy. Similar to Alzheimer disease, lvPPA also has amyloid and hyperphosphorylated tau accumulation.
History and Physical
Most individuals with PPA have progressive cognitive impairment or dementia with aphasia as the dominant symptom during the first couple of years of the disease course. This presentation differs from other dementia forms, which often have language involvement as a late symptom. Obtaining the history of a progressive language problem that worsens over time is crucial. The symptom differs from acute stroke, which has a sudden onset and is nonprogressive.
The neurologic examination in patients with PPA focuses on assessing language comprehension, expression, and fluency, including verbal and written communication skills. Additionally, cognitive functions such as memory, executive function, and visuospatial abilities are evaluated to rule out other neurodegenerative disorders. Motor function, sensory perception, and reflexes are also assessed to exclude neurological conditions mimicking PPA.
The clinical features and exam findings of each PPA variant are explained below.
History and Physical Examination Features of NfvPPA
The hallmark clinical features of nfvPPA are effortful speech and agrammatism. Effortful speech is characterized by slow, labored speech production, mainly due to a speech motor-planning deficit, ie, apraxia of speech (AOS).[22] Speech sound or phonemic errors, such as distortions, deletions, substitutions, insertions, and transpositions, may be present. Distortions are phonetic errors caused by AOS. Deletions, substitutions, insertions, and transpositions are phonemic errors that may arise from a motor speech impairment or phoneme selection deficit.[23][24] However, these 2 error types are often difficult to distinguish clinically. Research shows higher phonetic or phonemic error rates in patients with nfvPPA.[25] Other cognitive deficits may emerge as the disease progresses, including a decline in attentional resources, verbal working memory, executive functions, episodic memory, and praxis. Behavioral changes may also appear.[26]
The clinical criteria for diagnosing nfvPPA require at least 1 core feature, either agrammatism or effortful speech with inconsistent errors. Additionally, at least 2 of the following must be present: impaired comprehension of complex sentences, preserved single-word comprehension, and preserved object knowledge.
History and Physical Examination Features of SvPPA
Individuals with svPPA typically experience a gradual decline in their ability to understand and use words correctly, resulting in severe and progressive difficulties in word retrieval (anomia) and comprehension of single words. The patients' speech output is fluent. Naming and single-word comprehension deficits are more prominent for low-frequency or low-familiarity items (eg, "rhinoceros" vs. "dog") in the illness' earlier stages.[27] Patients often replace less frequent words with more familiar ones, typically using the superordinate category (eg, "animal" for "cat"). Anomia may also be observed in spontaneous speech, often empty and not very informative.[28]
Early in the disease course, patients with svPPA may struggle to understand unfamiliar words, often leading to anomia. These individuals frequently ask for the meaning of words as a symptom. The progression of semantic deficits leads to impaired object recognition affecting all sensory modalities, including vision, touch, olfaction, and gustation. Object familiarity strongly influences the ability to correctly identify objects, eg, a fork may be more familiar to the patient than a compass. Additionally, individuals with svPPA appear to have disproportionate difficulty understanding concrete concepts relative to abstract concepts.[29]
Episodic memory is relatively preserved in svPPA, especially when tasks with minimal conceptual loading are used.[30] Behavioral abnormalities are typically present in mid-to-late phases, including disinhibition, irritability, and food taste changes (eg, developing a preference for sweet foods). Lack of empathy, mental inflexibility, and compulsions, including clockwatching and intense interest in jigsaw puzzles, are also frequently noted.[31]
The current diagnostic guidelines identify anomia and single-word comprehension deficits as core features, and both are essential for diagnosis. At least 3 of the following diagnostic features must also be present: impaired object knowledge (especially for less common items), surface dyslexia or dysgraphia, intact repetition, and preserved speech production (grammar and motor skills).
History and Physical Examination Features of LvPPA
Patients with lvPPA typically present with word-finding difficulty, sentence repetition deficits, and, as the disease progresses, impaired sentence comprehension. Phonological impairments and, specifically, a phonological short-term memory deficit have been suggested to be the core of the syndrome.[32] Single-word repetition and comprehension remain largely spared. Longitudinal studies indicate faster cognitive decline in lvPPA than other variants, impacting not only language but also visuospatial abilities.[33]
To diagnose lvPPA, impairments in both single-word retrieval (in spontaneous speech and naming) and sentence repetition are required. At least 3 of the following features must also be present: phonological errors in spontaneous speech and naming, preserved single-word comprehension and object knowledge, intact motor speech, and absence of clear agrammatism.
The clinical features of the 3 PPA variants are summarized as follows:
| Clinical Features |
Nonfluent/Agrammatic Variant (NfvPPA) |
Semantic Variant (SvPPA) |
Logopenic Variant (LvPPA) |
|
Spontaneous speech |
Nonfluent, short grammatically flawed |
Fluent, profound impairment of object knowledge |
Fluent, with frequent word-finding pauses |
|
Naming |
Relatively normal with phonemic errors |
Highly impaired with paraphasic errors |
Impaired |
|
Repetition |
Nonfluent |
Relatively normal |
Impaired sentence repetition is a core feature |
|
Comprehension |
Relatively intact |
Severe impairment, even at single-word level |
Relatively intact |
|
Reading |
Intact for short items |
Significant alexia |
Intact for simple items |
Evaluation
Evaluating a patient with PPA symptoms involves obtaining a detailed history and specific speech and language testing. A simplified 2-step process has been developed for standardization and ease of clinical diagnosis. The first step requires the patient to meet PPA requirements designed by Mesulam, which include a set of inclusion and exclusion criteria. Afterward, the diagnostic features crafted by Gorno-Tempini aid in distinguishing between the various types of PPA.[34]
The Northwestern anagram test assesses grammar, often revealing impaired performance in patients with nfvPPA. The Montreal Cognitive Assessment test aids in evaluating cognitive decline, distinguishing it from dementia symptoms, but it must be used cautiously. Reduced verbal output and AOS in nfvPPA may lower Montreal Cognitive Assessment scores, as can depression symptoms. Cognitive assessment alongside detailed speech and language examination is crucial. Following diagnosis, the progressive aphasia severity scale helps clinicians track progression and symptom characterization in people with PPA.[35]
Imaging may also be helpful. Fludeoxyglucose positron emission tomography (FDG-PET) is often used with brain MRI to evaluate primary progressive aphasias. The Broca area and insular cortex are most affected in the nfvPPA, though temporal and parietal hypometabolism are usually absent.
Neuroimaging Findings
Brain MRI and FDG-PET scans are the most important neuroimaging studies for PPA. The findings in each PPA variant are explained below (see Image. Primary Progressive Aphasia Variants).
Neuroimaging for NfvPPA
Patients with nfvPPA typically exhibit more atrophy and hypometabolism, primarily affecting the dominant posterior frontal lobe. The left inferior frontal gyrus (pars opercularis) is considered the syndrome-specific epicenter in nfvPPA.[36][37] Additionally, the condition is associated with gray matter atrophy in the insula, premotor regions, supplementary motor area, and striatum. DWI reveals involvement of the dorsal language pathway, primarily comprising long-range white-matter fibers connecting frontal, subcortical, and parietal areas, in nfvPPA neurodegeneration.[38] Brain MRI has a low sensitivity at 29% but a high specificity at 91%. On the other hand, FDG-PET has roughly 67% sensitivity and 92% specificity.
Neuroimaging for SvPPA
Brain MRI and FDG-PET show atrophy and hypometabolism in the anterior temporal lobes, especially the temporal poles in patients with svPPA.[39] This focal anatomical damage makes neuroimaging a complementary tool in the diagnostic process for this PPA variant.[40] Brain MRI has good sensitivity (98%) and specificity (93%). By comparison, FDG-PET has close to 100% sensitivity and specificity. The damage is usually more significant in the left hemisphere during the disease's first stages.[41]
Neuroimaging for LvPPA
Anatomical damage in lvPPA is typically located in the posterior superior temporal and middle temporal gyri and the inferior parietal lobule.[42] This atrophy pattern is consistent with the classical anatomical model of the phonological loop. The condition's neurodegenerative pattern is highly similar to the one observed in early-onset Alzheimer disease.[43] Brain MRI has a low sensitivity at 57% but good specificity at 95%. FDG-PET is more sensitive at 92% and specific at 94%.
Treatment / Management
No medication has proven beneficial for PPA. Proper management of this condition begins with a detailed history and analysis of the patient’s speech and language components. Contrary to previous beliefs, speech and language therapy may be considerably beneficial. Referral to a speech and language pathologist is essential for properly diagnosing language disorders and planning the appropriate treatment strategy. Speech and language therapy is the most effective PPA intervention, especially for AOS symptoms.[44](A1)
Transcranial direct current stimulation is seen as a promising intervention to enhance language therapy outcomes in PPA. This treatment's low cost, high safety, and noninvasiveness justify research into its potential to augment behavioral interventions and slow language decline in PPA.[45]
Differential Diagnosis
The most important conditions that must be ruled out when considering PPA include the following:
- Dementia: A patient may or may not have aphasia as part of dementia. In contrast, PPA always has aphasia as the initial and primary symptom.
- Ischemic aphasia: An ischemic process will have symptoms of aphasia occurring more acutely. PPA has a slower course that can take years.
- Brain tumor: A brain tumor located at a site affecting speech and language may cause aphasia progressively as the lesion grows. Other complaints may include headache and sensorimotor weakness, both of which are often absent in PPA. Imaging can also differentiate a tumor from PPA.
- Alzheimer disease: Dementia is an initial and prominent sign of this condition. PPA rarely has dementia at the onset of the disease process but has aphasia as the primary and initial symptom.
- FTD: Dementia is the initial presenting symptom of FTD. In contrast, PPA always has aphasia as the primary and initial manifestation. Dementia may develop, though late in the illness course.
A thorough history, physical examination, and diagnostic testing can help differentiate PPA from these clinical entities.
Prognosis
PPA is progressive, and no cure currently exists. Thus, patients eventually lose the ability to speak and understand both written and spoken language. Individuals with PPA are typically expected to have predominantly aphasic and apraxic symptoms initially. However, other cognitive and behavioral impairments develop as the disease progresses, usually no earlier than 8 to 12 years after initial symptom onset.[46] Individual prognostication is somewhat unreliable because of PPA's heterogeneity.
Most patients with PPA live for 3 to 12 years after diagnosis. The mean survival from symptom onset for svPPA is 12 years, nfvPPA is 7.1 years, and lvPPA is 7.6 years. The most frequent cause of death is aspiration pneumonia.[47]
Complications
PPA's complications include the following:
- Loss of the ability to speak and write
- Loss of the ability to understand written and spoken language
- Depression
- Poor judgment
- Inappropriate social behavior
- Parkinsonism, hyperreflexia, and corticobasal syndrome may occur but rarely
Managing this condition's complications requires a multidisciplinary approach involving speech-language therapy, cognitive rehabilitation, psychosocial support, and caregiver education to optimize patient care and quality of life.
Consultations
Consultation with a neurologist, speech and language pathologist, speech therapist, social worker, and psychologist is necessary to optimize patient outcomes.
Deterrence and Patient Education
Primary preventive measures for PPA focus on reducing the risks associated with neurodegenerative diseases and promoting brain health. These measures include maintaining a healthy lifestyle with a proper diet, regular exercise, and mental stimulation through activities like reading and puzzle-solving. Managing cardiovascular risk factors like hypertension, diabetes, and obesity is crucial, as cardiovascular health is closely linked to brain health. Additionally, protecting against head injuries and maintaining social connections can help preserve cognitive function and reduce the risk of cognitive decline.
Secondary preventive measures for PPA involve early detection and management of underlying medical conditions potentially contributing to cognitive decline. Seeking medical advice if experiencing memory loss or language difficulties can lead to early diagnosis and intervention, which may help slow the progression of PPA and other neurodegenerative diseases. Regular medical check-ups and screenings can aid in detecting and managing risk factors such as hypertension, diabetes, and hypercholesterolemia.
Patients with PPA and their families have an increased depression risk due to the condition's potentially earlier onset and progressive nature compared to Alzheimer disease. Patients' awareness of their deficits can exacerbate psychological distress. Psychological evaluation, education, and support conferences can assist patients and families in managing expectations, developing coping strategies, and receiving emotional support. Without a cure, patients may rely on social support for assistance with daily activities. Early intervention with appropriate treatment and support services can improve outcomes for people with PPA and their caregivers.
Pearls and Other Issues
PPA presents a unique set of diagnostic and management challenges. Diagnosing this condition requires a comprehensive evaluation, including detailed language assessments and neuroimaging studies to identify specific neurodegenerative patterns. Subtyping PPA into its 3 main variants—nfvPPA, svPPA, and lvPPA—is crucial for tailoring interventions and predicting disease progression.
Semantic memory deficits and fluent but empty speech characterize svPPA. Interventions focus on augmenting residual semantic knowledge through strategies like semantic cueing and categorization tasks. In contrast, nfvPPA manifests with effortful, agrammatic speech, often accompanied by AOS. Treatment for nfvPPA may involve speech therapy targeting grammatical structures and articulatory planning alongside compensatory strategies like using simplified language. LvPPA, typified by word-finding difficulties and impaired phonological processing, benefits from interventions that enhance phonological awareness and word retrieval, including phonological cueing and repetition exercises.
Managing PPA requires a multidisciplinary approach. Collaborative efforts are essential for developing individualized treatment plans, providing support to patients and caregivers, and facilitating communication strategies to maintain functional abilities and quality of life throughout the disease course.
Enhancing Healthcare Team Outcomes
Patients with PPA are best managed with an interprofessional team approach. The relationships among the primary clinician, neurologist, and speech and language pathologist are critical. The precise evaluations provided by speech and language pathologists can more accurately diagnose a patient's presentation in a subject area with more fluid boundaries between particular diagnoses. The clinician can correlate the clinical findings with imaging findings to solidify the diagnosis. Treatment by speech and language pathologists provides the most significant potential for improvement. Proper management also requires close communication with the clinician to better monitor development and new findings that may need to be addressed several years after the initial symptom presentation. PPA may require psychological and psychiatric intervention for patients who develop significant depression later in the illness course.
Media
(Click Image to Enlarge)
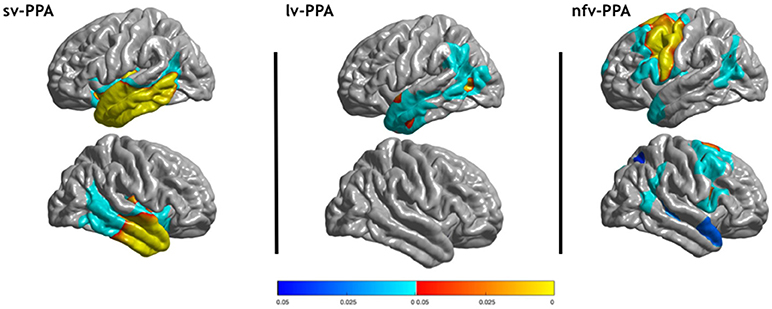
Primary Progressive Aphasia Variants. This illustration shows areas of reduced cortical thickness observed in semantic variant primary progressive aphasia (svPPA), logopenic variant primary progressive aphasia (lvPPA), and nonfluent variant primary progressive aphasia (nfvPPA) compared to healthy controls. Corrected p-values at the vertex- and cluster-levels are indicated with red/yellow and blue colors, respectively.
Contributed fromRoutier A, Habert MO, Bertrand A, et al. Structural, microstructural, and metabolic alterations in primary progressive aphasia variants. Front Neurol. 2018;9:766. doi: 10.3389/fneur.2018.00766.
References
Mesulam MM. Slowly progressive aphasia without generalized dementia. Annals of neurology. 1982 Jun:11(6):592-8 [PubMed PMID: 7114808]
Level 3 (low-level) evidenceGorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011 Mar 15:76(11):1006-14. doi: 10.1212/WNL.0b013e31821103e6. Epub 2011 Feb 16 [PubMed PMID: 21325651]
Rogalski E, Mesulam M. An update on primary progressive aphasia. Current neurology and neuroscience reports. 2007 Sep:7(5):388-92 [PubMed PMID: 17764628]
Habib M, Pelletier J, Khalil R. [Primary progressive aphasia (Mesulam syndrome)]. Presse medicale (Paris, France : 1983). 1993 May 1-8:22(16):757-64 [PubMed PMID: 8316530]
Bonner MF, Ash S, Grossman M. The new classification of primary progressive aphasia into semantic, logopenic, or nonfluent/agrammatic variants. Current neurology and neuroscience reports. 2010 Nov:10(6):484-90. doi: 10.1007/s11910-010-0140-4. Epub [PubMed PMID: 20809401]
Botha H, Josephs KA. Primary Progressive Aphasias and Apraxia of Speech. Continuum (Minneapolis, Minn.). 2019 Feb:25(1):101-127. doi: 10.1212/CON.0000000000000699. Epub [PubMed PMID: 30707189]
Ulugut H, Pijnenburg YAL. Frontotemporal dementia: Past, present, and future. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2023 Nov:19(11):5253-5263. doi: 10.1002/alz.13363. Epub 2023 Jun 28 [PubMed PMID: 37379561]
Chu M, Jiang D, Li D, Yan S, Liu L, Nan H, Wang Y, Wang Y, Yue A, Ren L, Chen K, Rosa-Neto P, Lu J, Wu L. Atrophy network mapping of clinical subtypes and main symptoms in frontotemporal dementia. Brain : a journal of neurology. 2024 Mar 1:():. pii: awae067. doi: 10.1093/brain/awae067. Epub 2024 Mar 1 [PubMed PMID: 38426222]
Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, Grinberg LT, Huang EJ, Trojanowski JQ, Meyer M, Henry ML, Comi G, Rabinovici G, Rosen HJ, Filippi M, Miller BL, Seeley WW, Gorno-Tempini ML. Typical and atypical pathology in primary progressive aphasia variants. Annals of neurology. 2017 Mar:81(3):430-443. doi: 10.1002/ana.24885. Epub 2017 Mar 20 [PubMed PMID: 28133816]
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta neuropathologica. 2011 Aug:122(2):137-53. doi: 10.1007/s00401-011-0839-6. Epub 2011 May 26 [PubMed PMID: 21614463]
Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, Halliday GM. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. Journal of neurology, neurosurgery, and psychiatry. 2014 Aug:85(8):865-70. doi: 10.1136/jnnp-2013-306948. Epub 2014 Jan 13 [PubMed PMID: 24421286]
Cioffi SM, Galimberti D, Barocco F, Spallazzi M, Fenoglio C, Serpente M, Arcaro M, Gardini S, Scarpini E, Caffarra P. Non Fluent Variant of Primary Progressive Aphasia Due to the Novel GRN g.9543delA(IVS3-2delA) Mutation. Journal of Alzheimer's disease : JAD. 2016 Sep 6:54(2):717-21. doi: 10.3233/JAD-160185. Epub [PubMed PMID: 27567822]
Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. The Lancet. Neurology. 2012 Jun:11(6):545-55. doi: 10.1016/S1474-4422(12)70099-6. Epub 2012 May 16 [PubMed PMID: 22608668]
Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL, Bigio EH, Weintraub S, Dickson DW, Hutton ML, Graff-Radford NR. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Archives of neurology. 2007 Jan:64(1):43-7 [PubMed PMID: 17210807]
Clark DG, Charuvastra A, Miller BL, Shapira JS, Mendez MF. Fluent versus nonfluent primary progressive aphasia: a comparison of clinical and functional neuroimaging features. Brain and language. 2005 Jul:94(1):54-60 [PubMed PMID: 15896383]
Santos-Santos MA, Rabinovici GD, Iaccarino L, Ayakta N, Tammewar G, Lobach I, Henry ML, Hubbard I, Mandelli ML, Spinelli E, Miller ZA, Pressman PS, O'Neil JP, Ghosh P, Lazaris A, Meyer M, Watson C, Yoon SJ, Rosen HJ, Grinberg L, Seeley WW, Miller BL, Jagust WJ, Gorno-Tempini ML. Rates of Amyloid Imaging Positivity in Patients With Primary Progressive Aphasia. JAMA neurology. 2018 Mar 1:75(3):342-352. doi: 10.1001/jamaneurol.2017.4309. Epub [PubMed PMID: 29309493]
Teichmann M, Migliaccio R, Kas A, Dubois B. Logopenic progressive aphasia beyond Alzheimer's--an evolution towards dementia with Lewy bodies. Journal of neurology, neurosurgery, and psychiatry. 2013 Jan:84(1):113-4. doi: 10.1136/jnnp-2012-302638. Epub 2012 Sep 11 [PubMed PMID: 22967721]
Level 3 (low-level) evidenceBekkhus-Wetterberg P, Brækhus A, Müller EG, Norvik MI, Winsnes IE, Wyller TB. Primary progressive aphasia. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2022 Nov 22:142(17):. doi: 10.4045/tidsskr.22.0100. Epub 2022 Nov 21 [PubMed PMID: 36416646]
Mouton A, Plonka A, Fabre R, Tran TM, Robert P, Macoir J, Manera V, Gros A. The course of primary progressive aphasia diagnosis: a cross-sectional study. Alzheimer's research & therapy. 2022 May 10:14(1):64. doi: 10.1186/s13195-022-01007-6. Epub 2022 May 10 [PubMed PMID: 35538502]
Level 2 (mid-level) evidencePengo M, Alberici A, Libri I, Benussi A, Gadola Y, Ashton NJ, Zetterberg H, Blennow K, Borroni B. Sex influences clinical phenotype in frontotemporal dementia. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2022 Sep:43(9):5281-5287. doi: 10.1007/s10072-022-06185-7. Epub 2022 Jun 8 [PubMed PMID: 35672480]
Marshall CR, Hardy CJD, Volkmer A, Russell LL, Bond RL, Fletcher PD, Clark CN, Mummery CJ, Schott JM, Rossor MN, Fox NC, Crutch SJ, Rohrer JD, Warren JD. Primary progressive aphasia: a clinical approach. Journal of neurology. 2018 Jun:265(6):1474-1490. doi: 10.1007/s00415-018-8762-6. Epub 2018 Feb 1 [PubMed PMID: 29392464]
Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer disease and associated disorders. 2007 Oct-Dec:21(4):S23-30 [PubMed PMID: 18090419]
Ash S, Evans E, O'Shea J, Powers J, Boller A, Weinberg D, Haley J, McMillan C, Irwin DJ, Rascovsky K, Grossman M. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013 Jul 23:81(4):329-36. doi: 10.1212/WNL.0b013e31829c5d0e. Epub 2013 Jun 21 [PubMed PMID: 23794681]
Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, Moore P, Gee J, Grossman M. Speech errors in progressive non-fluent aphasia. Brain and language. 2010 Apr:113(1):13-20. doi: 10.1016/j.bandl.2009.12.001. Epub 2010 Jan 13 [PubMed PMID: 20074786]
Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain : a journal of neurology. 2010 Jul:133(Pt 7):2069-88. doi: 10.1093/brain/awq129. Epub 2010 Jun 11 [PubMed PMID: 20542982]
Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, Miller BL, Gorno-Tempini ML. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006 Nov 28:67(10):1752-6 [PubMed PMID: 17130406]
Caine D, Breen N, Patterson K. Emergence and progression of 'non-semantic' deficits in semantic dementia. Cortex; a journal devoted to the study of the nervous system and behavior. 2009 Apr:45(4):483-94. doi: 10.1016/j.cortex.2007.07.005. Epub 2008 Feb 20 [PubMed PMID: 19231477]
Level 3 (low-level) evidenceGarrard P, Rentoumi V, Gesierich B, Miller B, Gorno-Tempini ML. Machine learning approaches to diagnosis and laterality effects in semantic dementia discourse. Cortex; a journal devoted to the study of the nervous system and behavior. 2014 Jun:55():122-9. doi: 10.1016/j.cortex.2013.05.008. Epub 2013 Jun 14 [PubMed PMID: 23876449]
Level 2 (mid-level) evidenceYi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in patients with semantic dementia. Neuropsychology. 2007 Jan:21(1):9-19 [PubMed PMID: 17201526]
Irish M, Bunk S, Tu S, Kamminga J, Hodges JR, Hornberger M, Piguet O. Preservation of episodic memory in semantic dementia: The importance of regions beyond the medial temporal lobes. Neuropsychologia. 2016 Jan 29:81():50-60. doi: 10.1016/j.neuropsychologia.2015.12.005. Epub 2015 Dec 9 [PubMed PMID: 26683384]
Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain : a journal of neurology. 2014 Apr:137(Pt 4):1241-53. doi: 10.1093/brain/awu003. Epub 2014 Feb 12 [PubMed PMID: 24523434]
Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008 Oct 14:71(16):1227-34. doi: 10.1212/01.wnl.0000320506.79811.da. Epub 2008 Jul 16 [PubMed PMID: 18633132]
Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: more than losing words. Neurology. 2013 Mar 5:80(10):897-903. doi: 10.1212/WNL.0b013e318285c15b. Epub 2013 Feb 6 [PubMed PMID: 23390170]
Vinogradova OM, Serov VV, Sivakov AE. [Clinico-morphologic characteristics of periodic disease]. Arkhiv patologii. 1975:37(2):70-5 [PubMed PMID: 1131061]
Henry ML, Grasso SM. Assessment of Individuals with Primary Progressive Aphasia. Seminars in speech and language. 2018 Jul:39(3):231-241. doi: 10.1055/s-0038-1660782. Epub 2018 Jun 22 [PubMed PMID: 29933490]
Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S, Santos-Santos MA, Miller ZA, Pakvasa M, Henry ML, Rosen HJ, Henry RG, Rabinovici GD, Miller BL, Seeley WW, Gorno-Tempini ML. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain : a journal of neurology. 2016 Oct:139(Pt 10):2778-2791 [PubMed PMID: 27497488]
Mandelli ML, Vitali P, Santos M, Henry M, Gola K, Rosenberg L, Dronkers N, Miller B, Seeley WW, Gorno-Tempini ML. Two insular regions are differentially involved in behavioral variant FTD and nonfluent/agrammatic variant PPA. Cortex; a journal devoted to the study of the nervous system and behavior. 2016 Jan:74():149-57. doi: 10.1016/j.cortex.2015.10.012. Epub 2015 Nov 14 [PubMed PMID: 26673947]
Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, Amirbekian B, Dronkers N, Miller BL, Henry RG, Gorno-Tempini ML. Frontal white matter tracts sustaining speech production in primary progressive aphasia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014 Jul 16:34(29):9754-67. doi: 10.1523/JNEUROSCI.3464-13.2014. Epub [PubMed PMID: 25031413]
Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001 Jul 24:57(2):216-25 [PubMed PMID: 11468305]
Yang J, Pan P, Song W, Shang HF. Quantitative meta-analysis of gray matter abnormalities in semantic dementia. Journal of Alzheimer's disease : JAD. 2012:31(4):827-33. doi: 10.3233/JAD-2012-120736. Epub [PubMed PMID: 22699847]
Level 1 (high-level) evidenceGorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004 Dec:10(6):426-36 [PubMed PMID: 15788282]
Level 3 (low-level) evidenceGorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of neurology. 2004 Mar:55(3):335-46 [PubMed PMID: 14991811]
Migliaccio R, Agosta F, Rascovsky K, Karydas A, Bonasera S, Rabinovici GD, Miller BL, Gorno-Tempini ML. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009 Nov 10:73(19):1571-8. doi: 10.1212/WNL.0b013e3181c0d427. Epub [PubMed PMID: 19901249]
Cadório I, Lousada M, Martins P, Figueiredo D. Generalization and maintenance of treatment gains in primary progressive aphasia (PPA): a systematic review. International journal of language & communication disorders. 2017 Sep:52(5):543-560. doi: 10.1111/1460-6984.12310. Epub 2017 Jan 24 [PubMed PMID: 28120406]
Level 1 (high-level) evidenceTippett DC, Hillis AE, Tsapkini K. Treatment of Primary Progressive Aphasia. Current treatment options in neurology. 2015 Aug:17(8):362. doi: 10.1007/s11940-015-0362-5. Epub [PubMed PMID: 26062526]
Mesulam MM. Primary progressive aphasia--differentiation from Alzheimer's disease. Annals of neurology. 1987 Oct:22(4):533-4 [PubMed PMID: 3324947]
Tastevin M, Lavoie M, de la Sablonnière J, Carrier-Auclair J, Laforce R Jr. Survival in the Three Common Variants of Primary Progressive Aphasia: A Retrospective Study in a Tertiary Memory Clinic. Brain sciences. 2021 Aug 24:11(9):. doi: 10.3390/brainsci11091113. Epub 2021 Aug 24 [PubMed PMID: 34573135]
Level 2 (mid-level) evidence