Introduction
Aortic regurgitation (AR), also known as aortic insufficiency, is a form of valvular heart disease in which the integrity of the aortic valve is compromised and leads to inadequate closure of the valve leaflets. A normal aortic valve is comprised of three semilunar cusps that attach to the aortic wall. Loss of function occurs when the valves themselves become diseased or if there is aortic root involvement. In AR, there is retrograde blood flow from the aorta into the left ventricle which occurs in diastole in the cardiac cycle. Chronic AR was initially described by Corrigan in 1832 in syphilitic patients who suffered from aortic root dilation. The clinical presentation of AR depends on its acuity of onset.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
As described above, aortic insufficiency may occur due to dysfunction linked to the aortic valve leaflets, aortic root, annulus, or ascending aorta. Typical etiologies responsible for acute AR include infective endocarditis, traumatic and non-traumatic rupture of the ascending aorta with aortic cusp prolapse, iatrogenic AR due to percutaneous aortic balloon valvuloplasty, prosthetic valve dysfunction, and perivalvular leak or dehiscence of a prosthetic valve.[1] Atypical causes of AR also include certain medications. For example, patients taking dopamine agonists such as bromocriptine (or other ergot alkaloids) have increased cardiac contraction, and increased stress is placed on the aortic valve, this is known to cause valvular fibrosis thereby putting them at higher risk for complications such as AR.
The etiologies responsible for chronic AR may be extensive, they include rheumatic heart disease (the most common cause in the developing world), infective endocarditis, myxomatous valve degeneration, congenital valve abnormalities (most commonly a bicuspid aortic valve while unicuspid and quadricuspid have also been reported), senile valvular calcifications, age-related dilatation of the aorta, ectasia of the aortic annulus, aortic dissection, aortitis/aortic root dilatation secondary to syphilis or giant cell arteritis, trauma, systemic hypertension, drug-induced valvulopathy, Whipple disease, osteogenesis imperfecta, and Crohn disease.[1][2]
Other rheumatologic processes involved are antiphospholipid syndrome, systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis, Reiter syndrome, psoriatic arthritis, relapsing polychondritis, Takayasu vasculitis, Marfan syndrome, Ehler-Danlos syndrome, and Behcet disease.[2]
Correlations have also been made between AR and Turner Syndrome. In one study of 253 patients with Turner syndrome from 7 to 67 years of age, AR was trivial, mild, and moderate to severe in 55%, 30%, and nearly 15% of the study population, respectively.[3]
Epidemiology
The Framingham Heart Study was a prospective epidemiologic study that began in 1948 as a means of determining risk factors for coronary heart disease. The original study cohort included 5,209 men and women from 28 to 62 years of age. In this patient cohort, aortic insufficiency had a prevalence of 4.9%, and 0.5% of patients were found to have moderate or greater severity AR.[4] The incidence and severity of aortic insufficiency increased with age, peaked in the fourth to sixth decades of life, occurs in 2% of people older than 70 years of age, and has been seen in 13% of men and 8.5% of women in the Framingham offspring study analyses.[4][5] The presentation of aortic insufficiency globally varies between industrialized countries and developing countries. Aortic insufficiency can often be seen in industrialized countries more commonly in older patients as a consequence of degenerative, insidious disease processes and linked to patients' comorbidities that put them at risk for developing AR. In developing countries, AR can present more commonly in younger patients with rapid onset, and rheumatic heart disease and infective endocarditis are two major contributors to AR development. Figures on the frequency of AR globally differ based on sex and geographic location.
Pathophysiology
Acute Aortic Insufficiency
In acute aortic insufficiency, the retrograde backflow of blood results in a quick rise in the left ventricular end-diastolic volume. The acute increase in preload and afterload does not allow the time for the left ventricle to acclimatize to the rapid increase in volume during diastole, and this also results in a sharp rise in left ventricular diastolic pressure. When the left ventricular diastolic pressure rises above the level of left atrial pressure, premature mitral valve closure and mitral regurgitation can be seen. Additionally, there will be increases in left atrial pressure and pulmonary capillary wedge pressure that cause pulmonary edema. Compensatory tachycardia may occur to blunt the decline in anterograde stroke volume, and concentric hypertrophy with increased wall thickness can occur.[6][7]
Chronic Aortic Insufficiency
In chronic aortic insufficiency, the left ventricle compensates for the diastolic regurgitation of blood from the aorta into the left ventricle by remodeling of the left ventricle via eccentric hypertrophy of the cardiomyocytes and left ventricular dilatation to allow for an increase in left ventricular stroke volume and a lesser increase in wall thickness than that which would be seen in acute aortic insufficiency. However, the left end-diastolic volume and pressure rise to a high degree such that the sarcomeres can become maximally stretched, left ventricle remodeling and compensation mechanisms fail, and interstitial fibrosis and a drop in left ventricular compliance occur.[1][6] The composite result of these changes is a decline in the initially compensated and elevated left ventricular stroke volume.
History and Physical
History
A thorough history and physical exam are essential parts of the diagnostic process. Upon history taking, patients with acute aortic insufficiency may complain of cough, palpitations, dyspnea on exertion, and chest pain. However, patients with chronic aortic regurgitation can often be asymptomatic for a lengthy amount of time before they exhibit exertional dyspnea, orthopnea, palpitations, paroxysmal nocturnal dyspnea, syncope, and/or chest pain.
Physical Exam
In general, aortic regurgitation diastolic murmurs are accentuated by maneuvers that increase afterload such as handgrip. Patients with acute aortic insufficiency can present as if they are experiencing a rapid onset of cardiogenic shock. They appear ill, hypotensive, and tachycardic. They can present with normal or mildly elevated pulse pressure, normal apical impulse, diminished S1 from early mitral valve closure, or an S3.
Patients with chronic aortic insufficiency will have a widened pulse pressure, may likely have a laterally and inferiorly displaced apical impulse, a high-frequency decrescendo diastolic murmur best heard at the 3rd or 4th intercostal space at the left sternal border, Austin Flint murmur, diminished S1, soft S2. If the pulse pressure is noticed to become more narrow and the patient has an S3, this may be a sign the patient has developed heart failure.
Listed below are various eponymous signs that have been described and associated with aortic insufficiency, but these may or may not always be seen [8].
- Austin Flint murmur: This is believed to occur due to the regurgitant jet of the aortic regurgitation causing premature closing of the mitral valve. This mimics functional mitral stenosis and is described as a low-pitched and rumbling mid to late diastolic or presystolic mitral murmur best heard at the apex.
- Pulsus bisferiens: A biphasic pulse with two systolic peaks seen on pressure tracings.
- Corrigan sign: A bounding pulse in large arteries such as the carotids that expands and suddenly collapses and empties between beats.
- De Musset sign: Head nodding with every heartbeat.
- Muller sign: Systolic pulsation of the uvula.
- Quincke sign: Repeatedly alternating blushing and blanching of capillaries in the nail folds upon palpation.
- Traube sign: Pistol shot sounds heard on the femoral artery upon compression of the artery.
- Waterhammer Pulse: Peripheral arterial pulses are rapidly dissipated or "bounding". This finding can be accentuated by raising the extremities.
Evaluation
The classification of aortic insufficiency, based on the 2017 American Society of Echocardiography guidelines, is as follows:
- In type 1 aortic insufficiency, the valve leaflets are functioning normally, but there is aortic dilation or perforation of the cusp(s). In type Ia aortic insufficiency, the sinotubular junction and ascending aorta are enlarged. In type 1b AR, the sinuses of Valsalva and the sinotubular junction are dilated. In type 1c aortic insufficiency, the aortic annulus is dilated. In type 1d aortic insufficiency, aortic cusp perforation is present.
- Type 2 aortic insufficiency is due to excessive leaflet tissue or commissural disruption that results in aortic cusp prolapse.
- Type 3 aortic insufficiency is due to various processes that result in restriction of the motion of the aortic leaflets.[9]
Chronic aortic insufficiency is classified into four different stages:
- Stage A: Patients at risk for aortic insufficiency.
- Stage B: Patients with progressive mild to moderate aortic insufficiency.
- Stage C: Patients with asymptomatic and severe aortic insufficiency
- Stage C1 patients have a left ventricular ejection fraction of more than 50% and mild to moderate left ventricular dilation with an end-systolic dimension less than 50 mm.
- Stage C2 patients have a left ventricular ejection fraction of less than 50% and severe left ventricular dilation with an end-systolic dimension more than 50 mm or an indexed left ventricular end-systolic dimension more than 25 mm/m2.
- Stage D: Patients with symptomatic and severe AR.
An electrocardiogram would show nonspecific ST-T wave changes, signs of left ventricular hypertrophy, or ST elevations or depressions if a myocardial infarction were associated with the patient's aortic insufficiency. A chest x-ray can show signs of pulmonary congestion or a widened mediastinum suggestive of aortic dissection.
However, the primary diagnostic test of choice for diagnosing aortic insufficiency is echocardiography, as it will provide a wealth of information about the aortic valve leaflets and surrounding apparatus, aorta, and the left ventricle. Echocardiography can identify the underlying cause of aortic insufficiency, the severity of regurgitant blood flow, and estimates of left ventricular size and systolic function. Transthoracic echocardiography assessment with color Doppler is necessary to characterize the regurgitant jet. Transesophageal echocardiography should be performed in cases with concern for aortic dissection or infective endocarditis. Some various echocardiographic measurements with different techniques combined, as mentioned below but not limited to this list, can help in accurately defining disease severity:[10][9]
Acute Aortic Insufficiency
- M-mode echocardiography showing early deceleration time on the mitral flow velocity curve and early mitral valve closure is indicative of elevated left ventricular end-diastolic pressure.
- An aortic velocity curve with a short half-time of less than 300 milliseconds indicates that there is rapid equilibration of the left ventricular and aortic diastolic pressures.
Chronic Aortic Insufficiency
Chronic aortic insufficiency is broken down into stages B, C, and D using the following valve hemodynamic information: jet width related to the left ventricular outflow tract, regurgitant blood volume (ml/beat), the regurgitant fraction (%), vena contracta measurement of the effective regurgitant orifice (cm), and effective regurgitant orifice area measurement (cm^2).
In aortic insufficiency patients with little to no symptoms, exercise stress testing can assess the presence of symptoms and their functional capacity. CT imaging, cardiac MRI, and coronary angiography may be useful in certain instances as well. CT imaging can help evaluate aortic disease. Cardiac MRI has a class 1 indication in stage B, C, and D moderate to severe chronic aortic insufficiency patients when suboptimal information is obtained from echocardiography, or the information obtained is discordant with clinical information. Cardiac catheterization can be used when noninvasive diagnostic modalities provide insufficient information, or are unavailable or contraindicated. If no cardiac MRI is available or is contraindicated due to the presence of implanted devices, the catheterization would help to understand the severity of aortic insufficiency.[10]
Treatment / Management
Acute Aortic Insufficiency
Hypertension can be controlled with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or dihydropyridine calcium channel blockers. Beta-blockers are recommended for managing aortic dissection, but they should be avoided in patients with severe aortic insufficiency as they can increase diastolic filling time and thereby allow for increased regurgitant blood flow. They block the compensatory tachycardia that is necessary to maintain adequate cardiac output. Antibiotics should be given for treating infective endocarditis; these patients should have their antibiotics tailored to the causative pathogens noted on blood cultures, and echocardiographic information should be reviewed to determine the need for any surgical interventions in addition to aortic valve replacement (AVR). Patients with acute aortic insufficiency due to infective endocarditis, aortic dissection, or trauma with hemodynamic instability require urgent surgical intervention.
Chronic Aortic Insufficiency
Medical therapy in chronic depends on the stage of the AR. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or dihydropyridine calcium channel blockers have a class I recommendation for managing hypertension in stage B and C patients. For stage C2 and D patients with symptoms and/or left ventricular dysfunction who cannot undergo surgery due to their comorbidities, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta-blockers have a class IIa recommendation as reasonable options.
Based on the 2014 valvular heart disease guidelines, AVR has a class I recommendation for patients with stage D AR, stage C2 aortic insufficiency with an ejection fraction <50%, or asymptomatic stage C AR but requiring cardiac surgery for other indications. Patients with stage C2 AR with an ejection fraction greater than or equal to 50% and left ventricular end-systolic dimension >50 mm have a class IIa recommendation for AVR. Patients with stage C AR with an ejection fraction greater than or equal to 50%, left ventricular end-diastolic dimension >65 mm, and low surgical risk have a class IIb recommendation for AVR. Patients with stage C AR with left ventricular end-systolic dimension >50 mm have a class IIa recommendation for AVR. Patients with stage C AR with an ejection fraction greater than or equal to 50%, left ventricular end-systolic dimension less than or equal to 50 mm, and left ventricular end-diastolic dimension less than or equal to 65 mm do not need AVR acutely. These patients, along with stage B AR patients not requiring cardiac surgery for another indication, should be watched carefully with periodic monitoring. However, stage B AR patients who need cardiac surgery for other indications have a class IIa recommendation for AVR.[11]
AVR is not recommended in patients with a left ventricular ejection fraction <35% and left ventricular end-diastolic dimension ≥70 mm due to poor postoperative outcomes.[11] While intervening in patients with symptoms or a decline in left ventricular ejection fraction has been validated, further work is needed to determine the optimal time to consider surgical consultation patients with asymptomatic AR, and if earlier intervention would benefit patients and provide good postoperative outcomes.[12](B3)
One study evaluating transcatheter aortic valve replacement (TAVR) and aortic valve repair in patients with isolated aortic insufficiency showed the following: (1) patients with bicuspid aortic valves had open surgical AVR more commonly than those with tricuspid aortic valves, but aortic root dilatation in either group and cusp prolapse in the bicuspid group favored aortic valve repair and (2) patients with tricuspid aortic valve cusp prolapse and restriction/retraction had an AVR.[13] This study highlights the notion that underlying etiologies and pathophysiological mechanisms affect clinical decision making on the types of surgical interventions considered for different patients with AR.
Aortic valve repair has also been mentioned as an option for AR. Those who consider aortic vale repair hope to preserve the aortic valve. Some report that aortic valve repair, either done alone or in conjunction with aortic root surgery, is a safe option with satisfactory postoperative outcomes, such as good long term survival and avoidance of repeat operations.[14] However, postoperative concerns are higher in those who undergo aortic valve repair vs. those who undergo AVR. For example, higher re-operation rates in patients with aortic insufficiency who underwent aortic repair than those who underwent AVR have been reported, and this shows that additional studies are needed to further validate aortic valve repair as a safe and effective option.[15](A1)
Differential Diagnosis
It is important to consider additional and alternative conditions that can be mistaken or overlooked when diagnosing aortic insufficiency. Aortic insufficiency has been reported to be mistaken for sepsis, pneumonia, or nonvalvular heart disease.[7] Additionally, recognizing the presence of AR in conjunction with other coexisting conditions is also important. The retrograde flow of blood in AR may result in congestive heart failure or be a consequence of aortic disease such as aortic dissection, so these must be recognized as well as this can impact clinical decision making. Pulmonary regurgitation can be considered as it too can present with a diastolic murmur, which can be seen with aortic insufficiency. One can differentiate between the two using differences in auscultation findings. A diastolic murmur of aortic insufficiency would increase with expiration, while a diastolic murmur of pulmonary regurgitation with underlying pulmonary hypertension would have a loud P2 and increase with inspiration since it is a right-sided heart murmur. Coronary heart disease resulting in myocardial infarction and/or congestive heart failure must also be considered in the differential.
Prognosis
Prognosis is dependent on the onset of symptoms and the progressions of the patient's aortic insufficiency. Patients with asymptomatic aortic regurgitation have a good outlook unless their disease progresses acutely. Patients with severe AR and preserved left ventricular ejection fraction who undergo prompt aortic valve replacement tend to have good long-term outcomes.[16] However, these patients need to be monitored carefully after valve replacement in case they require additional procedures, develop heart failure, or other negative outcomes. Those who develop decreased left ventricular ejection fraction and worsening symptoms have a poorer prognosis as they would be deemed unable to undergo aortic valve replacement based on current guideline recommendations.
Complications
Clinicians should be wary of multiple complications that can occur in the management of aortic insufficiency. Surgical management of aortic insufficiency is the gold standard for correcting aortic insufficiency, and the implementation of TAVR is influencing post-surgical outcomes. Initially, TAVR was contraindicated in pure native aortic insufficiency due to the lack of aortic valve annular calcification and difficulty with anchoring the implanted valves. Based on a multicenter registry developed in 2017 using 331 patients with aortic insufficiency to evaluate early generation vs. newer generation valve devices for TAVR in this population, TAVR was overall associated with relatively high rates of procedural complications; non-statistically significant post-procedural outcomes of conversion to conventional surgery, coronary obstruction, aortic root injury, and new permanent pacemaker implantation were reported more in the cohort of patients with newer generation devices.[17] The other reported complications reported overall were post-procedural aortic insufficiency, re-intervention, and need for a 2nd valve implantation, stroke, major or life-threatening bleeding, major vascular complications, and acute kidney injury.[17] Although this study shows some improvements in post-procedural outcomes when using TAVR in pure native aortic insufficiency, further studies are needed to if additional improvements in postoperative outcomes are possible that would warrant incorporating TAVR into current guidelines.
Deterrence and Patient Education
Patients should be educated on the symptoms that can be related to their aortic regurgitation. Patients who have not undergone surgery yet should be educated to follow up for serial echocardiograms depending on the severity of their aortic insufficiency. Patients with mild aortic insufficiency echocardiography can be performed every three to five years. Patients with moderate aortic insufficiency should have echocardiography performed every one to two years. Patients with severe aortic insufficiency should have echocardiography performed every six to twelve months or more frequently if left ventricular dilatation is progressively worsening. Patients should be told to follow up after surgery in approximately six weeks to three months to have echocardiography performed.[10]
Enhancing Healthcare Team Outcomes
Patients should be carefully evaluated by primary care doctors, cardiologists, nurses, and other healthcare personnel involved in patients' care to identify symptoms and underlying comorbidities that could be the contributing etiologies for the development of aortic regurgitation or post-interventional risk factors for morbidity and mortality. The difficulty in diagnosing the severity of aortic insufficiency is dependent on the use of various analytical methods via echocardiography by physicians. Cardiologists should establish appropriate follow up with patients based on the presence or lack of symptoms and involve cardiothoracic surgeons or interventional cardiologists for surgical or transcutaneous interventions, respectively, as soon as possible.
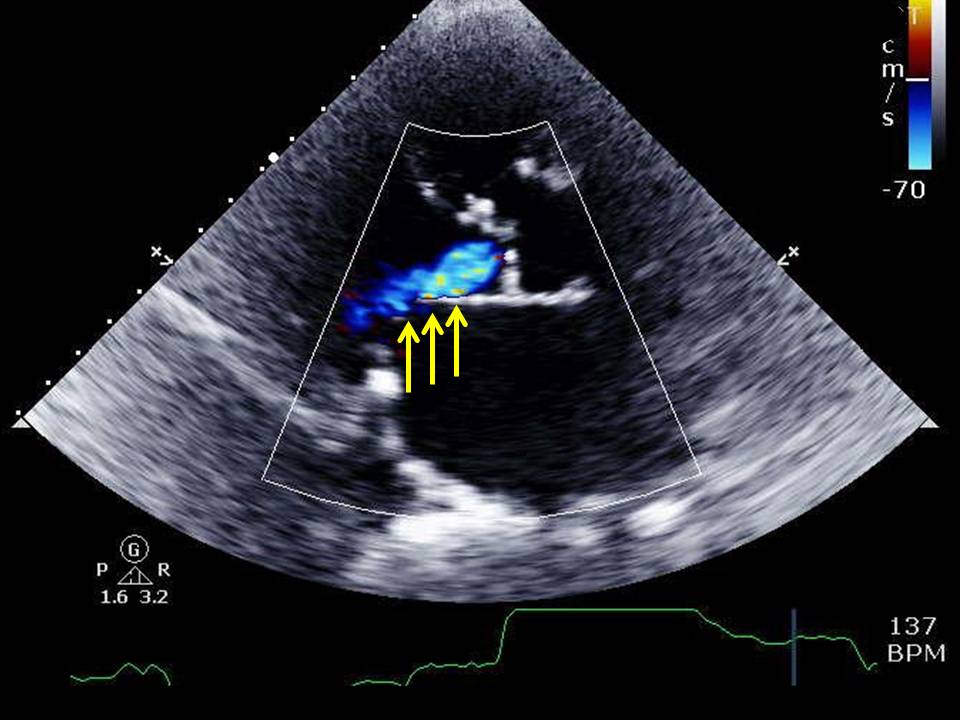
Media
(Click Image to Enlarge)
(Click Video to Play)
Aortic regurgitation
Contributed by Katherine Humphreys
(Click Video to Play)
References
Akinseye OA, Pathak A, Ibebuogu UN. Aortic Valve Regurgitation: A Comprehensive Review. Current problems in cardiology. 2018 Aug:43(8):315-334. doi: 10.1016/j.cpcardiol.2017.10.004. Epub 2017 Nov 2 [PubMed PMID: 29174586]
Enriquez-Sarano M, Tajik AJ. Clinical practice. Aortic regurgitation. The New England journal of medicine. 2004 Oct 7:351(15):1539-46 [PubMed PMID: 15470217]
Sachdev V, Matura LA, Sidenko S, Ho VB, Arai AE, Rosing DR, Bondy CA. Aortic valve disease in Turner syndrome. Journal of the American College of Cardiology. 2008 May 13:51(19):1904-9. doi: 10.1016/j.jacc.2008.02.035. Epub [PubMed PMID: 18466808]
Maurer G. Aortic regurgitation. Heart (British Cardiac Society). 2006 Jul:92(7):994-1000 [PubMed PMID: 16775114]
Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). The American journal of cardiology. 1999 Mar 15:83(6):897-902 [PubMed PMID: 10190406]
Flint N, Wunderlich NC, Shmueli H, Ben-Zekry S, Siegel RJ, Beigel R. Aortic Regurgitation. Current cardiology reports. 2019 Jun 3:21(7):65. doi: 10.1007/s11886-019-1144-6. Epub 2019 Jun 3 [PubMed PMID: 31161305]
Hamirani YS, Dietl CA, Voyles W, Peralta M, Begay D, Raizada V. Acute aortic regurgitation. Circulation. 2012 Aug 28:126(9):1121-6. doi: 10.1161/CIRCULATIONAHA.112.113993. Epub [PubMed PMID: 22927474]
Level 3 (low-level) evidenceAshrafian H. Pulsatile pseudo-proptosis, aortic regurgitation and 31 eponyms. International journal of cardiology. 2006 Mar 8:107(3):421-3 [PubMed PMID: 16503268]
Level 3 (low-level) evidenceZoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2017 Apr:30(4):303-371. doi: 10.1016/j.echo.2017.01.007. Epub 2017 Mar 14 [PubMed PMID: 28314623]
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014 Jun 10:63(22):e57-185. doi: 10.1016/j.jacc.2014.02.536. Epub 2014 Mar 3 [PubMed PMID: 24603191]
Level 1 (high-level) evidenceDong N, Jiang W, Yin P, Hu X, Wang Y. Predictors of Long-Term Outcome of Isolated Surgical Aortic Valve Replacement in Aortic Regurgitation With Reduced Left Ventricular Ejection Fraction and Extreme Left Ventricular Dilatation. The American journal of cardiology. 2020 May 1:125(9):1385-1390. doi: 10.1016/j.amjcard.2020.01.041. Epub 2020 Feb 8 [PubMed PMID: 32139161]
Bonow RO. Chronic mitral regurgitation and aortic regurgitation: have indications for surgery changed? Journal of the American College of Cardiology. 2013 Feb 19:61(7):693-701. doi: 10.1016/j.jacc.2012.08.1025. Epub 2012 Dec 19 [PubMed PMID: 23265342]
Level 3 (low-level) evidenceYang LT, Michelena HI, Maleszewski JJ, Schaff HV, Pellikka PA. Contemporary Etiologies, Mechanisms, and Surgical Approaches in Pure Native Aortic Regurgitation. Mayo Clinic proceedings. 2019 Jul:94(7):1158-1170. doi: 10.1016/j.mayocp.2018.11.034. Epub [PubMed PMID: 31272566]
Bisleri G. Aortic valve repair. Current opinion in cardiology. 2016 Nov:31(6):581-584 [PubMed PMID: 27583375]
Level 3 (low-level) evidenceWong CHM, Chan JSK, Sanli D, Rahimli R, Harky A. Aortic valve repair or replacement in patients with aortic regurgitation: A systematic review and meta-analysis. Journal of cardiac surgery. 2019 Jun:34(6):377-384. doi: 10.1111/jocs.14032. Epub 2019 Apr 6 [PubMed PMID: 30953445]
Level 1 (high-level) evidenceMentias A, Feng K, Alashi A, Rodriguez LL, Gillinov AM, Johnston DR, Sabik JF, Svensson LG, Grimm RA, Griffin BP, Desai MY. Long-Term Outcomes in Patients With Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. Journal of the American College of Cardiology. 2016 Nov 15:68(20):2144-2153. doi: 10.1016/j.jacc.2016.08.045. Epub [PubMed PMID: 27855803]
Yoon SH, Schmidt T, Bleiziffer S, Schofer N, Fiorina C, Munoz-Garcia AJ, Yzeiraj E, Amat-Santos IJ, Tchetche D, Jung C, Fujita B, Mangieri A, Deutsch MA, Ubben T, Deuschl F, Kuwata S, De Biase C, Williams T, Dhoble A, Kim WK, Ferrari E, Barbanti M, Vollema EM, Miceli A, Giannini C, Attizzani GF, Kong WKF, Gutierrez-Ibanes E, Jimenez Diaz VA, Wijeysundera HC, Kaneko H, Chakravarty T, Makar M, Sievert H, Hengstenberg C, Prendergast BD, Vincent F, Abdel-Wahab M, Nombela-Franco L, Silaschi M, Tarantini G, Butter C, Ensminger SM, Hildick-Smith D, Petronio AS, Yin WH, De Marco F, Testa L, Van Mieghem NM, Whisenant BK, Kuck KH, Colombo A, Kar S, Moris C, Delgado V, Maisano F, Nietlispach F, Mack MJ, Schofer J, Schaefer U, Bax JJ, Frerker C, Latib A, Makkar RR. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. Journal of the American College of Cardiology. 2017 Dec 5:70(22):2752-2763. doi: 10.1016/j.jacc.2017.10.006. Epub [PubMed PMID: 29191323]