Introduction
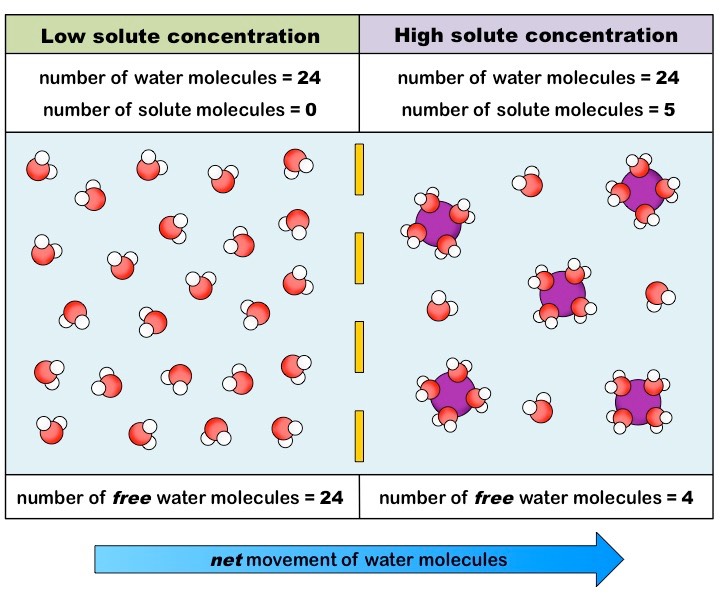
Osmosis, Greek for push, is the net movement of water across a semipermeable membrane (see Figure. Osmosis).[1][2] Across this membrane, water tends to move from an area of high concentration to an area of low concentration. It is important to emphasize that ideal osmosis requires only the movement of pure water across the membrane without any movement of solute particles across the semipermeable membrane. Osmosis can still occur with some permeability of solute particles, but the osmotic effect becomes reduced with greater solute permeability across the semipermeable membrane. It is also true that, at a specific moment, water molecules can move towards either the higher or lower concentration solutions, but the net movement of water is towards the higher solute concentration. The compartment with the highest solute and lowest water concentration has the greatest osmotic pressure. Osmotic pressure can be calculated using the Van 't Hoff equation, which states that osmotic pressure depends on the number of solute particles, temperature, and how well a solute particle can move across a membrane. Its measured osmolality can describe the osmotic pressure of a solution. The osmolality of a solution describes how many particles are dissolved in the solution. The reflection coefficient of a semipermeable membrane describes how well solutes permeate the membrane. This coefficient ranges from 0 to 1. A reflection coefficient of 1 means a solute is impermeable. A reflection coefficient of 0 means a solute can be freely permeable, and the solute cannot generate osmotic pressure across the membrane.[2] The compartment with the greatest osmotic pressure pulls water in and tends to equalize the solute concentration difference between the compartments. The physical driving force of osmosis is the increase in entropy generated by the movement of free water molecules. It is also thought that the interaction of solute particles with membrane pores generates a negative pressure, which is the osmotic pressure driving the water flow.[3] Reverse osmosis occurs when water is forced to flow in the opposite direction. In reverse osmosis, water flows into the compartment with lower osmotic pressure and higher water concentration. This flow is only possible by applying an external force to the system. Reverse osmosis is commonly used to purify drinking water and requires energy input.[4] The concept of osmosis should not be confused with diffusion. Diffusion is the net movement of particles from an area of high to low concentration. One can think of osmosis as a specific type of diffusion. Both osmosis and diffusion are passive processes and involve the movement of particles from an area of high to low concentration.[2][5]
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
The rate of osmosis always depends on the concentration of solute. The process is illustrated by comparing an environmental or external solution to the internal concentration found in the body. A hypertonic solution is any external solution with a high solute concentration and low water concentration compared to body fluids. In a hypertonic solution, the net movement of water is out of the body and into the solution. A cell placed into a hypertonic solution shrivels and dies by a process known as plasmolysis. An isotonic solution is any external solution with the same solute and water concentrations as body fluids. In an isotonic solution, no net movement of water takes place. A hypotonic tonic solution is any external solution with a low solute concentration and high water concentration compared to body fluids. In hypotonic solutions, there is a net water movement from the solution into the body. A cell placed into a hypotonic solution swells and expands until it eventually bursts through a process known as cytolysis. These three examples of different solute concentrations illustrate the spectrum of water movement based on solute concentration through osmosis. The body, therefore, must regulate solute concentrations to prevent cell damage and control the movement of water where needed.
Summary of Red Blood Cell Placed into Hypertonic, Isotonic, and Hypotonic Solutions
Hypertonic
A hypertonic solution has a higher solute concentration than the intracellular solute concentration. When a red blood cell is placed in any hypertonic solution, free water is moved out of the cell and into the solution. This movement occurs through osmosis because the cell has more free water than the solution. After the solutions are allowed to equilibrate, a cell with a lower overall volume results. The remaining volume inside the cell has a higher solute concentration, and the cell appears shriveled under the microscope. The solution is more dilute than originally. The overall process is known as plasmolysis.
Isotonic
An isotonic solution has the same solute concentration as the intracellular solute concentration. No net water movement occurs when a red blood cell is placed in an isotonic solution. The concentration of solute and water are equal both intracellularly and extracellularly; therefore, there is no net movement of water towards the solution or the cell. The cell and the environment around it are in equilibrium, and the cell should remain unchanged under the microscope.
Hypotonic
A hypotonic solution has a lower solute concentration than the intracellular solute concentration. When a red blood cell is placed in a hypotonic solution, free water moves into the cell. This situation results in an increased intracellular volume with a lower intracellular solute concentration. The solution ends up with a higher overall solute concentration. Under the microscope, the cell may appear engorged, and the cell membrane may eventually rupture. This overall process is known as cytolysis. Note that osmosis is a dynamic equilibrium, so, at any given moment, water molecular can momentarily flow toward any direction across the semipermeable membrane, but the overall net movement of all water molecules be from an area of high free water concentration to an area of low free water concentration.[5][6]
Clinical Significance
Water is known as the "universal solvent," and almost all life depends on it for survival. Therefore, though seemingly simple, the principle of osmosis plays a large role in almost all physiological processes. Osmosis is specifically important in maintaining homeostasis, which is the tendency of systems toward a relatively stable dynamic equilibrium. Biological membranes act as semipermeable barriers and allow for the process of osmosis to occur. Osmosis underlies almost all major processes in the body, including digestion, kidney function, nerve conduction, etc. It allows water and nutrient concentrations to be at equilibrium in all the body's cells. The underlying physical process regulates solute concentration in and out of cells and aids in excreting excess water out of the body.[2][7][8][9][10][11]
Media
(Click Image to Enlarge)
References
Chen J, Sabir S, Al Khalili Y. Physiology, Osmoregulation and Excretion. StatPearls. 2025 Jan:(): [PubMed PMID: 31082152]
Marbach S , Bocquet L . Osmosis, from molecular insights to large-scale applications. Chemical Society reviews. 2019 Jun 4:48(11):3102-3144. doi: 10.1039/c8cs00420j. Epub [PubMed PMID: 31114820]
Kiil F. Molecular mechanisms of osmosis. The American journal of physiology. 1989 Apr:256(4 Pt 2):R801-8 [PubMed PMID: 2705569]
Level 3 (low-level) evidenceGreenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P. Reverse osmosis desalination: water sources, technology, and today's challenges. Water research. 2009 May:43(9):2317-48. doi: 10.1016/j.watres.2009.03.010. Epub 2009 Mar 18 [PubMed PMID: 19371922]
Goodhead LK, MacMillan FM. Measuring osmosis and hemolysis of red blood cells. Advances in physiology education. 2017 Jun 1:41(2):298-305. doi: 10.1152/advan.00083.2016. Epub [PubMed PMID: 28526694]
Level 3 (low-level) evidenceMaldonado KA, Mohiuddin SS. Biochemistry, Hypertonicity. StatPearls. 2025 Jan:(): [PubMed PMID: 31082139]
Kiil F. Mechanism of osmosis. Kidney international. 1982 Feb:21(2):303-8 [PubMed PMID: 7069994]
Meir E, Perry J, Stal D, Maruca S, Klopfer E. How effective are simulated molecular-level experiments for teaching diffusion and osmosis? Cell biology education. 2005 Fall:4(3):235-48 [PubMed PMID: 16220144]
Schultz SG. Epithelial water absorption: osmosis or cotransport? Proceedings of the National Academy of Sciences of the United States of America. 2001 Mar 27:98(7):3628-30 [PubMed PMID: 11274376]
Level 3 (low-level) evidenceOgobuiro I, Tuma F. Physiology, Renal. StatPearls. 2025 Jan:(): [PubMed PMID: 30855923]
Trigo D, Smith KJ. Axonal morphological changes following impulse activity in mouse peripheral nerve in vivo: the return pathway for sodium ions. The Journal of physiology. 2015 Feb 15:593(4):987-1002. doi: 10.1113/jphysiol.2014.279331. Epub 2015 Jan 20 [PubMed PMID: 25524071]
Level 3 (low-level) evidence