Introduction
The nerve of the pterygoid canal, also known as the vidian nerve, supplies parasympathetic fibers to the nasal mucosa, palate, and lacrimal gland through the pterygopalatine ganglion. A vidian neurectomy, the sacrifice of this nerve, initially described by Golding-Wood in 1961, diminishes autonomic supply to the nasal cavity and reduces nasal secretions.[1][2]
The primary indication for a vidian neurectomy is vasomotor rhinitis, believed to stem from an imbalance between parasympathetic and sympathetic supply to the nasal mucosa. In the preendoscopic era, locating the vidian nerve was challenging, leading to suboptimal long-term outcomes and infrequent procedure utilization. Open approaches to the pterygopalatine fossa, like transantral and transpalatal exposures, were associated with significant morbidity, including ophthalmoplegia, orbital complications, and palatal fistulae.[3] In a groundbreaking development in 1991, Kamel and Zaher introduced endoscopic transnasal vidian neurectomy in cadaveric models. This innovation paved the way for modern surgical techniques, offering a more precise and less morbid approach.[4]
Clinical studies have reported improved nasal symptom outcomes with vidian neurectomy compared to medical management or alternative surgical procedures like turbinoplasty or septoplasty.[5] Despite the growing interest in vidian neurectomy, there remains limited evidence concerning its long-term results and potential complications. Further research is needed to comprehensively understand the procedure's efficacy and safety in the context of prolonged outcomes.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Anatomy of the Vidian Nerve
The vidian nerve is accompanied by the vidian artery and courses through the pterygoid canal (also known as the vidian canal), an osseous tunnel along the sphenoid sinus floor. The vidian nerve, originating from the convergence of parasympathetic fibers from the greater superficial petrosal nerve and sympathetic fibers from the deep petrosal nerve (derived from the internal carotid artery plexus), extends into the pterygopalatine ganglion located within the pterygopalatine fossa (see Image. Pterygopalatine Ganglion, Deep Petrosal Nerve, and Vidian Nerve). The vidian canal connects the foramen lacerum in the middle cranial fossa with the pterygopalatine fossa. This canal runs in a medial to lateral direction, traversing the sphenoid sinus floor to its funnel-shaped opening into the pterygopalatine fossa at the pterygoid "wedge."[6]
The pterygoid wedge, a valuable landmark for identification of the vidian canal, lies at the anterior junction of the pterygoid plates, which are located beneath the sphenoid sinus floor. Three openings exist along the floor of the sphenoid sinus, arranged from medial to lateral:
- Palatovaginal canal (transmitting the pharyngeal branch of the internal maxillary artery and the posterior pharyngeal nerve)
- Vidian canal
- Foramen rotundum (conveying the maxillary branch of the trigeminal nerve to the midface)
The pterygopalatine fossa, which houses the pterygopalatine ganglion, contains various nerves in addition to the vidian nerve. This fossa has an inverted pyramid shape and is located just behind the maxillary sinus. The pterygoid canal and the vidian nerve enter the fossa's posterior aspect. The maxillary division of the trigeminal nerve runs superior to the vidian nerve and the pterygopalatine ganglion and is connected to the ganglion via sensory fibers. Emanating from the ganglion are both somatic sensory and autonomic fibers, provided by the maxillary nerve (somatic sensory), the greater superficial petrosal nerve (parasympathetic), and the deep petrosal nerve (sympathetic). These branches include the greater and lesser palatine and nasopalatine nerves (sensory), the posterior nasal nerve, and other autonomic nerves to the lacrimal gland and the mucosal glands of the nose, palate, and pharynx. Knowing the location of these structures can help reduce the chances of surgical complications, such as numbness and xerophthalmia.
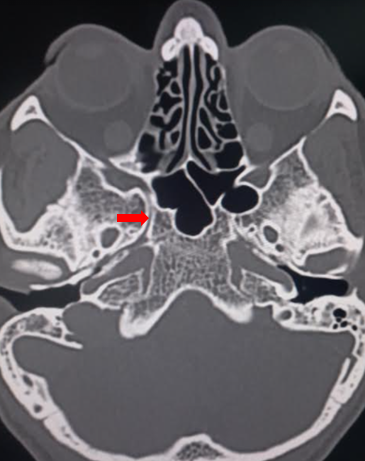
Before undertaking surgery in the area, it is crucial to understand the variations in the course and location of the vidian nerve relative to the sphenoid sinus. Radiographic studies indicate a medial to lateral course of the vidian canal from the pterygopalatine fossa to the foramen lacerum in 80% to 98% of cases (see Image. Vidian Canal).[7][8] The vidian canal is approximately 18 mm in length. The location of the vidian nerve within the sphenoid sinus exhibits significant variability, not only in its protrusion from the floor (inside the sphenoid corpus, partially protruding or connected by a bony stalk) but also in the angle formed by the floor with the nerve (flat, upsloping, downsloping, or inverted-V types).[9] Understanding these nuances is essential for ensuring surgical precision and minimizing potential complications.
Endoscopic Landmarks for the Vidian Canal
- The vidian canal is visible as an elevation along the lateral aspect of the sphenoid sinus floor.
- The opening of the vidian canal into the pterygopalatine fossa is located at the intersection between a line drawn along the posterior border of the palatine bone and a horizontal line drawn along the sphenoid sinus floor.
Surgical Relevance of the Vidian Canal
- Endoscopic management of juvenile nasopharyngeal angiofibromas
- Used as a landmark for the petrous carotid artery in expanded endonasal approaches
- Vidian neurectomy
Indications
A vidian neurectomy is indicated in the following cases:
Contraindications
There are no absolute contraindications to a vidian neurectomy. Relative contraindications to this procedure are skull base defects or tumors in the pterygomaxillary region.
Equipment
Equipment required for a vidian neurectomy includes:
- Functional endoscopic sinus surgery instrument set
- Endoscopic skull base instrument set, including endoscopic ligating clips, applicators, and an endoscopic drill system
- Endoscopes (0°, 45°, and 70°)
- Image-guided navigation system
- Epinephrine-soaked neurological sponges
- Nasal packing
Personnel
For a vidian neurectomy, a coordinated team approach involving specialized nurses in rhinology/endoscopic skull base surgery, an anesthesia provider, an endoscopic skull base surgeon, and an assistant is necessary.
Preparation
In preparation for surgery, obtaining a computed tomography scan of the paranasal sinuses with 1 mm contiguous axial, coronal, and sagittal views is necessary. During surgical planning, special attention is given to the position of the vidian canal in the sphenoid sinus, its relationship with the sphenoid corpus, the thickness of the bone covering the roof of the canal, and the angle formed between the floor of the sphenoid sinus and the canal.[9]
After orotracheal intubation, the patient is positioned in a semi-Fowler position (supine with the head of the bed elevated 30 to 45 degrees) with the head in a horseshoe rest or Mayfield pin holder. A pterygopalatine ganglion block is performed transorally via the greater palatine canal. The nasal cavity is prepared and decongested with oxymetazoline or epinephrine-soaked neurological sponges.
Technique or Treatment
Numerous descriptions in the literature of approaches to the vidian nerve include transantral via a Caldwell-Luc approach, transpalatal, transseptal mucoperichondrial, and endonasal routes. With advancements in endoscopic sinus surgery in the last 3 decades, the preferred route for vidian neurectomy has become the endoscopic endonasal route, using either the transsphenoidal or transnasal approach. The transsphenoidal approach is preferable in cases with a prominent vidian canal in the sphenoid sinus floor. However, both techniques can be combined in varying degrees to trace the vidian nerve from the sphenoid sinus to the pterygopalatine ganglion.
Surgical Steps of Transnasal or Retrograde Approaches
The surgical procedure begins by infiltrating the lateral wall of the nasal cavity anterior to the posterior end of the middle turbinate with a local anesthetic containing epinephrine. Subsequently, a U-shaped, posteriorly-based flap is raised over the palatine bone, positioned posterior to the posterior fontanelle of the maxillary sinus. The surgeon then identifies the ethmoidal crest of the palatine bone, a crucial landmark for locating the sphenopalatine foramen. After pinpointing the sphenopalatine artery, it is coagulated or clipped. The mucosal flap is elevated behind the foramen onto the face of the sphenoid sinus. Removing the posteroinferior margin of the sphenopalatine foramen eliminates the medial wall of the pterygopalatine fossa. The contents of the pterygopalatine fossa are laterally displaced to expose the vidian canal. Following clear visualization and identification of the vidian nerve, a 2- to 3-mm segment is excised using a sickle knife or scissors. Finally, the mucosal flap is repositioned and supported with a small piece of Gelfoam to complete the procedure.
Surgical Steps of Transsphenoidal or Anterograde Approaches
The identification of the sphenoid sinus ostium is carried out approximately 1.2 to 1.5 cm above the choana. To ensure the preservation of sphenopalatine artery branches inferolateral, a wide sphenoidotomy is performed, and the sphenoid rostrum is subsequently removed. Attention is directed to the potential risk of injury to the posterior septal branch of the sphenopalatine artery as it courses inferomedially, traversing superior to the choana and inferior to the sphenoid os. Following the thinning of the sphenoid sinus floor, the vidian nerve is identified on the lateral aspect of the floor using a 70-degree endoscope. If not readily visible, the canal is exposed using a pricking probe, Kerrison's rongeur, or drill. Subsequently, the nerve is transected with an angled probe, and a segment is excised. In the event of significant intraoperative bleeding, nasal packing is employed.
Pitfalls in Surgery
- Misidentification of the vidian canal
- The palatovaginal and vidian canals run along the sphenoid sinus floor; their proximity can easily confuse them. The palatovaginal canal's anterior opening is just a few millimeters away from the vidian canal's opening. However, there are some distinct differences between them. The palatovaginal canal is smaller than the vidian canal, and its opening is just medial to the vidian canal. Additionally, the palatovaginal canal transmits a smaller nerve known as the posterior pharyngeal nerve.
- Misidentification of the vidian nerve
- Due to their proximity, the posterior pharyngeal nerve can be mistaken for the vidian nerve. The posterior pharyngeal nerve exits the palatovaginal canal to run across the vidian canal in a lateral direction to join the pterygopalatine ganglion in the pterygopalatine fossa, while the vidian nerve, much greater in thickness, takes a slight lateral course before it joins the pterygopalatine ganglion.
- Incomplete resection of the vidian nerve
Complications
Immediate Complications
- Postoperative bleeding
- The bleeding usually comes from branches of the sphenopalatine artery, which can be controlled with nasal packing or cautery.
Long Term Complications
- Dry eye
- This is the most commonly reported complication, occurring between 35% to 72% of the time.
- Xerophthalmia is significantly more likely to occur after transsphenoidal approaches.
- Most studies reported the resolution of dry eyes within 1 to 5 months postoperatively.
- Palatal, gingival, and cheek numbness
- This occurs in 6.27% of patients.
- The incidence is higher with the pterygopalatine approach.
- Nasal crusting and dryness [11]
- This occurs in 3.7% of patients.
Clinical Significance
Surgical management of refractory rhinitis is still evolving, aiming to eliminate or substantially reduce parasympathetic innervation to the nasal mucosa. Endoscopic vidian neurectomy has proved to be an effective procedure with long-term (2 to 5 years) control of rhinorrhea.[11] To decrease complications of the procedure, particularly xerophthalmia and palate or cheek numbness, newer techniques, including posterior nasal neurectomy, are being studied.[12]
The posterior nasal nerve, a parasympathetic branch off the pterygopalatine ganglion, provides sensory and autonomic innervation to the nasal mucosa.[13] Sectioning of this nerve via an endoscopic approach provides symptomatic relief comparable to vidian neurectomy. However, with a lower likelihood of long-term complications due to injury to other nerves within the pterygopalatine fossa.[14] Because the posterior nasal nerve enters the nasal cavity just posterior to the middle and inferior turbinates, it is easily accessible endoscopically. In-office endoscopic ablation techniques are becoming more popular. The ablation modality most commonly employed is cryogenic, which appears to be as effective as vidian neurectomy but without a requirement for general anesthesia or as high a likelihood of causing xerophthalmia or cheek and palate numbness.[15][16] Radiofrequency and LASER ablation are also emerging as effective, safe alternative options.[17][18]
Enhancing Healthcare Team Outcomes
Concerning a vidian neurectomy, a collaborative healthcare team is essential for providing patient-centered care and optimizing outcomes. Surgeons and advanced practitioners must possess advanced endoscopic skills to identify and manipulate nasal structures, particularly the vidian nerve. Their responsibilities encompass accurate patient selection, meticulous execution of the surgical procedure, and effective postoperative management. Nurses play a crucial role in preoperative patient education, perioperative care, and postoperative monitoring, ensuring seamless transitions between different phases of care. Pharmacists contribute by reviewing medication regimens, managing pain effectively, providing guidance on potential drug interactions, and enhancing patient safety.Interprofessional communication is fundamental to the success of a vidian neurectomy. Physicians, advanced practitioners, nurses, pharmacists, and other health professionals must communicate clearly to share insights, discuss patient progress, and promptly address concerns. A cohesive approach to care coordination is vital, with each team member understanding their responsibilities and collaborating to implement a comprehensive care plan. This synergy ensures a patient-centric focus, minimizes the risk of complications, and optimizes team performance, contributing to enhanced patient safety and overall positive outcomes.
Media
(Click Image to Enlarge)
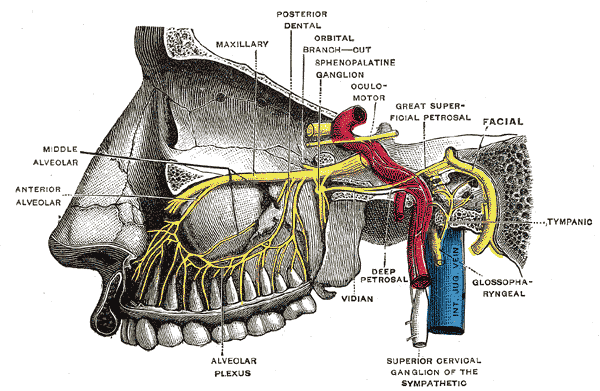
Pterygopalatine Fossa Nerves. Depiction of the pterygopalatine ganglion and the deep petrosal and Vidian nerves.
Henry Vandyke Carter, (plate 779), Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Konno A. Historical, pathophysiological, and therapeutic aspects of vidian neurectomy. Current allergy and asthma reports. 2010 Mar:10(2):105-12. doi: 10.1007/s11882-010-0093-3. Epub [PubMed PMID: 20425502]
GOLDING-WOOD PH. Observations on petrosal and vidian neurectomy in chronic vasomotor rhinitis. The Journal of laryngology and otology. 1961 Mar:75():232-47 [PubMed PMID: 13706533]
Golding-Wood PH. Vidian neurectomy: its results and complications. The Laryngoscope. 1973 Oct:83(10):1673-83 [PubMed PMID: 4758764]
Kamel R, Zaher S. Endoscopic transnasal vidian neurectomy. The Laryngoscope. 1991 Mar:101(3):316-9 [PubMed PMID: 2000022]
Tan G, Ma Y, Li H, Li W, Wang J. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Archives of otolaryngology--head & neck surgery. 2012 May:138(5):492-7. doi: 10.1001/archoto.2012.284. Epub [PubMed PMID: 22652948]
Kassam AB, Vescan AD, Carrau RL, Prevedello DM, Gardner P, Mintz AH, Snyderman CH, Rhoton AL. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. Journal of neurosurgery. 2008 Jan:108(1):177-83. doi: 10.3171/JNS/2008/108/01/0177. Epub [PubMed PMID: 18173330]
Level 3 (low-level) evidenceKim HS, Kim DI, Chung IH. High-resolution CT of the pterygopalatine fossa and its communications. Neuroradiology. 1996 May:38 Suppl 1():S120-6 [PubMed PMID: 8811698]
Level 2 (mid-level) evidenceVescan AD, Snyderman CH, Carrau RL, Mintz A, Gardner P, Branstetter B 4th, Kassam AB. Vidian canal: analysis and relationship to the internal carotid artery. The Laryngoscope. 2007 Aug:117(8):1338-42 [PubMed PMID: 17572642]
Level 2 (mid-level) evidenceLiu SC, Wang HW, Su WF. Endoscopic vidian neurectomy: the value of preoperative computed tomographic guidance. Archives of otolaryngology--head & neck surgery. 2010 Jun:136(6):595-602. doi: 10.1001/archoto.2010.72. Epub [PubMed PMID: 20566911]
Level 2 (mid-level) evidenceLiu SC, Kao MC, Huang YC, Su WF. Vidian Neurectomy for Management of Chronic Cluster Headache. Neurosurgery. 2019 May 1:84(5):1059-1064. doi: 10.1093/neuros/nyy136. Epub [PubMed PMID: 30535031]
Halderman A, Sindwani R. Surgical management of vasomotor rhinitis: a systematic review. American journal of rhinology & allergy. 2015 Mar-Apr:29(2):128-34. doi: 10.2500/ajra.2015.29.4141. Epub [PubMed PMID: 25785754]
Level 1 (high-level) evidenceIkeda K, Oshima T, Suzuki M, Suzuki H, Shimomura A. Functional inferior turbinosurgery (FITS) for the treatment of resistant chronic rhinitis. Acta oto-laryngologica. 2006 Jul:126(7):739-45 [PubMed PMID: 16803714]
Nishijima H, Kondo K, Toma-Hirano M, Iwasaki S, Kikuta S, Fujimoto C, Ueha R, Kagoya R, Yamasoba T. Denervation of nasal mucosa induced by posterior nasal neurectomy suppresses nasal secretion, not hypersensitivity, in an allergic rhinitis rat model. Laboratory investigation; a journal of technical methods and pathology. 2016 Sep:96(9):981-93. doi: 10.1038/labinvest.2016.72. Epub 2016 Jun 20 [PubMed PMID: 27322954]
Senanayake P, Wong E, McBride K, Singh N. Efficacy of Vidian Neurectomy and Posterior Nasal Neurectomy in the Management of Nonallergic Rhinitis: A Systematic Review. American journal of rhinology & allergy. 2022 Nov:36(6):849-871. doi: 10.1177/19458924221105933. Epub 2022 Jun 12 [PubMed PMID: 35695191]
Level 1 (high-level) evidenceOw RA, O'Malley EM, Han JK, Lam KK, Yen DM. Cryosurgical Ablation for Treatment of Rhinitis: Two-Year Results of a Prospective Multicenter Study. The Laryngoscope. 2021 Sep:131(9):1952-1957. doi: 10.1002/lary.29453. Epub 2021 Feb 22 [PubMed PMID: 33616224]
Level 2 (mid-level) evidenceKompelli AR, Janz TA, Rowan NR, Nguyen SA, Soler ZM. Cryotherapy for the Treatment of Chronic Rhinitis: A Qualitative Systematic Review. American journal of rhinology & allergy. 2018 Nov:32(6):491-501. doi: 10.1177/1945892418800879. Epub 2018 Sep 19 [PubMed PMID: 30229670]
Level 1 (high-level) evidenceEhmer D, McDuffie CM, Scurry WC Jr, McIntyre JB, Mehendale NH, Willis JH, Shealy RB, Watkins JP, Kakarlapudi VV. Temperature-Controlled Radiofrequency Neurolysis for the Treatment of Rhinitis. American journal of rhinology & allergy. 2022 Jan:36(1):149-156. doi: 10.1177/19458924211033400. Epub 2021 Aug 12 [PubMed PMID: 34382444]
Krespi YP, Wilson KA, Kizhner V. Laser ablation of posterior nasal nerves for rhinitis. American journal of otolaryngology. 2020 May-Jun:41(3):102396. doi: 10.1016/j.amjoto.2020.102396. Epub 2020 Jan 9 [PubMed PMID: 31948695]