Introduction
The entire nervous system forms via the process called neurulation in which neural tube and neural crest form initially. In the third week of embryogenesis, three germ layers arise, namely, ectoderm, mesoderm, and endoderm, through the process of gastrulation. The overlying ectoderm is induced and thickened by the notochord and the neural plate forms. The neural plate then gives rise to the neural tube by folding upon itself, which makes up the central nervous system, which includes the brain, brainstem, and the spinal cord. The brainstem and spinal cord are composed of plates separated by the sulcus limitans, which is in the fourth ventricle of the brain. It separates the alar plate, which gives rise to sensory neurons and a basal plate, which gives rise to motor neurons.[1][2][3]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The neural tube gives rise to three primary vesicles: Forebrain(Prosencephalon), Midbrain(Mesencephalon), and Hindbrain(Rhombencephalon). The prosencephalon divides into the Telencephalon and Diencephalon. The mesencephalon does not divide further. The rhombencephalon divides into the metencephalon and myelencephalon,[4][5][6]
The function of the Prosencephalon
- Basal ganglia: control of voluntary movements, cognition, emotion, eye movements, habitual, and perceptual learning
- Cerebral hemisphere: control of muscle movements, speech, thoughts, emotions, reading, writing, and learning.
- Hippocampus: regulates emotions associated with long-term memory and spatial navigation.
- Hypothalamus: synthesizes and releases neurohormones that control and regulate the pituitary gland.
- Infundibulum: connects the posterior pituitary gland to the hypothalamus.
- Lateral ventricles: contains cerebrospinal fluid and acts as a cushion for the brain while also aiding in the movement of nutrients and removal of waste.
- Pineal gland: produces and regulates melatonin, which regulates circadian rhythms.
- Thalamus: relays motor signals to the cerebral cortex, regulates consciousness, sleep, and alertness.
- Third ventricle: one of the four ventricles that aids in the protection of the brain from injury, and transports nutrients and waste.
The Function of the Mesencephalon
- Cerebral aqueduct: connects the third and fourth ventricle to allow cerebrospinal fluid to pass between them.
- Crus cerebri: transports nerve impulses from the cortex of the brain, brainstem, or lower part of the brain to the rest of the central nervous system.
- Tectum: responsible for the auditory and visual reflexes
- Tegmentum: responsible for controlling the basic body and limb movements
The Function of the Rhombencephalon
- Cerebellum: coordinates voluntary movements like posture, balance, coordination, and speech. It receives information from the sensory system, spinal cord, and other parts of the brain.
- The fourth ventricle: is filled with cerebrospinal fluid and aids in forming the central canal and protects the brain from trauma.
- Medulla oblongata: regulates breathing, digestion, swallowing, and blood vessel function.
- Pons: The pons is responsible for communication between different parts of the brain, sensations such as hearing, taste, and balance. It is also involved in the control of breathing.
- Spinal cord: connects the peripheral nervous system to the brain and sends nerve impulses through neurons into the brain.
Embryology
Gastrulation is the formation of the three embryonic germ layers or gastrula through the migration of epiblast cells. The gastrulation is initiated by primitive streak, by secreting fibroblast growth factor 8 (FGF8), which downregulates the E-cadherin, a protein that ties epiblast cell together. Now the epiblast cells are free to migrate through the primitive streak between the epiblast and hypoblast layers and form an intermediate cell layer called the intraembryonic mesoderm. The hypoblast is replaced by epiblast cells and form the endoderm. The remaining epiblast becomes the ectoderm.
Pre-notochordal cells inserting in the primitive node move cranially along the midline until they reach the prechordal plate. These pre-notochordal cells interspersed in the endoderm form the notochordal plate. Cells of the notochordal plate proliferate and get separated from the endoderm and a rod-like structure forms, which is called the notochord.
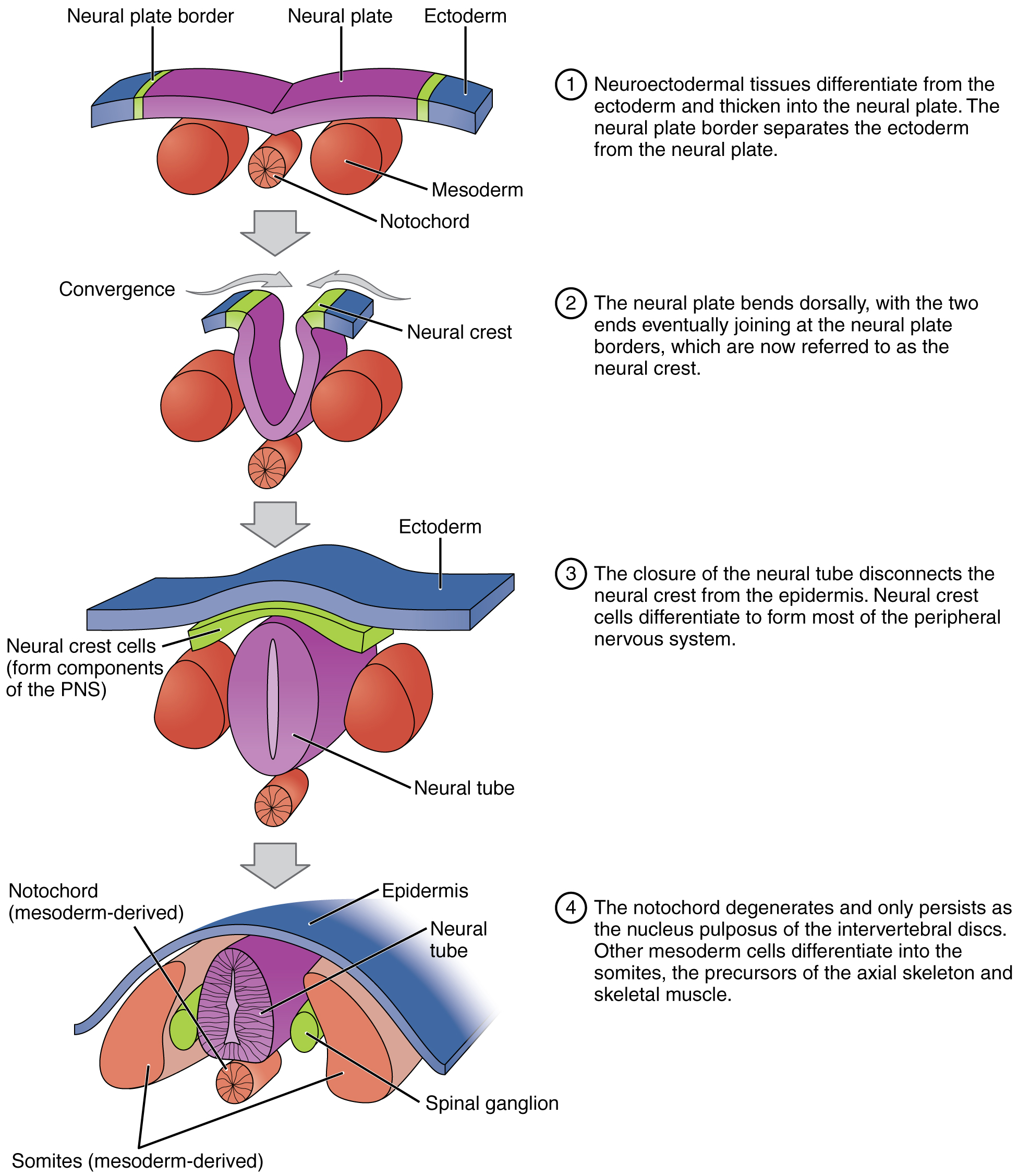
Notochord induces the neuroectoderm to become the neural plate by increasing the fibroblast growth factor(FGF) and inhibiting the bone morphogenic protein (BMP4) by the chordin, noggin, and follistatin in the cranial region and by WNT3a and FGF in the hindbrain and spinal cord region. These molecules are secreted by the primitive node, notochord, and prechordal mesoderm. The notochord itself forms the nucleus pulposus of the adult intervertebral disk.
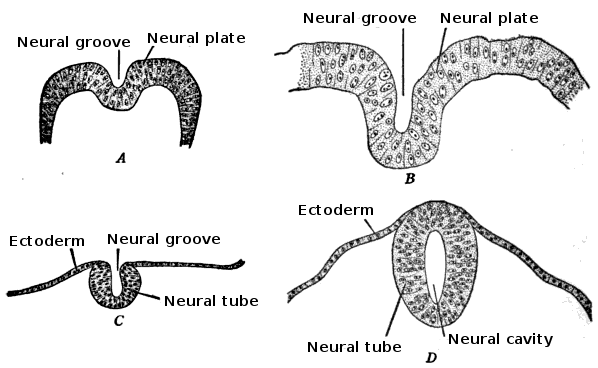
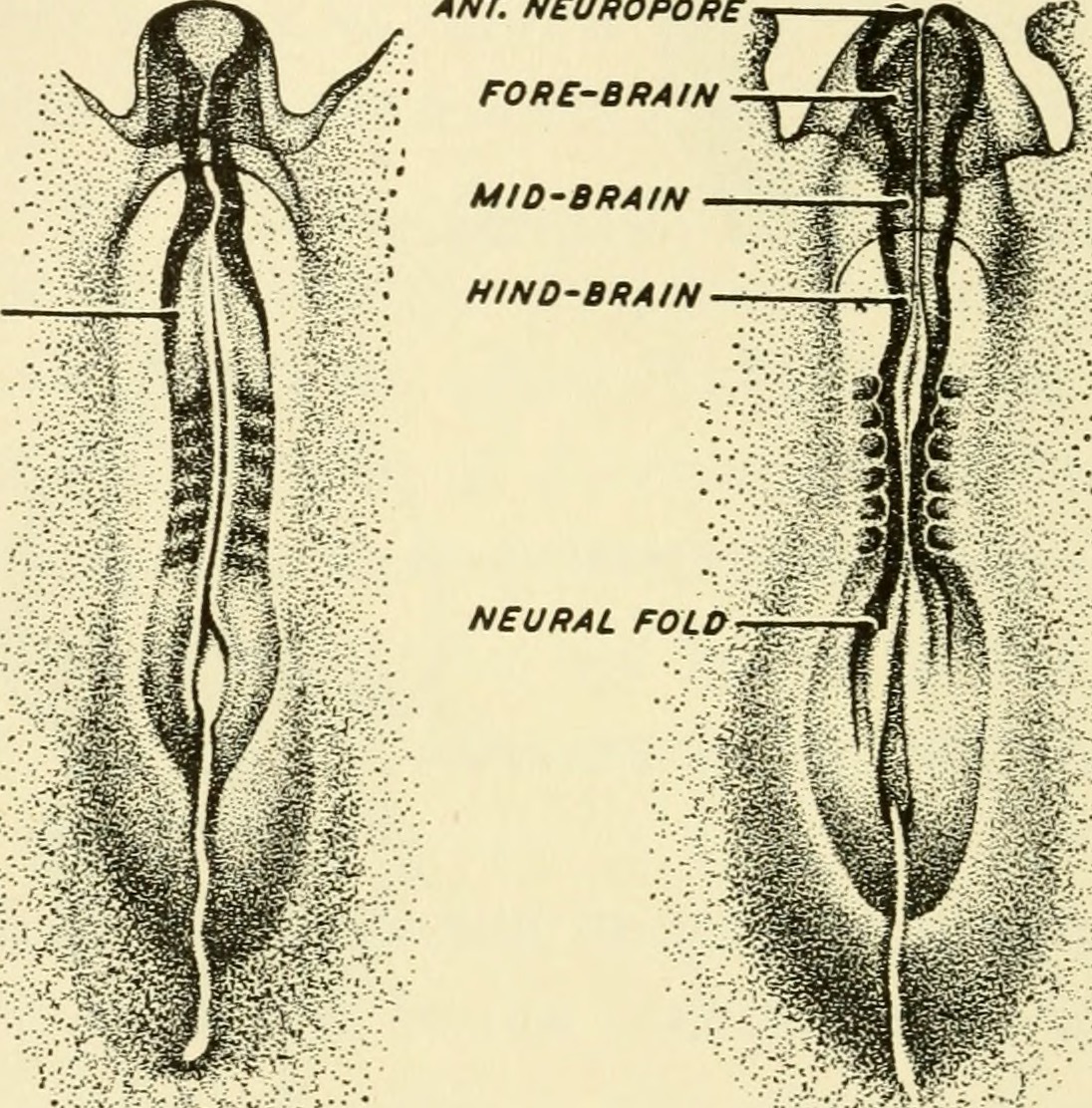
Primary neuralation: The lateral edges of the neural plate raise to form neural folds. The central area of the neural plate invaginates and becomes the neural groove. The neural folds converge and convert the neural groove into the neural tube. The fusion of the neural folds to form a neural tube requires a cadherin molecule; the neural plate changes E-cadherin expression to N-cadherin and increases N-cam formation to identify each cell as self and close the tube. This change in expression helps in separating the neural tube from the epidermis. During the folding of the neural tube, some cells which lie in the junction of the neural plate and ectoderm give raises to neural crest cells. Neural crest cells begin to appear due to the intermediate level of BMP4. The neural crest cells travel to a different location and form many cell types within the embryo. The neural tube is open anteriorly and posteriorly through which the lumen of the neural tube connects with the amniotic cavity. Anterior neuropore closes on day 25, which is the 18 to 20 somite stage. Posterior neuropore closes on day 28, which is the 25 somite stage.
Secondary neuralation: Mesenchyme cells coalesce into a solid cord that subsequently forms cavities that combine to form the hollow tube at the caudal end of the neural tube. This process creates filum terminale and conus medullaris.
The upper part of the neural tube becomes the adult brain, and the lower part of the neural tube becomes the adult spinal cord. The lumen of the neural tube forms the ventricular system and central canal of the brain and spinal cord, respectively. This process occurs during the seventeenth and twenty-first days of development. All components of the neural tube derive from the neural ectoderm.[7]
Blood Supply and Lymphatics
The brain receives blood supply from the internal carotid arteries and the vertebral arteries. The internal carotid artery branches off into the two main cerebral arteries- the anterior and middle cerebral arteries. The right and left vertebral artery come together at the level of the pons and join to form the basilar artery. The basilar artery connects the blood supply of the internal carotid arteries to form the circle of Willis, which is a circulatory anastomosis that provides collateral circulation to the cerebral circulation. This arrangement is important because if one of the vessels becomes occluded or narrowed, another vessel will preserve cerebral perfusion to avoid ischemia.[8]
The anterior and middle cerebral arteries then form the anterior circulation and give off branches that supply the structures of the forebrain such as the hippocampus, hypothalamus, and the internal capsule. The middle cerebral artery branches, namely the lenticulostriate arteries, specifically supply the basal ganglia and thalamus. The posterior circulation of the brain supplies both the midbrain and hindbrain structures. Branches from the posterior cerebral, basilar and vertebral arteries supply circulation to the posterior cortex, midbrain, and brainstem. The medial structures of the brainstem receive supply from the midline arteries, and the lateral arteries supply the lateral structures of the brainstem. The cerebellum, parts of the medulla, and pons obtain vascular supply via the posterior inferior cerebellar artery (PICA) and the anterior inferior cerebellar artery (AICA), which are also known as the dorsal-lateral arteries.[9][10]
Lymphatic drainage of the neural tube occurs through the meningeal lymphatic vessels, located parallel to the dural venous sinuses located in the central nervous system (CNS). These meningeal lymphatics drain immune cells, metabolic wastes, and excess fluid from the CNS and into the deep cervical lymph nodes, which are near the internal jugular vein.[11][12]
Clinical Significance
Neural tube defects occur during the fourth week due to the failure of the anterior or posterior neuropores to fuse, causing a persistent connection between the amniotic cavity and the spinal canal. Failure of neural tube closure is affected by genetic mutation or if the pregnant mother suffers exposure to certain environmental factors such as intake of harmful chemicals, i.e., .valproic acid, maternal infection, irradiation, maternal diabetes, as well as low folic acid intake during pregnancy.[13] There are five variants of neural tube defects; spina bifida occulta, meningocele, meningomyelocele, myeloschisis, and anencephaly, which is characterized by raise of alpha-fetoprotein and acetylcholinesterase in maternal serum.[14]
Spina bifida occulta: This condition is a failure of the caudal neuropore to close but does not herniate due to the dura being intact. It is usually seen at lower vertebral levels and is associated with a tuft of hair or dimpling of the skin at the level of the bony defect.[15] Normally, the sclerotomes on either side of the neural tube migrate medially and fuse between the surface ectoderm and neural tube to form vertebral arches and around the notochord to form vertebral bodies. In the case of spina bifida occulta, the primary defect is a failure of the surface ectoderm to separate from the neural tube in the caudal region. This defect impairs sclerotome migration and the formation of vertebral arches. The absence of sclerotomes in the caudal region can also lead to aberrant differentiation of the surface ectoderm and manifest as skin dimpling and a hair tuft, as seen here. Spina bifida occulta, which affects approximately 10% of the population, is usually asymptomatic.
Meningocele: the meninges, but no neural tissue herniates through the bony defect.
Meningomyelocele: both the meninges and the neural tissue herniate through the bony defect.[16]
Myeloschisis: exposure of unfused neural tissue without any skin covering it.
Anencephaly: This condition is a failure of the rostral neuropore to close, causing an open calvarium due to agenesis of the forebrain. Associated with polyhydramnios, which is the excessive accumulation of amniotic fluid.[17]
During the fifth and sixth weeks, failure of the right and left hemispheres to separate may occur due to a mutation in the sonic hedgehog signaling pathway resulting in holoprosencephaly. It is present in trisomy 13, Patau syndrome, and fetal alcohol syndrome. A moderate form of holoprosencephaly will result in cleft lip/palate, while a more severe form will result in cyclopia, which is characterized by the failure of the prosencephalon to divide the orbits into two eyes cavities properly.[18][19]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Moghadasi Boroujeni S, Koontz A, Tseropoulos G, Kerosuo L, Mehrotra P, Bajpai VK, Selvam SR, Lei P, Bronner ME, Andreadis ST. Neural crest stem cells from human epidermis of aged donors maintain their multipotency in vitro and in vivo. Scientific reports. 2019 Jul 5:9(1):9750. doi: 10.1038/s41598-019-46140-9. Epub 2019 Jul 5 [PubMed PMID: 31278326]
Duval N, Vaslin C, Barata TC, Frarma Y, Contremoulins V, Baudin X, Nedelec S, Ribes VC. BMP4 patterns Smad activity and generates stereotyped cell fate organization in spinal organoids. Development (Cambridge, England). 2019 Jul 25:146(14):. pii: dev175430. doi: 10.1242/dev.175430. Epub 2019 Jul 25 [PubMed PMID: 31239243]
Papaioannou E, Yánez DC, Ross S, Lau CI, Solanki A, Chawda MM, Virasami A, Ranz I, Ono M, O'Shaughnessy RFL, Crompton T. Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation. The Journal of clinical investigation. 2019 Jul 2:129(8):3153-3170. doi: 10.1172/JCI125170. Epub 2019 Jul 2 [PubMed PMID: 31264977]
Severino M, Huisman TAGM. Posterior Fossa Malformations. Neuroimaging clinics of North America. 2019 Aug:29(3):367-383. doi: 10.1016/j.nic.2019.03.008. Epub 2019 May 2 [PubMed PMID: 31256860]
Durcanova B, Appleton J, Gurijala N, Belov V, Giffenig P, Moeller E, Hogan M, Lee F, Papisov M. The Configuration of the Perivascular System Transporting Macromolecules in the CNS. Frontiers in neuroscience. 2019:13():511. doi: 10.3389/fnins.2019.00511. Epub 2019 May 28 [PubMed PMID: 31191221]
Badaloni A, Casoni F, Croci L, Chiara F, Bizzoca A, Gennarini G, Cremona O, Hawkes R, Consalez GG. Dynamic Expression and New Functions of Early B Cell Factor 2 in Cerebellar Development. Cerebellum (London, England). 2019 Dec:18(6):999-1010. doi: 10.1007/s12311-019-01051-3. Epub [PubMed PMID: 31273610]
Morimoto Y, Tamura R, Ohara K, Kosugi K, Oishi Y, Kuranari Y, Yoshida K, Toda M. Prognostic significance of VEGF receptors expression on the tumor cells in skull base chordoma. Journal of neuro-oncology. 2019 Aug:144(1):65-77. doi: 10.1007/s11060-019-03221-z. Epub 2019 Jun 25 [PubMed PMID: 31240525]
Spasojević G, Vujmilović S, Vujković Z, Gajanin R, Malobabić S, Ponorac N, Preradović L. Internal carotid and vertebral arteries diameters and their interrelationships to sex and left/right side. Folia morphologica. 2020:79(2):219-225. doi: 10.5603/FM.a2019.0071. Epub 2019 Jul 1 [PubMed PMID: 31257563]
Stojanović NN, Kostić A, Mitić R, Berilažić L, Radisavljević M. Association between Circle of Willis Configuration and Rupture of Cerebral Aneurysms. Medicina (Kaunas, Lithuania). 2019 Jul 3:55(7):. doi: 10.3390/medicina55070338. Epub 2019 Jul 3 [PubMed PMID: 31277348]
Anello MG, Miao TL, Pandey SK, Mandzia JL. Rare Bilateral Caudate Infarction in a Patient with a Common Circle of Willis Variant. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2019 Sep:46(5):593-594. doi: 10.1017/cjn.2019.233. Epub 2019 Jul 31 [PubMed PMID: 31230603]
Tamura R, Yoshida K, Toda M. Current understanding of lymphatic vessels in the central nervous system. Neurosurgical review. 2020 Aug:43(4):1055-1064. doi: 10.1007/s10143-019-01133-0. Epub 2019 Jun 18 [PubMed PMID: 31209659]
Level 3 (low-level) evidenceSon A, O'Donnell TF Jr, Izhakoff J, Gaebler JA, Niecko T, Iafrati MA. Lymphedema-associated comorbidities and treatment gap. Journal of vascular surgery. Venous and lymphatic disorders. 2019 Sep:7(5):724-730. doi: 10.1016/j.jvsv.2019.02.015. Epub 2019 Jun 24 [PubMed PMID: 31248833]
Yamaguchi Y, Miyazawa H, Miura M. Neural tube closure and embryonic metabolism. Congenital anomalies. 2017 Sep:57(5):134-137. doi: 10.1111/cga.12219. Epub 2017 May 31 [PubMed PMID: 28295633]
Sisman Y,Thomsen RH,Vestermark V,Krebs L, [Folate deficiency as a differential diagnosis to severe pre-eclampsia]. Ugeskrift for laeger. 2019 Jul 1; [PubMed PMID: 31267942]
Goto T, Sakai T, Sato N, Katoh S, Sairyo K. An Adolescent Athlete with Low Back Pain Associated with Spina Bifida Occulta at the Thoracolumbar Junction : A Case Report. The journal of medical investigation : JMI. 2019:66(1.2):199-200. doi: 10.2152/jmi.66.199. Epub [PubMed PMID: 31064941]
Level 3 (low-level) evidenceAlruwaili AA, M Das J. Myelomeningocele. StatPearls. 2023 Jan:(): [PubMed PMID: 31536302]
Struksnæs C,Blaas HK,Vogt C, Autopsy Findings of Central Nervous System Anomalies in Intact Fetuses Following Termination of Pregnancy After Prenatal Ultrasound Diagnosis. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2019 Jun 29; [PubMed PMID: 31256740]
Naikwadi A, Rege R, Hameed S. Antenatal sonographic diagnosis of semilobar holoprosencephaly with associated cleft lip and palate. BJR case reports. 2019 Feb:5(1):20180013. doi: 10.1259/bjrcr.20180013. Epub 2018 Oct 11 [PubMed PMID: 31131114]
Level 3 (low-level) evidenceCalloni SF,Caschera L,Triulzi FM, Disorders of Ventral Induction/Spectrum of Holoprosencephaly. Neuroimaging clinics of North America. 2019 Aug; [PubMed PMID: 31256862]