Introduction
Liver lesions have a broad spectrum of pathologies, ranging from benign lesions such as hemangiomas to malignant lesions such as primary hepatocellular carcinoma and metastasis. Imaging is a crucial step in diagnosing these conditions, as liver enzymes can be elevated in up to 9% of individuals in the USA.[1][2] A combination of medical history, serologic, and radiologic investigations can provide a diagnosis in most cases.[3] Liver lesions can be categorized into focal and diffuse liver lesions.
Focal liver lesions generally fall into 3 main clinical categories.
- Benign lesions that do not need treatment if they are asymptomatic, including hepatic hemangiomas, focal nodular hyperplasias, and benign liver cysts
- Benign lesions that require treatment, including hepatic adenoma, hepatic abscess, and hepatic adenomas
- Malignant lesions, including hepatocellular carcinoma, cholangiocarcinoma, hepatic angiosarcoma, and liver metastases
Diffuse liver lesions can be categorized into vascular, inflammatory, and storage diseases.
Benign liver lesions can be classified into 3 categories based on their tissue origin:
- Cholangiocellar: hepatic cyst, biliary cystadenoma, intraductal papillary neoplasm of the bile ducts, peribiliary cyst, intrahepatic bile adenoma (see Image. Hepatic Biloma).
- Hepatocellular: focal nodular hyperplasia, hepatic adenoma
- Mesenchymal: hemangioma, lipoma
Liver ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) are the primary imaging modalities to diagnose liver lesions. Postcontrast imaging can help distinguish lesions depending on their degree of vascularity and composition. Postcontrast hepatic imaging falls into 3 distinct phases: the arterial phase, the portal venous phase, and the delayed venous phase.[4] Ultrasound can be a method of choice as a screening modality, and contrast-enhanced multidetector CT (MDCT) is a modality of choice in most hepatic imaging.[5] MRI plays a role in better-characterizing lesions with equivocal features on US and CT.
Anatomy
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy
The most widely used anatomic classification of hepatic segments is the Couinaud classification, which describes 8 functionally independent liver segments based on vascularization, bile duct distribution, and lymphatic drainage.[6] Each segment is wedge-shaped, with the apex directed towards the hepatic hilum with some variability.[7] A single branch of the hepatic artery, portal vein, and bile duct enters through the apex into the hepatic segments. The hepatic vein runs between 2 adjacent segments and drains into the inferior vena cava (IVC). The middle hepatic vein runs from the inferior vena cava to the gallbladder fossa and divides the liver into the right and left lobes.[8] The right hepatic vein divides the right lobe into the anterior and posterior segments, and the falciform ligament divides the left lobe into medial and lateral segments. The portal vein divides segments horizontally into superior and inferior segments. Segment I is the unique caudate lobe, which can receive dual blood supply from the right and left portal vein and drains directly into IVC. Segments II, III, and IV are left hepatic lobe sections. Segments V, VI, VII, and VIII constitute the right lobe hepatic sections.[9]
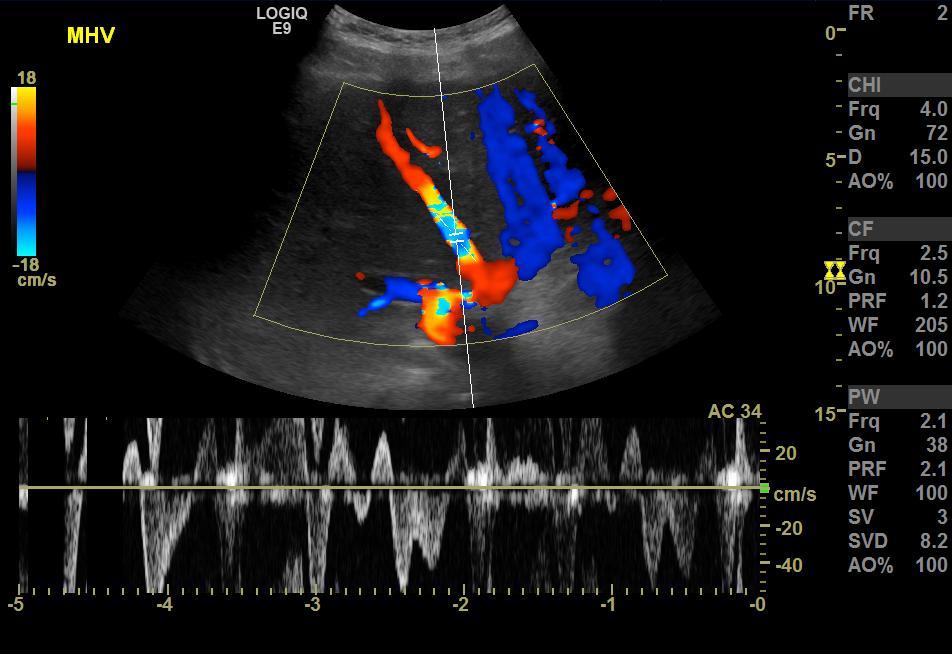
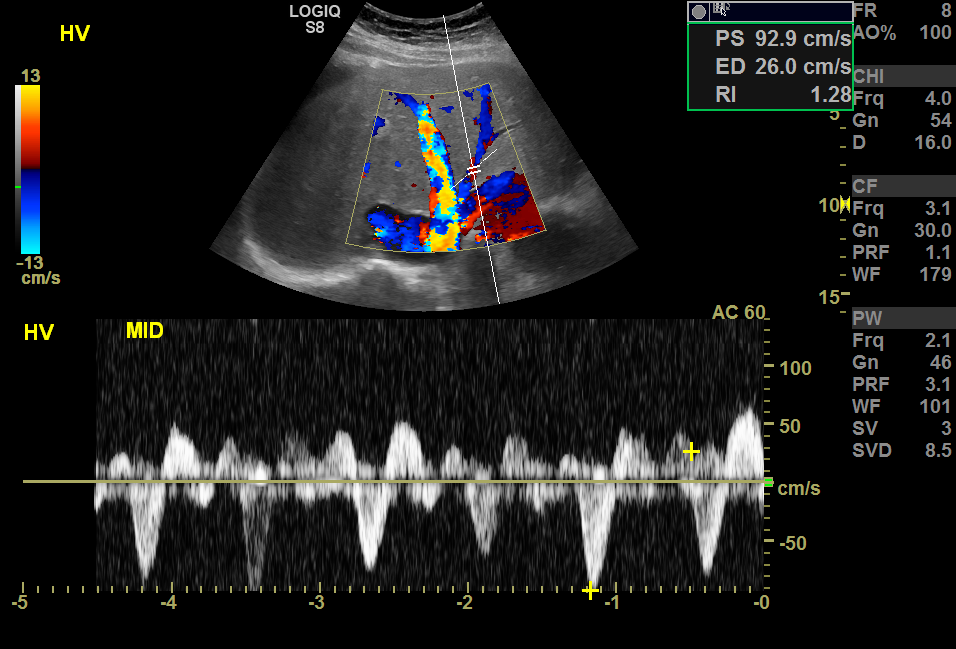
The hepatic blood supply is from the portal vein (about two-thirds), and the rest is from the hepatic artery; thus, maximum enhancement of the liver is attained during the portal venous phase, which is 60 to 120 seconds after arterial phase enhancement.[10] Tumors supplied from the hepatic artery enhance during the arterial phase, the principle in transarterial chemoembolization and chemoembolization.[11] Arterial embolization and treatment target tumors rather than normal hepatic cells.[12] The hepatic veins enter the inferior vena cava. They can be seen as anechoic tubes with thin walls on ultrasound (see Image. Color Doppler Ultrasound, Hepatic Veins With Hepatic Venous Waveforms). The portal triad, which includes the portal veins, hepatic arteries, and bile ducts surrounded by fibrofatty tissue, can be seen as echogenic foci throughout the liver.[13]
The portal vein forms from the junction of the superior mesenteric artery and splenic veins behind the neck of the pancreas.[14] Inferior mesenteric, gastric, and cystic veins drain into the portal vein. It divides into the right hepatic vein and porta hepatis. Although the portal vein provides 75% of the liver's blood supply, it provides 50% of its oxygen supply.[15] Portal hypertension or hepatic venous pressure gradient is a portosystemic pressure gradient. Different pathologies can cause portal hypertension, including portal vein thrombosis, cirrhosis, viral hepatitis, Budd-Chiari syndrome, and congestive heart failure.[16] Ultrasound manifestations of portal hypertension are a dilated portal vein (>13 mm), a dilation of the splenic and superior mesenteric veins, the presence of collateral vessels between the portal and systemic pathways, splenomegaly, ascites, and the presence of biphasic or reversed flow (hepatofugal flow) on Doppler US which is diagnostic and pathognomonic for portal hypertension.[17] Portal hypertension can be appreciated in CT and MRI with similar manifestations on ultrasound and the presence of contrast in the paraumbilical vein, which is pathognomonic.
Hepatomegaly and liver cirrhosis are 2 main pathologies that distort the liver anatomy. Cardiac disease, including congestive heart failure, restrictive cardiomyopathy, and right-sided valvular disease, can cause hepatomegaly and dilation of hepatic veins, called passive hepatic congestion.[18] It is due to hepatic venous drainage impairment and stasis of blood in hepatic parenchyma. Ultrasound manifestations of passive hepatic congestion are right hepatic lobe enlargement, inferior vena cava dilatation, and hepatic vein enlargement.[19] Changes in the hepatic vein and IVC velocity pattern can be appreciated in color Doppler ultrasound with loss of normal triphasic flow and flattening of the waveform in hepatic veins.[20] Congestive right heart failure also can cause painless, diffuse wall thickening of the gallbladder.[21][22] The most common etiologies of cirrhosis are alcohol, followed by viral hepatitis, cryptogenic infection, vascular pathologies, and metabolic disorders.[23] The most common ultrasound changes in cirrhosis are a nodular appearance on the surface, heterogeneous echotexture, relative enlargement of the caudate lobe compared to the right lobe, and atrophy of the medial segment of the left lobe. The presence of portal hypertension imaging changes increases the sensitivity and specificity of cirrhosis based on the ultrasound changes, but these usually happen in the advanced and late stages of cirrhosis.[3]
Plain Films
Plain films are rarely helpful in assessing hepatic pathology except for evaluating hepatomegaly when the liver shadow extends beyond the shadow of the right kidney.
Computed Tomography
CT imaging of the liver can be done with different protocols, including unenhanced, single-phase, dual-phase, and triphasic contrast-enhanced protocols.[24] Each of these liver CT protocols is important in evaluating different liver pathologies. The single-phase contrast-enhanced CT is generally the modality of choice in the portal venous phase in CT, which is typically 70 seconds after intravenous (IV) contrast injection, and the liver has the maximum enhancement. It can mainly provide information about diffuse liver pathologies, such as liver cirrhosis and hypovascular metastatic liver disease. The dual-phase contrast-enhanced CT is the primary imaging modality conducted in the portal venous and late arterial phases, which can be obtained approximately 35 seconds after IV injection and might provide better information about hypervascular lesions.[25]
This protocol can help with hypervascular metastatic lesions like renal cell carcinoma, breast cancer, melanoma, and endocrine tumors and preoperative evaluation for partial hepatic resection. It can provide the referring surgeon with information about liver anatomy and vasculature. Triphasic contrast-enhanced CT constitutes non-enhanced arterial and portal venous or arterial, venous, and delayed phases.[26] Arterial phase enhancement begins approximately 20 to 25 seconds after IV contrast injection. Hypervascular pathologies, like most benign and malignant hepatic lesions, can be appreciated in this phase. Triphasic protocols usually are used in patients with possible cirrhosis and hepatocellular carcinoma (HCC).[27] HCC is a hypervascular lesion that enhances during the arterial phase and has a fast wash-out during the portal venous phase.[28] Cholangiocarcinoma is 1 of the liver pathologies that can be appreciated better on a triphasic CT technique, with non-enhanced, portal venous phase and delayed phase (10 to 15 minutes) hyperenhancement due to fibrous tissue.[29] See Image. Cholangiocarcinoma.
Fatty liver disease is a common condition that can present with different patterns. Because of its important clinical consequences in the long term, radiologists should provide detailed descriptions of fatty changes. The 6 main important patterns of fatty liver infiltrations are diffuse, focal, geographic, subcapsular, multifocal, and perivascular. Fatty liver changes are hyperechoic lesions compared to the spleen and renal cortex.[30] Hepatic steatosis, either diffuse or focal, is best evaluated on a non-contrast CT of the abdomen. In a non-contrast study, the liver measures at least 10 Hounsfield units (HU) less than the spleen in hepatic steatosis.[31] On a contrast-enhanced CT scan, the liver and spleen have different rates of contrast uptake. In the portal venous phase, a hepatic attenuation of 25 HU less than the spleen suggests steatosis.[32] Fatty liver disease on MRI can be appreciated by the signal drop in gradient-echo T1-weighted out-of-phase images compared to in-phase.[33]
Diffuse fatty liver is the most common form of liver steatosis. Diffuse steatosis with hepatomegaly and increased caudate-to-right lobe ratio increase the possibility of non-alcoholic steatohepatitis (NASH).[34] Focal hepatic steatosis can have similar imaging features as specific benign and malignant liver lesions, and MRI can help differentiate different entities. Areas in the liver that are more susceptible to focal hepatic steatosis are perivascular and subcapsular regions adjacent to the hepatic hilum or falciform ligament, which are the same areas for focal hepatic sparing in the diffuse fatty infiltration as a result of altered intrahepatic blood flow with aberrant venous system and no portal inflow.[35]
Geographic fatty liver disease can occur secondary to some parenchymal disease like cholangitis, or involvement of the right liver lobe can be related to the feeding of this part of the liver by the superior mesenteric vein, which has lipogenic materials from gastrointestinal tracts. Subcapsular fat deposition can be visible in insulin-dependent diabetes patients due to fat accumulation in the subcapsular region secondary to higher insulin concentration.[36] Multifocal fat deposition can resemble metastasis, abscesses, lymphoma, sarcoidosis, and hemangiomatosis in the ultrasound and CT, but MRI can differentiate the presence of fat with the signal drop in out-of-phase images.[37] Fat deposition around the portal vein or hepatic vein can have similar ultrasound and CT imaging characteristic features as Budd-Chiari syndrome, periportal edema, and MRI can differentiate fat deposition from other pathologies.
Non-contrast CT can be useful for evaluating and detecting iron and calcium deposition in the liver. Amyloidosis and Wilson disease mainly have nonspecific liver imaging features. Amyloidosis, abnormal deposition of amyloid fibrils, can have a nonspecific liver imaging manifestation like diffuse hepatomegaly, diffuse or focal areas of hypoattenuation, heterogeneous appearance, or periportal involvement.[38] Wilson disease is an autosomal recessive disorder that causes the accumulation of copper in different organs, such as the basal ganglia, cornea, and liver. The CT manifestation of Wilson disease in the liver can be nodular areas of hyperattenuation due to fatty infiltration and copper accumulation in the liver.[39]
The presence of gas in the portal vein can have various etiologies, and it should be differentiated from pneumobilia. Portal venous gas is peripheral in location, while pneumobilia is central.[40] It manifests as echogenic foci in the portal vein lumen in the ultrasound and foci of low density in the liver, portal vein, and its branches in CT. Pneumobillia is the presence of gas in the biliary tree, which is more central. Portal venous gas can be caused by umbilical vein catheterization, necrotizing enterocolitis, or postoperative findings in corrective bowel surgery in children. It can be related to ischemic bowel, inflammatory bowel disease, upper and lower endoscopic procedures, and intra-abdominal sepsis in adults.[41] Pneumobillia can be seen in the setting of recent biliary instrumentation, an incompetent sphincter of Oddi, biliary-enteric surgical anastomosis, and spontaneous biliary-enteric fistula.[42]
The hepatic abscess, often a cystic-appearing lesion, usually arises from an infectious process in the gastrointestinal system that carries the infection to the liver via the portal venous system. Escherichia coli is the most common microorganism.[43] The imaging characteristic of a hepatic abscess is a double target sign, a central area of low attenuation surrounded by 2 layers of rings.[44] The inner layer can appear as a high attenuation rim, with early and persistent delayed contrast enhancement, and the outer layer enhances only in the delayed phase. Hepatic abscess imaging features on T2-weighted MRI can demonstrate an irregular wall with late enhancement and central area of T2 hyperintensity.[45] Due to disseminated candidiasis, fungemia is another infection process that can occur in immunocompromised patients. Liver and spleen involvement is visible in CT as multiple small hypoattenuating lesions, which may have ring-enhancing and look like microabscesses. Although MRI has better sensitivity and specificity, CT has been used more frequently because it is less expensive and more accessible.[46]
Magnetic Resonance
MRI has advantages and disadvantages compared to ultrasound and CT. Lack of ionizing radiation, higher cross-sectional resolution, presence of extracellular and hepatocyte-specific contrast agents, and the ability to give better and more accurate information about the diffuse liver lesion and characterization of focal liver lesions make MRI the best imaging modality in liver imaging. However, it costs more, takes longer to acquire images, and requires patient cooperation, such as breath-holding, to have better and fewer motion artifacts (see Image. Aliasing Artifact).[47]
The liver MRI protocol consists of multiple sequences before and post-contrast. Post-contrast phases include the arterial phase, portal venous phase, equilibrium phase, hepatobiliary delayed phase, and later delayed phase.[48] Hepatobiliary-specific contrast agents that are used in liver MRI are mangafodipir trisodium (Mn-DPDP), gadobenate dimeglumine (Gd-BOPTA), and gadoxetic acid (Gd-EOB-DTPA). These contrast agents have specific characteristics such as specific receptors and transporters for uptake, different excretion percentages via the biliary and renal systems, method of administration, and side effects. These hepatocyte-specific contrasts can better help identify and characterize small liver lesions, especially well-differentiated HCC. It can be used to distinguish FNH from hepatic adenoma. Hepatic adenoma is visible in the hepatobiliary phase as a hypointense lesion versus FNH that is iso or hyperintense when evaluating hepatic disease during HCC surveillance and post-liver transplant evaluation. It can also differentiate tumors with a hepatocellular origin from nonhepatocellular ones.[49] Contrast agents secreted into bile can differentiate hepatocellular adenoma (HCA) by early heterogeneous enhancement in T1-weighted fat-suppressed arterial phase MRI and subsequent contrast washout in the portal venous phase. Because HCA has no bile ducts, uptake of biliary-specific contrast is minimal in the delayed phase.[50]
The most sensitive imaging technique to assess the presence and degree of hepatic steatosis is in and out gradient recalled echo (GRE) MRI. However, a liver biopsy is the gold standard method to evaluate the presence of inflammatory and early fibrotic changes.[50] Magnetic resonance elastography is a newer technique introduced originally as ultrasound elastography, which can measure liver stiffness and degree of fibrosis.[51] Magnetic resonance elastography does not have the possible sampling bias in liver biopsy. It has better sensitivity and specificity compared to ultrasound elastography, which has limitations in obese patients and patients with ascites. Iron overload and accumulation in hepatic and hepatic Kupffer cells can occur in hemochromatosis and hemosiderosis. The liver is the primary organ of deposition and can present as hepatomegaly as the first sign of disease.[52] It can be seen as hypointense compared to paraspinal muscle signal in T1 and T2 Weighted and GRE T2 weighting MRI.[53]
Hepatic hemangioma is the most common benign liver lesion that can be categorized into typical and atypical hemangiomas. A typical hepatic hemangioma can be appreciated on non-contrast CT as a hypo-attenuating lesion most of the time. In arterial phase contrast-enhanced CT, these neoplasms present as a lesion with nodular enhancement in the periphery. Portal venous and delayed phases show continuous enhancement of the periphery with contrast filling the central part.[54] Hemangioma is hypointense on T1 imaging and hyperintense on T2 imaging. Gadolinium-enhanced contrast T1 imaging often shows nodular enhancement of the periphery with a progressive enhancement of the central part and retaining the contrast in delayed imaging. Hepatobiliary contrast T1 imaging is non-specific and may not be helpful. Two main categories for atypical hemangiomas are giant hepatic hemangiomas and flash-filling hepatic hemangiomas. Giant hemangiomas are remarkably large but non-neoplastic vascular lesions with radiologic manifestations similar to hepatic hemangiomas.[55] Flash-filling hepatic hemangiomas/venous malformations are smaller (mostly less than 2 cm) and have a hyperechoic appearance in the ultrasound.[56] They are hypo- or iso-dense in noncontract imaging and demonstrate a fast and homogenous enhancement in the arterial phase while retaining the contrast in the late phase. Flash-filling hemangiomas have similar imaging manifestations in CEUS and gadolinium-enhanced contrast MRI with quick, homogenous enhancement and no washout in the late phase.
Focal nodular hyperplasia (FNH) is the second most common benign liver mass with a hepatocellular origin. It has a central scar with fat and fibrous tissue contents and an increased density of hepatocytes in about 80% of FNH.[57] MRI has better sensitivity and specificity in diagnosing FNH than CT and ultrasound. FNH consists of hepatocytes, bile ducts, and Kupffer cells in an abnormal alignment. The ultrasound appearance of FNH includes subtle changes in the liver contour or parenchymal echogenicity.[57] FNH is iso- or hypointense in nonenhanced T1 imaging and has a homogenous enhancement on the arterial phase. FNH can be appreciated in multiphasic MRI with hyperintense and delayed enhancement in T2 weighted images due to retaining contrast in hepatocytes because of a central scar.[57]
Hepatic adenomas are rare and benign liver tumors with a hepatocellular origin, most commonly related to long-term oral contraceptive use.[58] Other factors precipitating hepatic adenomas are anabolic steroids, glycogen storage diseases, obesity, and metabolic syndrome.[59] Although hepatic adenomas are benign liver tumors, they have a potential risk of bleeding and malignant degeneration.[60] Surgical resection is the recommended treatment for hepatic adenomas. For patients in which resection is not feasible or not preferable, modifying risk factors, serial imaging, and monitoring alpha-fetoprotein levels are possible management options. Hepatic arterial embolization can be considered in select cases.[61]
A hepatic cyst is a common hepatic lesion with a cholangiocellular origin. Benign hepatic cyst(s) can be found in around 5% of the general population.[62] Its size can vary from microscopic to sometimes greater than 20 cm in diameter.[63] Ultrasound can characterize hepatic cysts as anechoic lesions with thin walls and the presence of posterior acoustic enhancement, which confirms the fluid content of the cyst and very thin septa in some cases. CT imaging can be appreciated as an area of low internal attenuation with a thin wall and the possible presence of thin septa. Cysts can be appreciated in the MRI as an area of homogenous low signal in T1-weighted imaging, homogenous high internal signal in T2-weighted imaging, and absence of enhancement with contrast.[64] See Image. Hepatic Cysts.
Hepatocellular carcinoma (HCC) is the most common primary liver tumor. Cirrhosis and chronic hepatitis are the major risk factors for the development of HCC. Alpha-fetoprotein becomes elevated in two-thirds of HCC. Elevated alpha-fetoprotein in the presence of cirrhosis is suggestive of HCC and can be seen in 70% of patients with HCC.[65][66] HCC can appear on ultrasound from focal hypoechoic, hyperechoic, and heterogeneous lesions. Most HCCs are hypervascular on color Doppler. In contrast-enhanced ultrasound, HCC can be appreciated with enhancement in the arterial phase and washout on the portal venous phase.[67] MRI and CT have 82% and 56% sensitivity in detecting HCC, respectively.[68][69] In CT, the enhancement pattern is similar to contrast-enhanced ultrasound. Because of the possible arterioportal shunt in HCC, focal fatty changes might be seen as focal fat deposition in the normal liver or focal fat sparing around the HCC in diffuse hepatic steatosis. HCC results from a regenerative nodule's progression to dysplastic nodules and high-grade dysplastic nodules to low-grade HCC. The portal vein feeds regenerative and dysplastic nodules, which are indistinguishable from each other on imaging, although dysplastic nodules are premalignant, and regenerative nodules are not. Most regenerative and dysplastic nodules are hypo-intense in T2- T2-weighted images and have variable intensity in T1- T1-weighted images. Regenerative nodules and low-grade dysplastic nodules are isointense in contrast-enhanced MRI.[70] [71]
Hepatocellular carcinoma (HCC) receives blood from the hepatic artery compared to healthy hepatic tissue that gets supply from the portal vein. Because of this characteristic, HCC can be appreciated in multiphasic, contrast-enhanced MRI by increasing contrast enhancement in the arterial phase and contrast washout in the portal phase with peripheral rim enhancement. HCC appears slightly hyperintense T2-weighted unenhanced MRI. The hepatic artery and portal vein feed cholangiocarcinoma, so there is an increasing contrast enhancement in both arterial and venous phases.[50][70]
Fibrolamellar carcinoma is a subtype of HCC and has a better prognosis than the typical HCC. These generally occur in younger patients and do not demonstrate elevated alpha-fetoprotein levels. Fibrolamellar HCC on imaging is usually large at diagnosis and does not have a capsule. Fibrolamellar carcinoma appears on T1- and T2-weighted MRI as a large, heterogeneous mass with central hypointensity due to central fibrotic scar.[72]
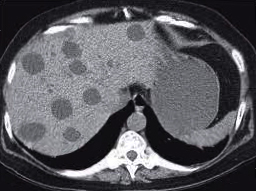
Metastatic liver lesions are usually hypointense on T1-weighted imaging and hyperintense on T2-weighted images. Most metastatic liver tumors are hypovascular, and the portal venous phase is the phase for visualization. Some metastatic tumors, including renal cell carcinoma, melanoma, and sarcoma, are hypervascular. These liver metastases can be appreciated best in the arterial phase.[73]
Ultrasonography
Ultrasonography is often the first imaging modality in evaluating and screening for focal and diffuse liver pathologies due to its availability, low cost, and noninvasive.[74] Liver pathology on ultrasound can present with different patterns such as generalized hypoechogenicity, as seen in acute hepatitis and diffuse malignant lesions, generalized hyperechogenicity, as seen in diffuse hepatic steatosis, and anechoic/hyperechoic lesions as seen in various benign and malignant lesions. Benign hyperechoic lesions include hemangioma, hepatic adenoma, focal nodular hyperplasia, and focal fatty changes. Malignant hyperechoic lesions include hepatic metastases from colorectal, renal cell carcinoma, melanoma, cholangiocarcinoma, and HCC.[75] Ultrasonography has limitations, including interobserver variability and decreased accuracy in patients with large body habitus.[49]
Ultrasonography, in combination with techniques and tools, has a significant role in diagnosing and treating different liver pathologies, for example:
- Ultrasound with color Doppler can provide information about the liver vasculature; for example, it can provide information about the portal vein flow and direction. Normally the portal vein blood flows away from the liver (hepatopetal flow). Retrograde flow from the periphery to the central area can suggest liver cirrhosis with portal hypertension. Doppler ultrasound can assess the patency of the portal vein before transplant and evaluate hepatic artery thrombosis after the transplant.[76]
- The contrast-enhanced US (CEUS) usage has grown during the past few years. CEUS needs considerable training, and it is very dependent on operator skills. It can have 95.8% sensitivity and 83.1% specificity in diagnosing focal liver lesions in healthy livers.[77] CEUS can provide information about vessel architecture, microcirculation within the focal liver lesions, and the malignant versus benign nature of the tumors.[78]
- A percutaneous liver biopsy is possible under ultrasound or CT guidance. Ultrasound is the modality of choice for both non-focal liver biopsy and focal, targeted biopsy in select cases with a good target window. A CT-guided biopsy is mainly reserved for lesion(s) not visible on ultrasound.[79] Indications for focal liver biopsies are unknown focal liver lesions and the presence of metastasis in the liver without a specific source. Indications for non-focal liver biopsy are the staging of parenchymal diseases like cirrhosis, primary biliary cirrhosis, and non-alcoholic fatty liver disease, determining the presence of rejection after liver transplant, assessment of hepatic storage disease, and the presence of abnormal liver function tests without other findings of any specific etiology. In cases of coagulopathy and severe ascites, transjugular liver biopsy is the alternate choice of percutaneous biopsy.[80]
- Ultrasound-guided percutaneous drainage can be useful for any abnormal fluid collection, such as abscesses of different etiologies. There are some advantages and disadvantages in comparison to CT-guided percutaneous drainage. The advantages are no exposure to ionizing radiation, flexible study with more control on the needle insertion, and less personnel needed for the procedure. It has limitations in deep lesions not visible on ultrasound, and bowel gas may limit the field of view.[81]
- Endoscopic ultrasound (EUS) has become a diagnostic and therapeutic method in different gastrointestinal and pancreaticobiliary diseases, including diagnosis and therapeutic use in portal hypertensive diseases like esophageal varices, EUS-guided focal liver lesions biopsy, EUS-guided portal venous access, EUS-guided portal vein embolization to induce lobar atrophy in the liver, and EUS-guided portal vein injection of chemotherapy. EUS is more sensitive than cross-sectional imaging in identifying small lesions less than 10 mm.[82]
- Ultrasound-based elastography is a noninvasive method to assess hepatic fibrosis instead of liver biopsy and predict complications in patients with liver cirrhosis. The most common techniques are the shear wave elastography (SWE) technique and acoustic radiation force impulse techniques. Shear wave elastography can be performed using several methods, including transient elastography, point-SWE, and 2-dimensional (2D)-SWE.[83]
A cirrhotic liver can appear hyperechoic on ultrasound. The sensitivity of diagnosing cirrhosis by ultrasound can vary and be as high as 91%.[84] The presence of signs and symptoms of portal hypertension, such as increased spleen or portal vein diameter, can increase the sensitivity of ultrasound in later stages of cirrhosis.[3] A hypervascular nodule in CEUS has a higher specificity for small HCC, less than 2 cm, than CT and MRI studies. Liver metastasis appearance mainly depends on the primary source of the tumor. It can appear as hypoechoic or hyperechoic lesions. The presence of a hyperechoic halo sign is suggestive of a malignant lesion.[85] In CEUS, metastasis has variable echogenicity in the arterial phase, but the washout appearance on the portal phase can help detect malignant lesions.[86]
One of the important applications of the Doppler ultrasound is in post-transplant patients. Hepatic arterial thrombosis is the most common vascular complication after a liver transplant. The resistive index (RI) is a Doppler parameter to evaluate it, measured by subtraction of peak systolic velocity from end-diastolic velocity, divided by peak systolic velocity.[87] The RI normal range is between 0.55 to 0.8. Low RI is more specific in patients with a liver transplant, which can be related to partial arterial obstruction in the proximal part of the artery, peripheral vascular shunts, and portal hypertension. In comparison, high RI can be related to microvascular compression, disease, or physiologic.[88]
Hemangioma is the most common benign liver lesion. It is usually an incidental finding in asymptomatic patients as the most common hyperechoic liver lesion.[54] It can appear on ultrasound as a hyperechoic lesion with a distinct border and peripheral feeding vessel on color Doppler.[56][89] A hemangioma appears in the arterial phase of CEUS with peripheral nodular enhancement, portal venous phase, and late phase with continuous contrast filling and changing to a hyperechoic lesion. In asymptomatic patients without any history of malignancy, diagnosis of hemangioma can be made solely based on imaging manifestation of a single, well-defined, homogenously hyperechoic lesion in the ultrasound; no further workup is necessary.[90]
The imaging manifestation of viral hepatitis in ultrasound can range from normal to nonspecific findings of hepatomegaly, gallbladder wall thickening, fluid accumulation around the portal vein, or generalized hypoechogenicity.[91] The diagnosis is mainly made based on clinical features and serology testing.
Nuclear Medicine
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are the nuclear imaging techniques used more frequently to diagnose liver malignancy and evaluate response to treatment metastasis, recurrence, and prognosis. F-fluorodeoxyglucose (FDG)-PET and C-acetate PET are more common nuclear imaging modalities in diagnosing liver malignancy.[92] Although it has high sensitivity, the false positive rate is high due to detecting any focal hypermetabolism area. The sensitivity is low in the detection of lesions smaller than 1 cm. Transarterial radioembolization and selective internal radiotherapy are theragnosis methods in the nuclear medicine field, and PET or SPECT is used simultaneously for diagnosis and treatment.[93]
Angiography
Multidetector computed tomography angiography (MDCT) is an imaging method to assess liver neoplasm for staging and surgical planning by providing information about hepatic vascular anatomy and parenchymal pathology. Biphasic hepatic CT with arterial and portal venous phases can appreciate hypervascular tumors with increased sensitivity than the portal phase alone. MDCT angiography has been the imaging modality of choice for living donor liver transplantation and evaluating any arterial complications after the transplant.[94]
Transarterial chemoembolization therapy (TACE) is a method in which chemotherapy is administered directly into the hepatic artery, feeding the tumor and resulting in necrosis. Its primary use is treating HCC and specific liver metastasis, such as colorectal metastases and cholangiocarcinoma. It can also be useful for unresectable HCC.[95]
Patient Positioning
Ultrasound is the main liver imaging technique, and patient positioning and operator expertise are important factors in optimizing the study.[96]
Clinical Significance
The first imaging modality to evaluate liver pathology depends on the patient's clinical situation, the availability of different modalities, the technician's familiarity with the test, and the physician's familiarity with the test. Ultrasound and CT remain the first imaging modality to assess the diffuse and focal liver lesion for screening and characterization. Based on the findings of ultrasound and CT, further workup and imaging can occur.
Hepatic steatosis or fatty liver is the most common abnormal imaging finding in liver imaging, which can be related to alcoholic liver disease and nonalcoholic liver disease, including obesity, insulin resistance, diabetes, hyperlipidemia, and hypertension. Nonalcoholic fatty liver disease can progress from simple fatty liver disease to nonalcoholic steatohepatitis (NASH) and cirrhosis. Regardless of etiology, ultrasound findings of fatty liver disease include increased echogenicity of the liver compared to the renal cortex, loss of normal echogenicity of the portal triad in the periphery of the liver, and poor visualization of the diaphragm. Transient elastography is the most validated technique with more sensitivity and specificity (87% and 91%, respectively) in diagnosing cirrhosis than diagnosing significant fibrosis (70% and 84%, respectively).[97]
Risk stratification is essential in patients with liver cirrhosis to estimate complications risks such as portal hypertension consequences and liver cancer. Liver serology and elastography are noninvasive methods to determine patients with advanced liver cirrhosis. A liver biopsy can provide information about the stages of fibrosis. Elastography is a method that can show the increased stiffness of the parenchyma. Among the different approaches to elastography, vibration-controlled transient elastography is the most common type used worldwide.[3]
Liver Imaging Reporting and Data System (LI-RADS) is a comprehensive and standardized system for reporting and classifying liver lesions at an increased risk of developing into HCC.[98] LIRAD categorizes lesions into definitely benign, probably benign, probably or malignant, but not HCC specific, intermediate probability of malignancy, probably HCC, and HCC. It guides radiologists, clinicians, and surgeons for surveillance guidelines and recommendations if a biopsy is necessary.
Media
(Click Image to Enlarge)
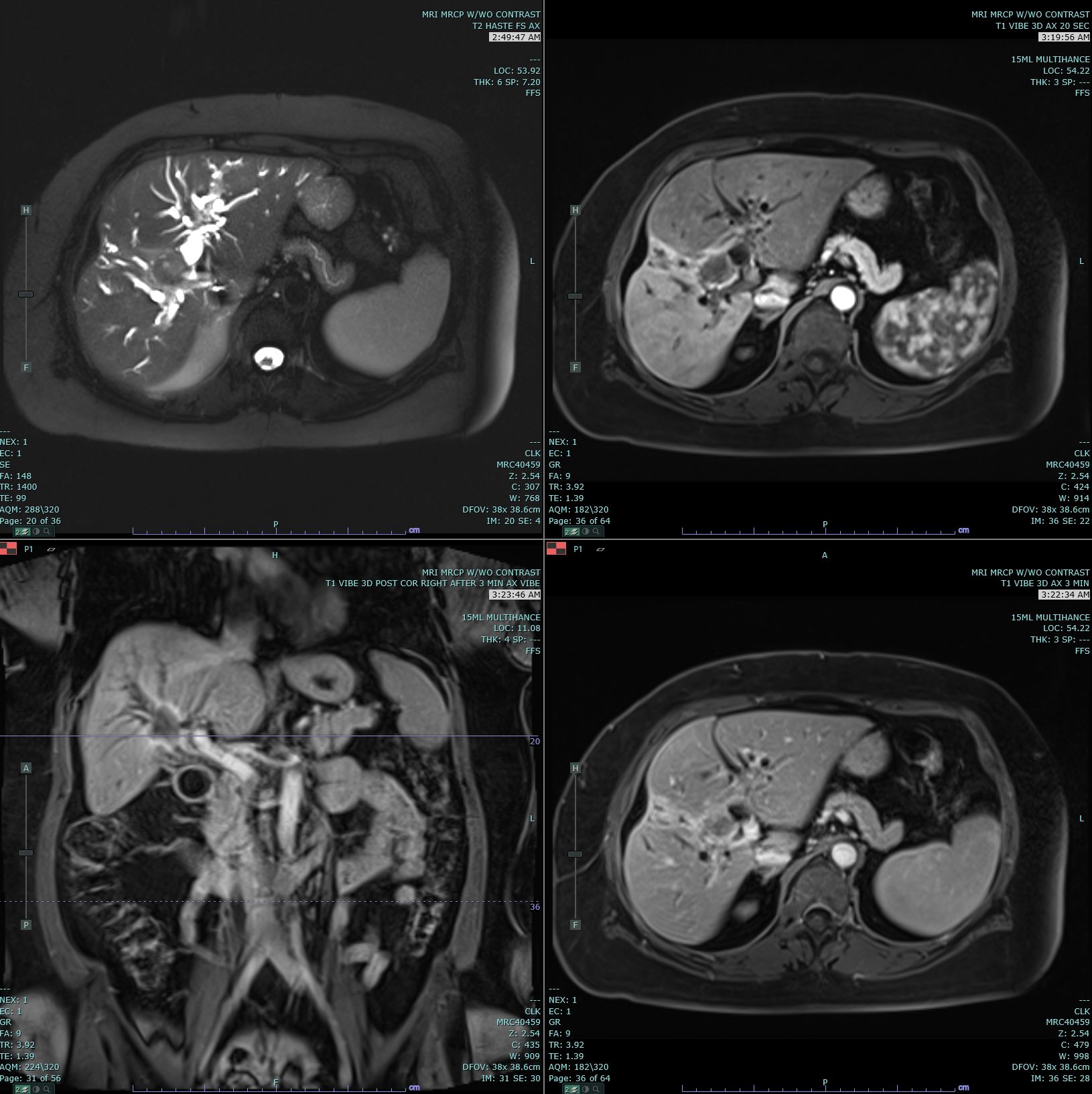
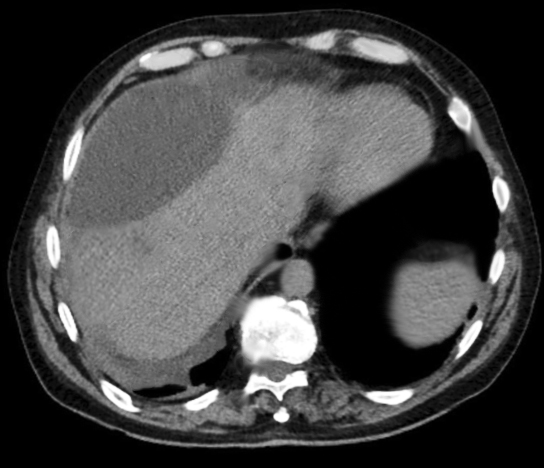
Cholangiocarcinoma. Axial T2 Haste, 20s, and 3-minute post-contrast T1 Vibe images are submitted. There is marked biliary dilatation, greatest in the left biliary tree. A delayed enhancing hilar mass is identified, which is biopsy proven to be cholangiocarcinoma. This mass has increased T2 signal in comparison to the background liver parenchyma. Mild capsular retraction in the right hepatic lobe is noted.
Contributed by M Smith, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scandinavian journal of gastroenterology. 1986 Jan:21(1):109-13 [PubMed PMID: 3952445]
Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. The American journal of gastroenterology. 2006 Jan:101(1):76-82 [PubMed PMID: 16405537]
Level 2 (mid-level) evidenceTapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. The New England journal of medicine. 2017 Aug 24:377(8):756-768. doi: 10.1056/NEJMra1610570. Epub [PubMed PMID: 28834467]
Maniam S,Szklaruk J, Magnetic resonance imaging: Review of imaging techniques and overview of liver imaging. World journal of radiology. 2010 Aug 28; [PubMed PMID: 21160685]
Level 3 (low-level) evidenceZhang YN, Fowler KJ, Hamilton G, Cui JY, Sy EZ, Balanay M, Hooker JC, Szeverenyi N, Sirlin CB. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. The British journal of radiology. 2018 Sep:91(1089):20170959. doi: 10.1259/bjr.20170959. Epub 2018 Jun 6 [PubMed PMID: 29722568]
Level 3 (low-level) evidenceFasel JH, Majno PE, Peitgen HO. Liver segments: an anatomical rationale for explaining inconsistencies with Couinaud's eight-segment concept. Surgical and radiologic anatomy : SRA. 2010 Oct:32(8):761-5. doi: 10.1007/s00276-010-0626-4. Epub 2010 Jan 29 [PubMed PMID: 20111966]
Fischer L, Cardenas C, Thorn M, Benner A, Grenacher L, Vetter M, Lehnert T, Klar E, Meinzer HP, Lamadé W. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. Journal of computer assisted tomography. 2002 Nov-Dec:26(6):962-7 [PubMed PMID: 12488744]
Lafortune M,Madore F,Patriquin H,Breton G, Segmental anatomy of the liver: a sonographic approach to the Couinaud nomenclature. Radiology. 1991 Nov; [PubMed PMID: 1924786]
Pauli EM, Staveley-O'Carroll KF, Brock MV, Efron DT, Efron G. A handy tool to teach segmental liver anatomy to surgical trainees. Archives of surgery (Chicago, Ill. : 1960). 2012 Aug:147(8):692-3. doi: 10.1001/archsurg.2012.689. Epub [PubMed PMID: 22911067]
Itoh S, Ikeda M, Achiwa M, Satake H, Iwano S, Ishigaki T. Late-arterial and portal-venous phase imaging of the liver with a multislice CT scanner in patients without circulatory disturbances: automatic bolus tracking or empirical scan delay? European radiology. 2004 Sep:14(9):1665-73 [PubMed PMID: 15067427]
Level 1 (high-level) evidenceBrancatelli G, Federle MP, Baron RL, Lagalla R, Midiri M, Vilgrain V. Arterially enhancing liver lesions: significance of sustained enhancement on hepatic venous and delayed phase with magnetic resonance imaging. Journal of computer assisted tomography. 2007 Jan-Feb:31(1):116-24 [PubMed PMID: 17259843]
Rammohan A,Sathyanesan J,Ramaswami S,Lakshmanan A,Senthil-Kumar P,Srinivasan UP,Ramasamy R,Ravichandran P, Embolization of liver tumors: Past, present and future. World journal of radiology. 2012 Sep 28 [PubMed PMID: 23024842]
Nakashima J, Bordoni B. Anatomy, Abdomen and Pelvis: Hepatoduodenal Ligament. StatPearls. 2024 Jan:(): [PubMed PMID: 32119375]
Schmidt S, Demartines N, Soler L, Schnyder P, Denys A. Portal vein normal anatomy and variants: implication for liver surgery and portal vein embolization. Seminars in interventional radiology. 2008 Jun:25(2):86-91. doi: 10.1055/s-2008-1076688. Epub [PubMed PMID: 21326549]
Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World journal of gastroenterology. 2010 Dec 28:16(48):6046-57 [PubMed PMID: 21182219]
Miñano C,Garcia-Tsao G, Clinical pharmacology of portal hypertension. Gastroenterology clinics of North America. 2010 Sep; [PubMed PMID: 20951924]
Kennedy P, Bane O, Hectors SJ, Fischman A, Schiano T, Lewis S, Taouli B. Noninvasive imaging assessment of portal hypertension. Abdominal radiology (New York). 2020 Nov:45(11):3473-3495. doi: 10.1007/s00261-020-02729-7. Epub 2020 Sep 14 [PubMed PMID: 32926209]
Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. American heart journal. 2000 Jul:140(1):111-20 [PubMed PMID: 10874271]
Wells ML, Fenstad ER, Poterucha JT, Hough DM, Young PM, Araoz PA, Ehman RL, Venkatesh SK. Imaging Findings of Congestive Hepatopathy. Radiographics : a review publication of the Radiological Society of North America, Inc. 2016 Jul-Aug:36(4):1024-37. doi: 10.1148/rg.2016150207. Epub 2016 Jun 10 [PubMed PMID: 27284758]
Afif AM, Chang JP, Wang YY, Lau SD, Deng F, Goh SY, Pwint MK, Ooi CC, Venkatanarasimha N, Lo RH. A sonographic Doppler study of the hepatic vein, portal vein and hepatic artery in liver cirrhosis: Correlation of hepatic hemodynamics with clinical Child Pugh score in Singapore. Ultrasound (Leeds, England). 2017 Nov:25(4):213-221. doi: 10.1177/1742271X17721265. Epub 2017 Aug 3 [PubMed PMID: 29163657]
Desautels CN, Tierney DM, Rossi F, Rosborough TK. Case report: an unrecognized etiology of transient gallbladder pain in heart failure diagnosed with internist-performed point-of-care ultrasound. Critical ultrasound journal. 2015:7():2. doi: 10.1186/s13089-014-0019-8. Epub 2015 Jan 21 [PubMed PMID: 25852843]
Level 3 (low-level) evidenceTeefey SA, Baron RL, Bigler SA. Sonography of the gallbladder: significance of striated (layered) thickening of the gallbladder wall. AJR. American journal of roentgenology. 1991 May:156(5):945-7 [PubMed PMID: 2017956]
Level 2 (mid-level) evidenceSmith A, Baumgartner K, Bositis C. Cirrhosis: Diagnosis and Management. American family physician. 2019 Dec 15:100(12):759-770 [PubMed PMID: 31845776]
Kartalis N, Brehmer K, Loizou L. Multi-detector CT: Liver protocol and recent developments. European journal of radiology. 2017 Dec:97():101-109. doi: 10.1016/j.ejrad.2017.10.026. Epub 2017 Oct 27 [PubMed PMID: 29153359]
Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014 Aug:272(2):322-44. doi: 10.1148/radiol.14130091. Epub [PubMed PMID: 25058132]
Sween S, Samar C, Binu SM. Triple-phase MDCT of liver: Scan protocol modification to obtain optimal vascular and lesional contrast. The Indian journal of radiology & imaging. 2018 Jul-Sep:28(3):315-319. doi: 10.4103/ijri.IJRI_75_18. Epub [PubMed PMID: 30319208]
Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer imaging : the official publication of the International Cancer Imaging Society. 2013 Feb 8:12(3):530-47. doi: 10.1102/1470-7330.2012.0044. Epub 2013 Feb 8 [PubMed PMID: 23400006]
Kojiro M, Roskams T. Early hepatocellular carcinoma and dysplastic nodules. Seminars in liver disease. 2005:25(2):133-42 [PubMed PMID: 15918142]
Ringe KI, Wacker F. Radiological diagnosis in cholangiocarcinoma: Application of computed tomography, magnetic resonance imaging, and positron emission tomography. Best practice & research. Clinical gastroenterology. 2015 Apr:29(2):253-65. doi: 10.1016/j.bpg.2015.02.004. Epub 2015 Feb 17 [PubMed PMID: 25966426]
Wu M, Sharma PG, Grajo JR. The Echogenic Liver: Steatosis and Beyond. Ultrasound quarterly. 2020 Sep 21:37(4):308-314. doi: 10.1097/RUQ.0000000000000510. Epub 2020 Sep 21 [PubMed PMID: 32956242]
Ahmed AM, Ebid ME, Ajlan AM, Al-Mallah MH. Low-dose attenuation correction in diagnosis of non-alcoholic fatty liver disease. Abdominal radiology (New York). 2017 Oct:42(10):2454-2459. doi: 10.1007/s00261-017-1166-8. Epub [PubMed PMID: 28470401]
Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics : a review publication of the Radiological Society of North America, Inc. 2006 Nov-Dec:26(6):1637-53 [PubMed PMID: 17102041]
Kalra N, Duseja A, Das A, Dhiman RK, Virmani V, Chawla Y, Singh P, Khandelwal N. Chemical shift magnetic resonance imaging is helpful in detecting hepatic steatosis but not fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Annals of hepatology. 2009 Jan-Mar:8(1):21-5 [PubMed PMID: 19221529]
Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR. American journal of roentgenology. 2009 Jul:193(1):14-27. doi: 10.2214/AJR.09.2601. Epub [PubMed PMID: 19542391]
Yoshikawa J, Matsui O, Takashima T, Sugiura H, Katayama K, Nishida Y, Tsuji M. Focal fatty change of the liver adjacent to the falciform ligament: CT and sonographic findings in five surgically confirmed cases. AJR. American journal of roentgenology. 1987 Sep:149(3):491-4 [PubMed PMID: 3303875]
Level 3 (low-level) evidenceDécarie PO, Lepanto L, Billiard JS, Olivié D, Murphy-Lavallée J, Kauffmann C, Tang A. Fatty liver deposition and sparing: a pictorial review. Insights into imaging. 2011 Oct:2(5):533-538 [PubMed PMID: 22347973]
Bader TR, Braga L, Semelka RC. Exophytic benign tumors of the liver: appearance on MRI. Magnetic resonance imaging. 2001 Jun:19(5):623-8 [PubMed PMID: 11672619]
Özcan HN, Haliloğlu M, Sökmensüer C, Akata D, Özmen M, Karçaaltıncaba M. Imaging for abdominal involvement in amyloidosis. Diagnostic and interventional radiology (Ankara, Turkey). 2017 Jul-Aug:23(4):282-285. doi: 10.5152/dir.2017.16484. Epub [PubMed PMID: 28498108]
Schilsky ML. Wilson Disease: Diagnosis, Treatment, and Follow-up. Clinics in liver disease. 2017 Nov:21(4):755-767. doi: 10.1016/j.cld.2017.06.011. Epub 2017 Aug 10 [PubMed PMID: 28987261]
Cordella A, Bertolini G. Multiphase multidetector-row CT reveals different patterns of hepatic portal venous gas and pneumobilia. Veterinary radiology & ultrasound : the official journal of the American College of Veterinary Radiology and the International Veterinary Radiology Association. 2021 Jan:62(1):68-75. doi: 10.1111/vru.12928. Epub 2020 Nov 27 [PubMed PMID: 33245597]
Abboud B, El Hachem J, Yazbeck T, Doumit C. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World journal of gastroenterology. 2009 Aug 7:15(29):3585-90 [PubMed PMID: 19653334]
Sherman SC, Tran H. Pneumobilia: benign or life-threatening. The Journal of emergency medicine. 2006 Feb:30(2):147-53 [PubMed PMID: 16567248]
Level 3 (low-level) evidenceKhim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. British medical bulletin. 2019 Dec 11:132(1):45-52. doi: 10.1093/bmb/ldz032. Epub [PubMed PMID: 31836890]
Méndez RJ, Schiebler ML, Outwater EK, Kressel HY. Hepatic abscesses: MR imaging findings. Radiology. 1994 Feb:190(2):431-6 [PubMed PMID: 8284394]
Level 2 (mid-level) evidenceMortelé KJ, Segatto E, Ros PR. The infected liver: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Jul-Aug:24(4):937-55 [PubMed PMID: 15256619]
Level 3 (low-level) evidenceMasood A, Sallah S. Chronic disseminated candidiasis in patients with acute leukemia: emphasis on diagnostic definition and treatment. Leukemia research. 2005 May:29(5):493-501 [PubMed PMID: 15755501]
Lima PH, Fan B, Bérubé J, Cerny M, Olivié D, Giard JM, Beauchemin C, Tang A. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR. American journal of roentgenology. 2019 Jul:213(1):17-25. doi: 10.2214/AJR.18.20341. Epub 2019 Apr 17 [PubMed PMID: 30995098]
Coenegrachts K. Magnetic resonance imaging of the liver: New imaging strategies for evaluating focal liver lesions. World journal of radiology. 2009 Dec 31:1(1):72-85. doi: 10.4329/wjr.v1.i1.72. Epub [PubMed PMID: 21160723]
O'Neill EK, Cogley JR, Miller FH. The ins and outs of liver imaging. Clinics in liver disease. 2015 Feb:19(1):99-121. doi: 10.1016/j.cld.2014.09.006. Epub 2014 Oct 29 [PubMed PMID: 25454299]
Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, Gambarini S, Donato F, Colagrande S. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012 Feb:262(2):520-9. doi: 10.1148/radiol.11101742. Epub [PubMed PMID: 22282184]
Manduca A, Bayly PJ, Ehman RL, Kolipaka A, Royston TJ, Sack I, Sinkus R, Van Beers BE. MR elastography: Principles, guidelines, and terminology. Magnetic resonance in medicine. 2021 May:85(5):2377-2390. doi: 10.1002/mrm.28627. Epub 2020 Dec 9 [PubMed PMID: 33296103]
Shankaran K, Gill HH, Desai HG. Genetic hemochromatosis presenting as asymptomatic hepatomegaly. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 1994 Apr:13(2):64-5 [PubMed PMID: 8206540]
Level 3 (low-level) evidenceLabranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D, Billiard JS, Yokoo T, Tang A. Liver Iron Quantification with MR Imaging: A Primer for Radiologists. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018 Mar-Apr:38(2):392-412. doi: 10.1148/rg.2018170079. Epub [PubMed PMID: 29528818]
Klotz T, Montoriol PF, Da Ines D, Petitcolin V, Joubert-Zakeyh J, Garcier JM. Hepatic haemangioma: common and uncommon imaging features. Diagnostic and interventional imaging. 2013 Sep:94(9):849-59. doi: 10.1016/j.diii.2013.04.008. Epub 2013 Jun 21 [PubMed PMID: 23796395]
Hoekstra LT, Bieze M, Erdogan D, Roelofs JJ, Beuers UH, van Gulik TM. Management of giant liver hemangiomas: an update. Expert review of gastroenterology & hepatology. 2013 Mar:7(3):263-8. doi: 10.1586/egh.13.10. Epub [PubMed PMID: 23445235]
Mamone G, Di Piazza A, Carollo V, Cannataci C, Cortis K, Bartolotta TV, Miraglia R. Imaging of hepatic hemangioma: from A to Z. Abdominal radiology (New York). 2020 Mar:45(3):672-691. doi: 10.1007/s00261-019-02294-8. Epub [PubMed PMID: 31686179]
Hussain SM, Terkivatan T, Zondervan PE, Lanjouw E, de Rave S, Ijzermans JN, de Man RA. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Jan-Feb:24(1):3-17; discussion 18-9 [PubMed PMID: 14730031]
Haring MPD, Gouw ASH, de Haas RJ, Cuperus FJC, de Jong KP, de Meijer VE. The effect of oral contraceptive pill cessation on hepatocellular adenoma diameter: A retrospective cohort study. Liver international : official journal of the International Association for the Study of the Liver. 2019 May:39(5):905-913. doi: 10.1111/liv.14074. Epub 2019 Mar 19 [PubMed PMID: 30773766]
Level 2 (mid-level) evidenceShreenath AP, Grant LM, Kahloon A. Hepatocellular Adenoma. StatPearls. 2024 Jan:(): [PubMed PMID: 30020636]
Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008 Apr:21(4):491-7. doi: 10.1038/modpathol.2008.8. Epub 2008 Feb 1 [PubMed PMID: 18246041]
Nasser F, Affonso BB, Galastri FL, Odisio BC, Garcia RG. Minimally invasive treatment of hepatic adenoma in special cases. Einstein (Sao Paulo, Brazil). 2013 Dec:11(4):524-7 [PubMed PMID: 24488396]
Level 3 (low-level) evidenceSeo JK, Kim SH, Lee SH, Park JK, Woo SM, Jeong JB, Hwang JH, Ryu JK, Kim JW, Jeong SH, Kim YT, Yoon YB, Lee KU, Kim SH, Kim MA. Appropriate diagnosis of biliary cystic tumors: comparison with atypical hepatic simple cysts. European journal of gastroenterology & hepatology. 2010 Aug:22(8):989-96. doi: 10.1097/MEG.0b013e328337c971. Epub [PubMed PMID: 20300006]
Level 2 (mid-level) evidenceAsuquo M, Nwagbara V, Agbor C, Otobo F, Omotoso A. Giant simple hepatic cyst: a case report and review of relevant literature. African health sciences. 2015 Mar:15(1):293-8. doi: 10.4314/ahs.v15i1.40. Epub [PubMed PMID: 25834563]
Level 3 (low-level) evidenceAnderson MA, Dhami RS, Fadzen CM, Molina G, Taylor MS, Deshpande V, Qadan M, Catalano OA, Ferrone CR, Mojtahed A. CT and MRI features differentiating mucinous cystic neoplasms of the liver from pathologically simple cysts. Clinical imaging. 2021 Aug:76():46-52. doi: 10.1016/j.clinimag.2021.01.036. Epub 2021 Feb 3 [PubMed PMID: 33549919]
Mizejewski GJ. Levels of alpha-fetoprotein during pregnancy and early infancy in normal and disease states. Obstetrical & gynecological survey. 2003 Dec:58(12):804-26 [PubMed PMID: 14668662]
Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernández-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC cancer. 2007 Feb 8:7():28 [PubMed PMID: 17288606]
Numata K, Luo W, Morimoto M, Kondo M, Kunishi Y, Sasaki T, Nozaki A, Tanaka K. Contrast enhanced ultrasound of hepatocellular carcinoma. World journal of radiology. 2010 Feb 28:2(2):68-82. doi: 10.4329/wjr.v2.i2.68. Epub [PubMed PMID: 21160920]
Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. Journal of hepatology. 2021 Jul:75(1):108-119. doi: 10.1016/j.jhep.2021.01.041. Epub 2021 Feb 3 [PubMed PMID: 33548385]
Level 1 (high-level) evidenceTakayasu K, Furukawa H, Wakao F, Muramatsu Y, Abe H, Terauchi T, Winter TC 3rd, Sakamoto M, Hirohashi S. CT diagnosis of early hepatocellular carcinoma: sensitivity, findings, and CT-pathologic correlation. AJR. American journal of roentgenology. 1995 Apr:164(4):885-90 [PubMed PMID: 7726041]
Level 2 (mid-level) evidenceWillatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008 May:247(2):311-30. doi: 10.1148/radiol.2472061331. Epub [PubMed PMID: 18430871]
Bolog N, Andreisek G, Oancea I, Mangrau A. CT and MR imaging of hepatocellular carcinoma. Journal of gastrointestinal and liver diseases : JGLD. 2011 Jun:20(2):181-9 [PubMed PMID: 21725516]
Ganeshan D, Szklaruk J, Kundra V, Kaseb A, Rashid A, Elsayes KM. Imaging features of fibrolamellar hepatocellular carcinoma. AJR. American journal of roentgenology. 2014 Mar:202(3):544-52. doi: 10.2214/AJR.13.11117. Epub [PubMed PMID: 24555590]
Namasivayam S, Martin DR, Saini S. Imaging of liver metastases: MRI. Cancer imaging : the official publication of the International Cancer Imaging Society. 2007:7(1):2-9 [PubMed PMID: 17293303]
Phisalprapa P, Supakankunti S, Charatcharoenwitthaya P, Apisarnthanarak P, Charoensak A, Washirasaksiri C, Srivanichakorn W, Chaiyakunapruk N. Cost-effectiveness analysis of ultrasonography screening for nonalcoholic fatty liver disease in metabolic syndrome patients. Medicine. 2017 Apr:96(17):e6585. doi: 10.1097/MD.0000000000006585. Epub [PubMed PMID: 28445256]
Minami Y, Kudo M. Hepatic malignancies: Correlation between sonographic findings and pathological features. World journal of radiology. 2010 Jul 28:2(7):249-56. doi: 10.4329/wjr.v2.i7.249. Epub [PubMed PMID: 21160664]
Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Apr:9(4):746-57. doi: 10.1111/j.1600-6143.2008.02541.x. Epub 2009 Mar 2 [PubMed PMID: 19298450]
Level 1 (high-level) evidenceStrobel D, Seitz K, Blank W, Schuler A, Dietrich C, von Herbay A, Friedrich-Rust M, Kunze G, Becker D, Will U, Kratzer W, Albert FW, Pachmann C, Dirks K, Strunk H, Greis C, Bernatik T. Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2008 Oct:29(5):499-505. doi: 10.1055/s-2008-1027806. Epub [PubMed PMID: 19241506]
Level 1 (high-level) evidenceBattaglia V, Cervelli R. Liver investigations: Updating on US technique and contrast-enhanced ultrasound (CEUS). European journal of radiology. 2017 Nov:96():65-73. doi: 10.1016/j.ejrad.2017.08.029. Epub 2017 Sep 21 [PubMed PMID: 29103478]
Vijayaraghavan GR, David S, Bermudez-Allende M, Sarwat H. Imaging-guided Parenchymal Liver Biopsy: How We Do It. Journal of clinical imaging science. 2011:1():30. doi: 10.4103/2156-7514.82082. Epub 2011 Jun 15 [PubMed PMID: 21966627]
Adnan A, Sheth RA. Image-guided Percutaneous Biopsy of the Liver. Techniques in vascular and interventional radiology. 2021 Dec:24(4):100773. doi: 10.1016/j.tvir.2021.100773. Epub 2021 Oct 3 [PubMed PMID: 34895710]
Smith BC, Desmond PV. Outpatient liver biopsy using ultrasound guidance and the Biopty gun is safe and cost effective. Australian and New Zealand journal of medicine. 1995 Jun:25(3):209-11 [PubMed PMID: 7487687]
Shah ND, Baron TH. Endoscopic ultrasound and the liver: current applications and beyond. Journal of hepato-biliary-pancreatic sciences. 2018 Mar:25(3):171-180. doi: 10.1002/jhbp.528. Epub 2018 Jan 15 [PubMed PMID: 29334698]
Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, Taouli B. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology. 2018 Mar:286(3):738-763. doi: 10.1148/radiol.2018170601. Epub [PubMed PMID: 29461949]
Simonovský V. The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. The British journal of radiology. 1999 Jan:72(853):29-34 [PubMed PMID: 10341686]
Level 1 (high-level) evidenceWernecke K, Henke L, Vassallo P, von Bassewitz DB, Diederich S, Peters PE, Edel G. Pathologic explanation for hypoechoic halo seen on sonograms of malignant liver tumors: an in vitro correlative study. AJR. American journal of roentgenology. 1992 Nov:159(5):1011-6 [PubMed PMID: 1329455]
Dănilă M, Popescu A, Sirli R, Sporea I, Martie A, Sendroiu M. Contrast enhanced ultrasound (CEUS) in the evaluation of liver metastases. Medical ultrasonography. 2010 Sep:12(3):233-7 [PubMed PMID: 21203602]
Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003 Jun:9(6):612-20 [PubMed PMID: 12783404]
García-Criado A, Gilabert R, Salmerón JM, Nicolau C, Vilana R, Bianchi L, Buñesch L, García-Valdecasas JC, Rimola A, Brú C. Significance of and contributing factors for a high resistive index on Doppler sonography of the hepatic artery immediately after surgery: prognostic implications for liver transplant recipients. AJR. American journal of roentgenology. 2003 Sep:181(3):831-8 [PubMed PMID: 12933490]
Level 2 (mid-level) evidenceBajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T. Hepatic hemangioma -review-. Journal of medicine and life. 2015:8 Spec Issue(Spec Issue):4-11 [PubMed PMID: 26361504]
Ito H, Tsujimoto F, Nakajima Y, Igarashi G, Okamura T, Sakurai M, Nobuoka S, Otsubo T. Sonographic characterization of 271 hepatic hemangiomas with typical appearance on CT imaging. Journal of medical ultrasonics (2001). 2012 Apr:39(2):61-8. doi: 10.1007/s10396-011-0339-2. Epub 2012 Jan 12 [PubMed PMID: 27278845]
Park SJ, Kim JD, Seo YS, Park BJ, Kim MJ, Um SH, Kim CH, Yim HJ, Baik SK, Jung JY, Keum B, Jeen YT, Lee HS, Chun HJ, Kim CD, Ryu HS. Computed tomography findings for predicting severe acute hepatitis with prolonged cholestasis. World journal of gastroenterology. 2013 Apr 28:19(16):2543-9. doi: 10.3748/wjg.v19.i16.2543. Epub [PubMed PMID: 23674857]
Level 2 (mid-level) evidenceBlechacz B, Gores GJ. Positron emission tomography scan for a hepatic mass. Hepatology (Baltimore, Md.). 2010 Dec:52(6):2186-91. doi: 10.1002/hep.24002. Epub [PubMed PMID: 20967825]
Eo JS, Paeng JC, Lee DS. Nuclear imaging for functional evaluation and theragnosis in liver malignancy and transplantation. World journal of gastroenterology. 2014 May 14:20(18):5375-88. doi: 10.3748/wjg.v20.i18.5375. Epub [PubMed PMID: 24833867]
Price M, Patino M, Sahani D. Computed Tomography Angiography of the Hepatic, Pancreatic, and Splenic Circulation. Radiologic clinics of North America. 2016 Jan:54(1):55-70. doi: 10.1016/j.rcl.2015.08.009. Epub [PubMed PMID: 26654391]
Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer treatment reviews. 2019 Jan:72():28-36. doi: 10.1016/j.ctrv.2018.11.002. Epub 2018 Nov 12 [PubMed PMID: 30447470]
Kruskal JB, Newman PA, Sammons LG, Kane RA. Optimizing Doppler and color flow US: application to hepatic sonography. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 May-Jun:24(3):657-75 [PubMed PMID: 15143220]
Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007 Oct:5(10):1214-20 [PubMed PMID: 17916549]
Level 1 (high-level) evidenceChernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018 Dec:289(3):816-830. doi: 10.1148/radiol.2018181494. Epub 2018 Sep 25 [PubMed PMID: 30251931]