Stentless Autograft/Homograft Aortic Valve Replacement
Stentless Autograft/Homograft Aortic Valve Replacement
Introduction
A valve is a mobile, thin sheet of tissue that ensures unidirectional flow through a passage. In the heart, valves can become insufficient or stenosed, leading to various health conditions. Valvular heart disease is a significant public health issue, affecting approximately 2.5% of the population. Among the various types of valvular heart disease, aortic valve disease, including aortic stenosis and regurgitation, is particularly prevalent. Damaged aortic valves are managed through medications, aortic valve repair, or surgical or transcatheter aortic valve replacement.[1][2] Selecting an appropriate valve replacement strategy is crucial and depends on multiple factors, such as patient preference, age, anticoagulation tolerance, risk of valve deterioration, anticoagulation status, and future pregnancy plans.
Prosthetic heart valves used for aortic valve replacement are either mechanical or bioprosthetic. Mechanical heart valves include disc, bileaflet, and ball-and-cage types; bioprosthetic valves are categorized into xenografts (from animals), homografts (from the same species), and autografts (from the same individual). Bioprosthetic valves may be stented on metallic support or directly attached to the aorta.[3] Stentless auto- and homograft aortic valve replacements have emerged as important surgical options for patients requiring valve replacement, offering distinct benefits compared to traditional stented prosthetic valves.
Although stent placement can reduce the aortic valve lumen and is not hemodynamically favorable, it does not significantly impact the clinical outcomes of stentless autograft and homograft valve types.[4] This finding underscores the importance of considering hemodynamic performance and other patient-specific factors when selecting a valve replacement strategy. Moreover, different types of bioprosthetic valves exhibit similar mortality and reoperation rates, further highlighting the need for individualized patient care.[5] Transcatheter aortic valve replacement offers a safer alternative for patients who aren't candidates for open-heart valve replacement surgery. However, stentless autograft and homograft aortic valve replacements remain valuable options for many patients, especially younger individuals and those with specific clinical indications.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Aortic Valve
The aortic valve is 1 of the 4 valves of the heart, located between the left ventricle (LV) and the ascending aorta. This tricuspid valve has 3 cusps or leaflets of different sizes—right, left, and noncoronary.[6] The noncoronary cusp can range from 14 to 28 mm, while the left and right coronary cusps can range from 12 to 25 mm. Results from one study found a mean geometric height of 19 mm for the tricuspid aortic valve.[7] These leaflets are thin, flexible, and attached to the aortic annulus, a fibrous ring that provides structural support. When the LV contracts, the aortic valve opens to allow blood to flow from the heart into the aorta and then to the rest of the body. During diastole, when the LV relaxes, the aortic valve closes to prevent the backflow of blood into the ventricle.
Aortic Regurgitation
Aortic regurgitation (AR) is the backflow of blood from the aorta into the LV during diastole due to inadequate aortic valve closure. Causes include:
- Valve leaflet abnormalities: Abnormalities include a congenital bicuspid valve (eg, having 2 leaflets instead of 3), degenerative changes, or endocarditis.
- Aortic root dilation: Conditions like Marfan syndrome, aortic dissection, or hypertension can cause dilation of the aortic root, pulling the leaflets apart and preventing proper closure.
Regurgitant flow increases the volume load on the LV, causing it to dilate and eventually hypertrophy. This volume overload leads to elevated left ventricular end-diastolic pressure, decreased cardiac output, and progressive LV dysfunction. AR leads to a diastolic blowing-type murmur in the third intercostal space on the left side along the sternal border. Patients with AR may experience symptoms such as palpitations, fatigue, dyspnea, and, in severe cases, heart failure.[8]
The American College of Cardiology/American Heart Association (ACC/AHA) provide guidelines that categorize AR into 4 stages: A, B, C, and D, based on valve anatomy, valve hemodynamics, LV response, and symptoms. The stages are:
- Stage A: At risk for AR
- Patients with conditions that predispose them to AR, such as bicuspid aortic valve, aortic dilatation, or history of endocarditis
- No significant valve dysfunction or regurgitation
- Stage B: Progressive AR
- Mild to moderate AR
- Normal LV size, function
- Asymptomatic or mild symptoms
- Echocardiographic findings:
- Effective regurgitant orifice area (EROA) <0.10 cm²
- Regurgitant volume <30 mL/beat
- Regurgitant fraction <30%
- Left ventricular end-diastolic dimension typically <60 mm
- Stage C: Asymptomatic severe AR
- C1: Asymptomatic severe AR with normal LV function
- Severe AR without symptoms
- Normal left ventricular ejection fraction (LVEF) ≥50%
- Echocardiographic findings:
- EROA ≥0.30 cm²
- Regurgitant volume ≥60 mL/beat
- Regurgitant fraction ≥50%
- Left ventricular end-diastolic diameter (LVEDD) ≥60 mm, but LVEF ≥50%
- C2: Asymptomatic severe AR with LV dysfunction
- Severe AR without symptoms
- LV dysfunction (LVEF <50%)
- Echocardiographic findings:
- The same criteria for AR severity as C1
- LVEDD ≥70 mm or LVEF <50%
- C1: Asymptomatic severe AR with normal LV function
- Stage D: Symptomatic severe AR
- D1: Symptomatic severe AR with normal LV function
- Severe AR with symptoms such as dyspnea, fatigue, or palpitations
- Normal LV function (LVEF ≥50%)
- Echocardiographic findings:
- EROA ≥0.30 cm²
- Regurgitant volume ≥60 mL/beat
- Regurgitant fraction ≥50%
- LVEDD ≥60 mm, but LVEF ≥50%
- D2: Symptomatic severe AR with LV dysfunction
- Severe AR with symptoms
- LV dysfunction (LVEF <50%)
- Echocardiographic findings:
- The same criteria for AR severity as D1
- LVEDD ≥70 mm or LVEF <50%
- D1: Symptomatic severe AR with normal LV function
Aortic Stenosis
Aortic stenosis (AS) is characterized by narrowing of the aortic valve opening, which restricts blood flow from the LV into the aorta. This condition can be caused by several factors, including:
- Congenital defects
- Bicuspid aortic valve
- Calcific degeneration
- Age-related calcification and hardening of the valve leaflets
- Rheumatic fever
- An inflammatory disease that can cause scarring and fusion of the valve leaflets
The narrowed valve increases resistance to blood flow, leading to higher pressure within the LV. To overcome this resistance, the LV compensates by hypertrophying. Over time, this increased workload can lead to LV dysfunction, heart failure, and symptoms such as angina, syncope, fatigue, and dyspnea. AS leads to a crescendo-decrescendo systolic murmur in the right-second intercostal space that radiates to the carotids.
The diagnosis of AS typically begins with a physical examination and is confirmed through echocardiography (ECG). Findings on ECG, such as LV hypertrophy with strain and left atrial enlargement, are nonspecific. Two-dimensional ECG can reveal a thickened aortic valve, reduced leaflet mobility, and concentric LV hypertrophy. An ECG can also quantify the severity of AS, assess the LV size and the presence of concurrent aortic or mitral regurgitation, and estimate pulmonary systolic pressure. Chest radiography may show a normal cardiac size due to concentric hypertrophy in AS, but cardiomegaly may appear once LV systolic failure occurs. Calcification of the aortic valve, pulmonary congestion, and poststenotic dilation of the aorta are other nonspecific findings. Cardiac catheterization is indicated to evaluate coexistent coronary disease or when aortic valve replacement is indicated.
The 2020 ACC/AHA guidelines categorize AS into 4 stages defined by valve anatomy, hemodynamics, changes in the LV and vasculature, and the presence or absence of symptoms. The key hemodynamic parameters for staging include the maximum transaortic velocity (Vmax), mean pressure gradient (ΔP) across the aortic valve, and aortic valve area (AVA). The transvalvular pressure gradient and cardiac output are determined at catheterization, and the AVA is calculated using the Gorlin equation. The stages are as follows:
- Stage A: At risk for AS
- Characterized by aortic valve sclerosis or a congenital anomaly
- Vmax <2 m/s
- No hemodynamic consequences
- No symptoms
- Stage B: Progressive hemodynamic obstruction
- Involves mild to moderate calcification/fibrosis
- Mild (Vmax 2.0–2.9 m/s, ΔP <20 mm Hg) to moderate AS (Vmax 3.0–3.9 m/s, ΔP 20–39 mm Hg)
- Normal LVEF, early diastolic dysfunction
- No symptoms
- Stage C: Asymptomatic severe AS
- Severe leaflet calcification/fibrosis or congenital stenosis of the aortic valve, with strongly reduced leaflet motion
- C1: Asymptomatic severe AS with normal LV function
- Severe AS without symptoms
- LVEF ≥50%
- Vmax ≥4 m/s or mean ΔP ≥40 mm Hg
- C2: Asymptomatic severe AS with LV systolic dysfunction
- LVEF <50%
- Vmax ≥4 m/s or ΔP ≥40 mm Hg
- C1: Asymptomatic severe AS with normal LV function
- Severe leaflet calcification/fibrosis or congenital stenosis of the aortic valve, with strongly reduced leaflet motion
- Stage D: Symptomatic Severe AS
- Severe leaflet calcification/fibrosis or congenital stenosis, which significantly restricts leaf motion/opening
- D1: Symptomatic severe high-gradient AS
- Vmax ≥4 m/s or mean ΔP ≥40 mm Hg
- AVA is typically ≤1.0 cm2
- LV hypertrophy and diastolic dysfunction are present
- May also be pulmonary hypertension
- Symptoms: exertional dyspnea, decreased exercise tolerance or heart failure, exertional angina, and exertional syncope or presyncope
- D2: Symptomatic severe low-flow, low-gradient AS with reduced LVEF
- Resting aortic Vmax <4 m/s or mean ΔP <40 mm Hg and an AVA ≤1.0 cm2
- LV hypertrophy and diastolic dysfunction are present
- LVEF <50%
- Symptoms: heart failure, angina, and syncope or presyncope.
- D3: Symptomatic severe low-gradient AS with normal LVEF
- Resting aortic Vmax <4 m/s or mean ΔP <40 mm Hg and an AVA ≤1.0 cm2
- LVEF ≥50%
- Stroke volume index <35 mL/m2
- Symptoms: heart failure, angina, and syncope or presyncope
- D1: Symptomatic severe high-gradient AS
- Severe leaflet calcification/fibrosis or congenital stenosis, which significantly restricts leaf motion/opening
Notably, any damage to the heart valves, including AR and AS, puts patients at risk for endocarditis. Characteristic features of endocarditis are fever, pulmonary infarcts, arterial emboli, Janeway lesions, splinter hemorrhages, conjunctival hemorrhages, painful Osler nodes, Roth spots, glomerulonephritis, new-onset heart murmur, and positive blood cultures. Duke criteria are used to assess the probability of endocarditis.[9]
Indications
The indications for stentless autograft/homograft aortic valve replacement include the following:
Aortic Regurgitation
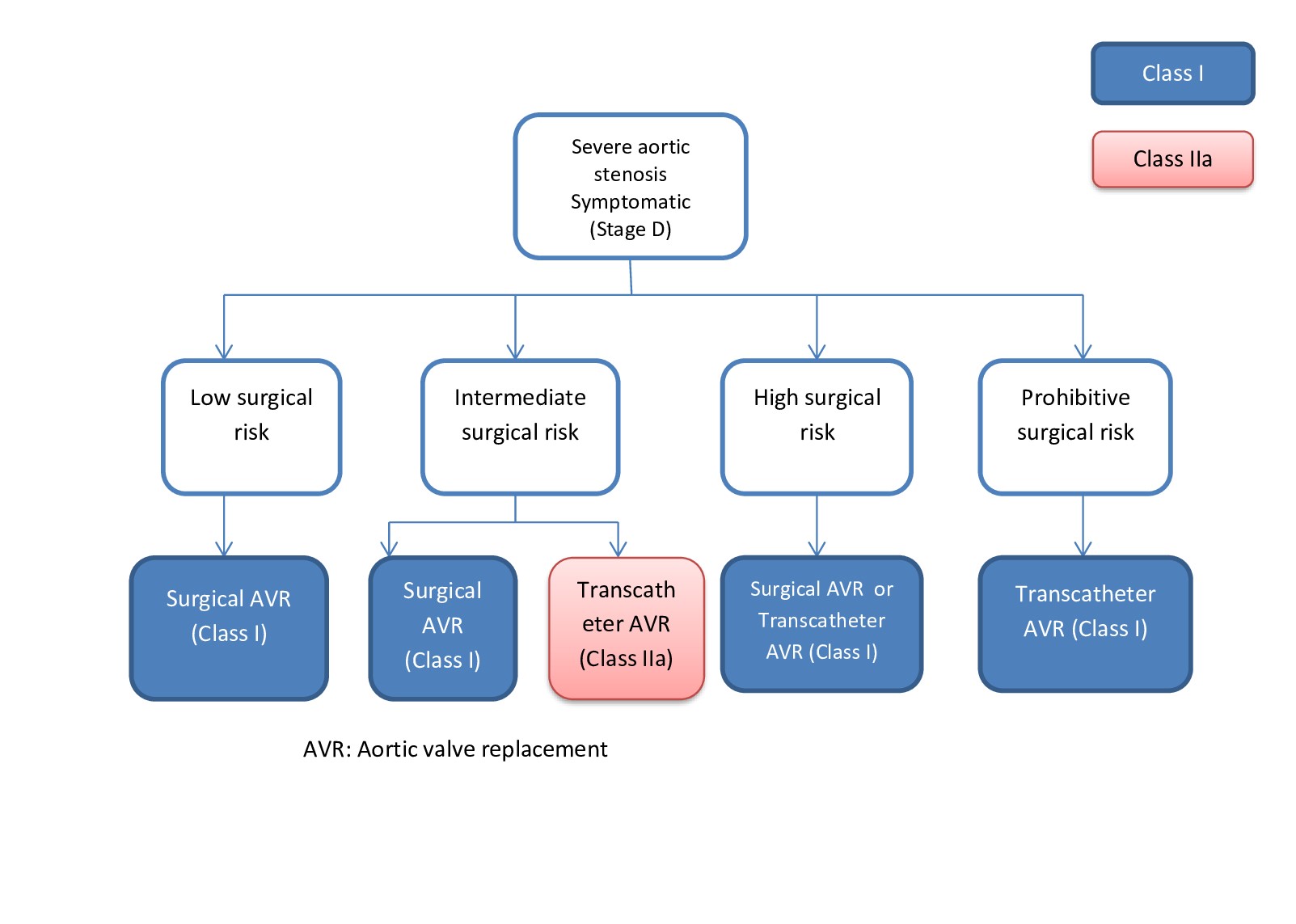
AR is initially treated with diuretics and vasodilators for symptomatic management. The following are indications for aortic valve replacement in patients with AR:
- Stage D AR
- Stage C2 AR with LVEF <50%, left ventricular end-systolic dimension >50 mm, or LVEDD >65 mm
- Stage B, C, or D AR undergoing any other cardiac surgery [2]
Current data are insufficient to indicate a specific type of prosthesis for aortic valve replacement. However, the following points favor a bioprosthetic heart valve:
- Contraindications, noncompliance, or refractory to anticoagulant drugs
- Fully informed patients' desire
- Patients with mechanical valve thrombosis
- Low risk of valve redo operations
- Future pregnancy plans
- Being 65 or older [10]
Aortic Stenosis
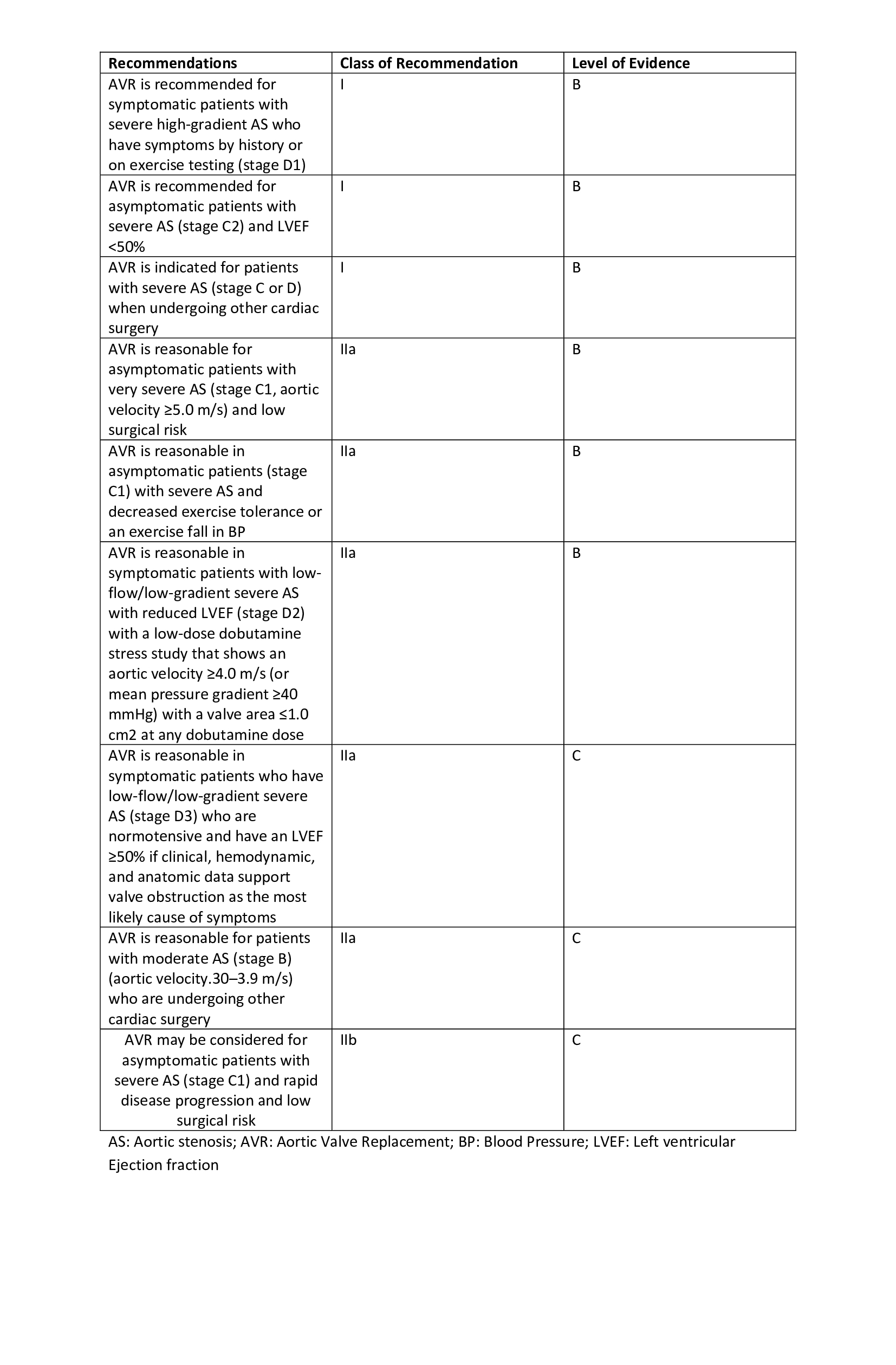
AS is initially managed symptomatically with beta-blockers (eg, atenolol, carvedilol, metoprolol), calcium channel blockers (eg, verapamil, diltiazem), diuretics (eg, furosemide, thiazides), or angiotensin-converting enzyme (ACE) inhibitors. The following are commonly used indications for aortic valve replacement in patients with AS:
- Stage D1 AS
- Stage C2 AS; LVEF <50%
- Stage C1 AS; low exercise tolerance
- Stage C1 AS; Vmax ≥4.5 m/s with low surgical risk
- Stage C or D AS undergoing any other cardiac surgery [11]
Graft Choice
Autograft aortic valve replacement is performed using the Ross procedure, which is particularly favored for children and young adults (see Image. Ross Procedure). This technique uses the patient's pulmonary valve to replace the defective aortic valve, which is subsequently replaced by a bovine jugular vein conduit in patients younger than 6 or in older patients with a decellularized pulmonary homograft.[12][13] This approach leverages the potential of the pulmonic valve to grow and regenerate, along with a lower risk of endocarditis.[12]
Homografts, sourced from cadaveric or brain-dead heart donors, are preferred in cases of aortic valve endocarditis, especially with periannular abscesses, as the periannular graft tissue can patch these defects. They are also recommended for patients with a history of intravenous drug use with endocarditis due to their higher risk of reoperations.[14] Xenografts, on the other hand, are readily available in all sizes.[15]
Contraindications
Common contraindications for the Ross procedure include connective tissue diseases (eg, Marfan and Ehlers-Danlos syndrome) and inflammatory conditions like rheumatoid arthritis and systemic lupus erythematosus. These conditions often involve the pulmonary aorta or pulmonary valve, making them unsuitable candidates for this procedure. Additional contraindications include multiple vessel coronary artery disease, severely decreased LV function or any existing pulmonary valve pathology. There are no specific contraindications for aortic valve homografts other than the availability of the donor and the preference of the surgeon and patient. However, young age is considered a relative contraindication for homograft valve replacement due to the potential for future growth and the need for reoperation.[15][16]
Equipment
Aortic heart valve replacement, including the Ross procedure, is performed via open median sternotomy. The heart valves are initially assessed with echocardiography. Essential equipment for the procedure includes:
- Hegar dilator is used to measure the autografts.
- Polypropylene sutures are used to attach the graft valve securely.
- Dacron tube is employed to extend the pulmonary valve.[17][18]
- Cardiopulmonary bypass machine is essential for maintaining circulation and oxygenation during the surgery.
- Aortic cross-clamp is used to isolate the aorta and control blood flow.
- Surgical instruments are standard instruments such as scalpels, scissors, forceps, and needle holders.
Personnel
A team consisting of an anesthesiologist, cardiac surgeon, cardiologist, surgery assistant, operation room assistant, radiologist, nurses, and additional staff members is required to perform an aortic heart valve replacement surgery.[19]
Preparation
The main goal of preparation is sterility. Before starting the procedure, the chest is shaved and sterilized. The patient is draped based on the institution's protocols, and other general anesthesia-related preparations are done. Important landmarks (eg, jugulum and xiphoid process) are identified and marked before starting the procedure.[19][20]
Technique or Treatment
Aortic valve replacement via the Ross procedure is performed through a median sternotomy with bicaval and aortic cannulation. Cardioplegia is typically achieved with cold intermittent techniques, followed by warm reperfusion before releasing the aortic clamp. The common adventitia between the pulmonary root and aorta is dissected to access the muscular infundibulum. The aortic and pulmonary valves are inspected before extraction. The route of coronary arteries and their branches is carefully considered during these procedures.[14] The pulmonary valve is carefully extracted, ensuring it remains intact and the first septal coronary artery is preserved. The Hegar dilator is used to measure the autograft.
Next, the diseased aortic valve is removed, and the annulus is measured to ensure a match with the graft. Annuloplasty is performed if there is any mismatch. The valve leaflets are then attached to the aorta using polypropylene sutures. After the autograft implantation, the left coronary button is anastomosed first, followed by the others. The distal anastomosis of the pulmonary graft is performed first, followed by the distal anastomosis of the aortic graft. The final step is securing the proximal portion of the pulmonary graft with a running suture.[18][21]
Homograft aortic valve replacement is performed through a median sternotomy, followed by cardioplegia. During the procedure, the surgeon removes all infected tissue and the damaged aortic valve. This debridement process can sometimes create a defect at the mitroaortic junction. The anterior mitral leaflet or a part of a leaflet from the graft is used to fill the defect. The atrium is then sutured, and a pericardial patch is applied if needed.[17]
Complications
About 30% of patients experience pulmonary autograft dilatation after the Ross procedure. Patients younger than 25 almost always require autograft reintervention at some point in their lives.Younger age is also associated with right ventricular outflow tract conduit degeneration. Older patients face a 32% to 68% risk of reintervention due to graft valve-related complications. The Ross procedure has comparatively lower rates of bleeding, thrombosis, and endocarditis-related complications than other types of graft replacements and is associated with a smaller number of early and late mortalities.[22][23]
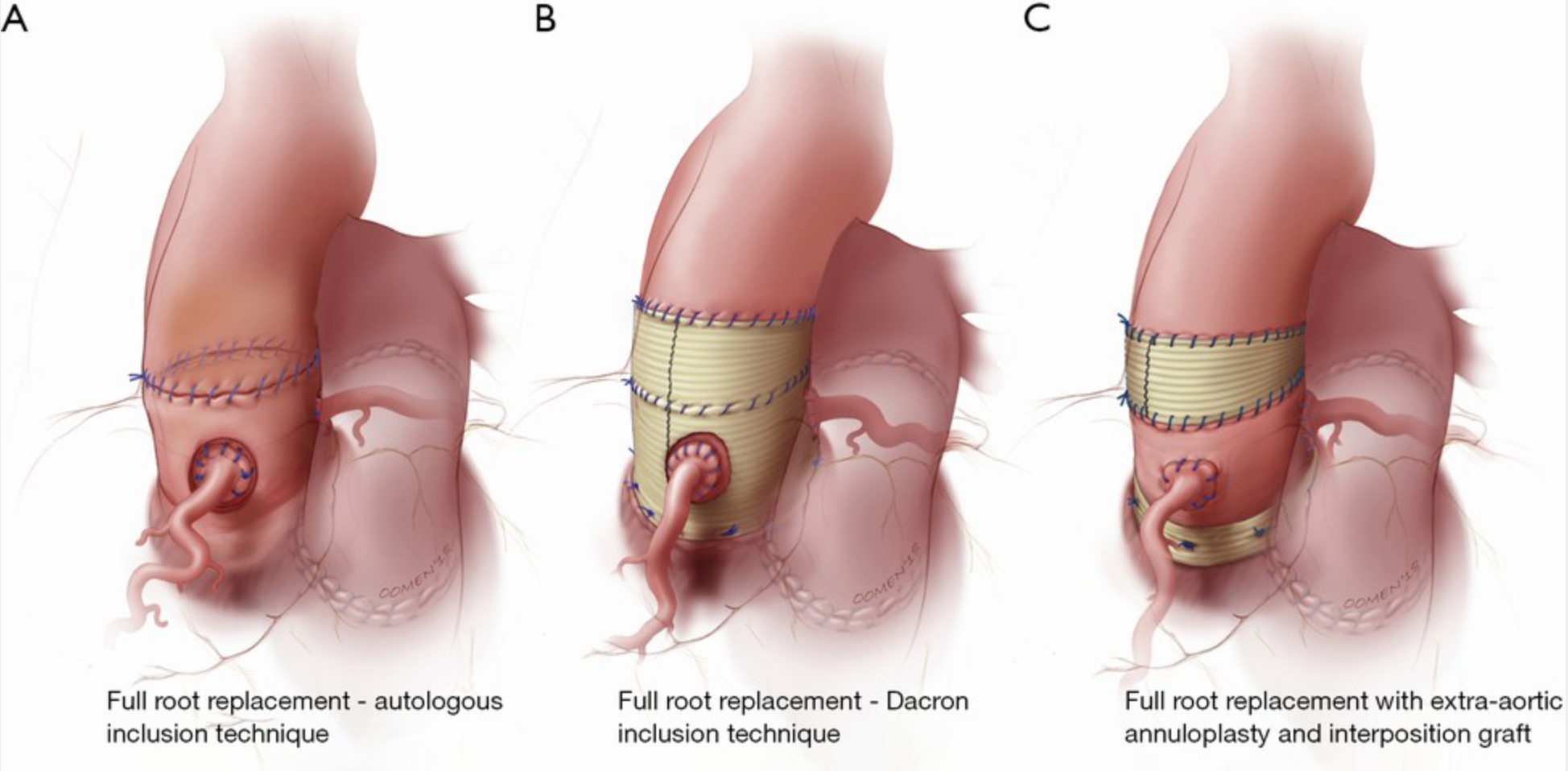
The Melbourne group has extensive experience with autologous support of the pulmonary autograft root using the patient's aorta, known as the inclusion technique. They reported long-term outcomes for this technique in 322 consecutive patients. Before implanting the pulmonary autograft, the native aorta and aortic root remnants were modified to achieve specific measurements for the sinotubular junction and aortic annulus: 24 to 26 mm for men and 22 to 24 mm for women. The long-term results are remarkable, showing stable neoaortic dimensions up to 15 years post-surgery. Only 1.5% of patients developed a maximum aortic root size exceeding 40 mm in diameter, with none surpassing 43 mm. Previous study results indicated that patients with preoperative AR and those with a large aortic annulus were at a higher risk for developing larger neoaortic root diameters during follow-up and were more likely to require reoperation. Despite these risks, the overall freedom from autograft reoperation at 18 years was 96%, with no reoperations due to autograft dilatation.[24]
Homograft aortic valve replacement may lead to early mortality, but the incidence is low. Due to the rupture of a graft leaflet, calcification of the graft valve, or poor coaptation of leaflets, structural deterioration of the graft may occur in about 20 years. Early results from the prospective Australasian Resuscitation In Sepsis Evaluation, ARISE, trial and registry, which includes 223 patients, show outcomes similar to those in another study. The ARISE trial reports outcomes after an average follow-up of 1.54 ± 0.81 years for the prospective arm and 2.60 ± 2.13 years for the registry cohort. In the study's analysis, 36% of the patients were also part of the early results of the prospective trial.
Additionally, 38% of the registry cohort were pediatric patients, resulting in a lower mean age of 28.7 ± 19.8 years compared to 47.0 ± 11.4 years in the study cohort. The authors of the ARISE trial reported very low rates of adverse events, similar to the study. Five years postprocedure, the reported rates of freedom from mortality, endocarditis, reoperations, bleeding, and thromboembolic events were 98.2 ± 0.9%, 97.3 ± 2.2%, 90.8 ± 4.0%, 99.5 ± 0.5%, and 99.5 ± 0.5%, respectively.[25][26]
Clinical Significance
Valvular heart disease poses a significant public health challenge, affecting approximately 2.5% of the population, with AS and AR constituting a considerable portion of valvular heart disease cases. The prevalence of AS varies by region and notably increases with age, affecting 0.2% of individuals in their 50s and up to 9.8% of those 80 to 89. The most common congenital heart defect, the bicuspid aortic valve, has a prevalence of 0.5% to 0.8% and often necessitates surgical intervention in younger patients, typically around age 50. Additionally, significant AR affects 0.5% of the population, with its frequency increasing with age.[27]
Despite the Ross procedure being introduced over 5 decades ago, its application in adult clinical practice remains debated. However, substantial evidence highlights its benefits. The primary advantages include improved long-term survival, avoidance of anticoagulant therapy, a low incidence of endocarditis, and an infrequent need for reintervention. The living tissue of the autograft maintains native valve physiology and retains contractile and neurohumoral responsiveness, leading to superior hemodynamics and an enhanced quality of life. These characteristics make the Ross procedure ideal for pediatric and young patients, with pediatric cases showing lower mortality and a lower rate of reinterventions than other graft procedures.[12]
Homograft aortic valve replacement, while complex, yields favorable clinical outcomes, particularly in adults and patients planning future pregnancies.[28] Compared to cryopreserved nondecellularized homografts, decellularized homografts are anticipated to offer improved durability, especially given the limited longevity of cryopreserved grafts in younger patients. Study results have demonstrated that decellularized homografts exhibit significantly higher freedom from explantation and lower rates of structural valve degeneration 10 years post-intervention.[29] For instance, results from a 2016 study by Helder et al reported that 51% of 41 patients who received decellularized homografts between 2002 and 2004 were free from reoperation after 10 years. Despite differences in decellularization techniques and small sample sizes, extended follow-up studies are crucial for these grafts. An intrinsic benefit of decellularized homografts is their low immunogenicity, which is particularly important for patients who may require organ transplantation.[27]
Enhancing Healthcare Team Outcomes
Stentless autograft/homograft aortic valve replacement requires a high level of skill, strategy, and coordinated care from an interprofessional team to ensure optimal patient-centered care and outcomes. Physicians and surgeons must possess advanced surgical skills and a deep understanding of cardiovascular anatomy and pathology to successfully perform these complex procedures. Advanced clinicians and nurses are critical in perioperative care, including patient education, preoperative assessments, and postoperative monitoring. Their ability to identify complications early and provide timely interventions is crucial for patient safety. Pharmacists contribute by managing anticoagulation therapy, ensuring proper medication reconciliation, and providing guidance on postoperative medications to prevent infections and manage pain.
Effective interprofessional communication is essential for enhancing team performance and patient outcomes. Regular interdisciplinary meetings and clear, concise handoffs ensure that all team members are informed about the patient's condition and care plan. Care coordination, including collaboration between cardiologists, cardiothoracic surgeons, anesthesiologists, and rehabilitation specialists, helps streamline the patient's journey from preoperative evaluation to postoperative recovery. This integrated approach not only enhances patient safety by reducing the risk of errors but also promotes a seamless transition of care—ultimately improving the overall patient experience and outcomes. By fostering a culture of teamwork and continuous communication, healthcare professionals can deliver high-quality, patient-centered care in stentless autograft/homograft aortic valve replacement.
Nursing, Allied Health, and Interprofessional Team Interventions
The aortic valve replacement procedure is major cardiac surgery. There should not be any communication gap between the team members. A thorough assessment of the patient before, during, and after the procedure leads to better outcomes. Negligence can lead to preventable adverse events. Appropriate care provides better results after heart valve replacement. Phase 2 randomized clinical trial results have shown better VO2 max (maximum oxygen utilized during exercise) in patients with cardiac rehabilitation.[30]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017 Jun 20:135(25):e1159-e1195. doi: 10.1161/CIR.0000000000000503. Epub 2017 Mar 15 [PubMed PMID: 28298458]
Level 1 (high-level) evidenceNishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 10:129(23):2440-92. doi: 10.1161/CIR.0000000000000029. Epub 2014 Mar 3 [PubMed PMID: 24589852]
Level 1 (high-level) evidenceMathew P, Kanmanthareddy A. Prosthetic Heart Valve. StatPearls. 2024 Jan:(): [PubMed PMID: 30725672]
Shultz BN, Timek T, Davis AT, Heiser J, Murphy E, Willekes C, Hooker R. A propensity matched analysis of outcomes and long term survival in stented versus stentless valves. Journal of cardiothoracic surgery. 2017 May 31:12(1):45. doi: 10.1186/s13019-017-0608-2. Epub 2017 May 31 [PubMed PMID: 28569201]
Dagenais F, Cartier P, Voisine P, Desaulniers D, Perron J, Baillot R, Raymond G, Métras J, Doyle D, Mathieu P. Which biologic valve should we select for the 45- to 65-year-old age group requiring aortic valve replacement? The Journal of thoracic and cardiovascular surgery. 2005 May:129(5):1041-9 [PubMed PMID: 15867778]
Silver MA, Roberts WC. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. The American journal of cardiology. 1985 Feb 1:55(4):454-61 [PubMed PMID: 3155899]
Matsushima S, Karliova I, Gauer S, Miyahara S, Schäfers HJ. Geometry of cusp and root determines aortic valve function. Indian journal of thoracic and cardiovascular surgery. 2020 Jan:36(Suppl 1):64-70. doi: 10.1007/s12055-019-00813-2. Epub 2019 Apr 6 [PubMed PMID: 33061186]
Thomas SL, Heaton J, Makaryus AN. Physiology, Cardiovascular Murmurs. StatPearls. 2024 Jan:(): [PubMed PMID: 30247833]
Topan A, Carstina D, Slavcovici A, Rancea R, Capalneanu R, Lupse M. Assesment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Clujul medical (1957). 2015:88(3):321-6. doi: 10.15386/cjmed-469. Epub 2015 Jul 1 [PubMed PMID: 26609264]
Level 3 (low-level) evidenceBaumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal. 2017 Sep 21:38(36):2739-2791. doi: 10.1093/eurheartj/ehx391. Epub [PubMed PMID: 28886619]
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014 Jun 10:63(22):2438-88. doi: 10.1016/j.jacc.2014.02.537. Epub 2014 Mar 3 [PubMed PMID: 24603192]
Level 1 (high-level) evidenceHerrmann JL, Stram AR, Brown JW. Ross Procedure: How to Do It and How to Teach It. World journal for pediatric & congenital heart surgery. 2019 Sep:10(5):624-627. doi: 10.1177/2150135119852324. Epub [PubMed PMID: 31496411]
Yokoyama Y, Kuno T, Toyoda N, Fujisaki T, Takagi H, Itagaki S, Ibrahim M, Ouzounian M, El-Hamamsy I, Fukuhara S. Ross Procedure Versus Mechanical Versus Bioprosthetic Aortic Valve Replacement: A Network Meta-Analysis. Journal of the American Heart Association. 2023 Jan 3:12(1):e8066. doi: 10.1161/JAHA.122.027715. Epub 2022 Dec 24 [PubMed PMID: 36565200]
Level 1 (high-level) evidenceByrne JG, Rezai K, Sanchez JA, Bernstein RA, Okum E, Leacche M, Balaguer JM, Prabhakaran S, Bridges CR, Higgins RS. Surgical management of endocarditis: the society of thoracic surgeons clinical practice guideline. The Annals of thoracic surgery. 2011 Jun:91(6):2012-9. doi: 10.1016/j.athoracsur.2011.01.106. Epub [PubMed PMID: 21620012]
Level 1 (high-level) evidenceLuciani GB, Santini F, Mazzucco A. Autografts, homografts, and xenografts: overview on stentless aortic valve surgery. Journal of cardiovascular medicine (Hagerstown, Md.). 2007 Feb:8(2):91-6 [PubMed PMID: 17299289]
Level 3 (low-level) evidenceOury JH. Clinical aspects of the Ross procedure: indications and contraindications. Seminars in thoracic and cardiovascular surgery. 1996 Oct:8(4):328-35 [PubMed PMID: 8899918]
Solari S, Mastrobuoni S, De Kerchove L, Navarra E, Astarci P, Noirhomme P, Poncelet A, Jashari R, Rubay J, El Khoury G. Over 20 years experience with aortic homograft in aortic valve replacement during acute infective endocarditis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2016 Dec:50(6):1158-1164. doi: 10.1093/ejcts/ezw175. Epub 2016 May 26 [PubMed PMID: 27229671]
Concha M, Aranda PJ, Casares J, Merino C, Alados P, Muñoz I, Gonzalez JR, Ribes R, Villalba R. The Ross procedure. Journal of cardiac surgery. 2004 Sep-Oct:19(5):401-9 [PubMed PMID: 15383050]
Brown KN, Kanmanthareddy A. Ross Procedure for Aortic Valve Replacement. StatPearls. 2024 Jan:(): [PubMed PMID: 30725934]
Reser D, Caliskan E, Tolboom H, Guidotti A, Maisano F. Median sternotomy. Multimedia manual of cardiothoracic surgery : MMCTS. 2015:2015():. pii: mmv017. doi: 10.1093/mmcts/mmv017. Epub 2015 Jul 17 [PubMed PMID: 26188337]
Conklin LD, Reardon MJ. Technical aspects of the Ross procedure. Texas Heart Institute journal. 2001:28(3):186-9 [PubMed PMID: 11678251]
Berdajs D. Ross procedure for everyone. Swiss medical weekly. 2012:142():w13641. doi: 10.4414/smw.2012.13641. Epub 2012 Jul 23 [PubMed PMID: 22826114]
Etnel JRG, Grashuis P, Huygens SA, Pekbay B, Papageorgiou G, Helbing WA, Roos-Hesselink JW, Bogers AJJC, Mokhles MM, Takkenberg JJM. The Ross Procedure: A Systematic Review, Meta-Analysis, and Microsimulation. Circulation. Cardiovascular quality and outcomes. 2018 Dec:11(12):e004748. doi: 10.1161/CIRCOUTCOMES.118.004748. Epub [PubMed PMID: 30562065]
Level 2 (mid-level) evidenceSkillington PD, Mokhles MM, Takkenberg JJ, Larobina M, O'Keefe M, Wynne R, Tatoulis J. The Ross procedure using autologous support of the pulmonary autograft: techniques and late results. The Journal of thoracic and cardiovascular surgery. 2015 Feb:149(2 Suppl):S46-52. doi: 10.1016/j.jtcvs.2014.08.068. Epub 2014 Sep 17 [PubMed PMID: 25439787]
Coti I, Wenda S, Andreeva A, Kocher A, Laufer G, Fischer G, Andreas M. Donor-specific HLA antibodies after fresh decellularized vs cryopreserved native allograft implantation. HLA. 2020 Nov:96(5):580-588. doi: 10.1111/tan.14077. Epub 2020 Oct 13 [PubMed PMID: 32975376]
Fukushima S, Tesar PJ, Pearse B, Jalali H, Sparks L, Fraser JF, Pohlner PG. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. The Journal of thoracic and cardiovascular surgery. 2014 Jul:148(1):65-72.e2. doi: 10.1016/j.jtcvs.2013.07.038. Epub 2013 Sep 8 [PubMed PMID: 24021951]
Level 2 (mid-level) evidenceAndreeva A, Coti I, Werner P, Scherzer S, Kocher A, Laufer G, Andreas M. Aortic Valve Replacement in Adult Patients with Decellularized Homografts: A Single-Center Experience. Journal of clinical medicine. 2023 Oct 24:12(21):. doi: 10.3390/jcm12216713. Epub 2023 Oct 24 [PubMed PMID: 37959179]
Nappi F, Nenna A, Petitti T, Spadaccio C, Gambardella I, Lusini M, Chello M, Acar C. Long-term outcome of cryopreserved allograft for aortic valve replacement. The Journal of thoracic and cardiovascular surgery. 2018 Oct:156(4):1357-1365.e6. doi: 10.1016/j.jtcvs.2018.04.040. Epub 2018 Apr 18 [PubMed PMID: 29759737]
Helder MR, Kouchoukos NT, Zehr K, Dearani JA, Maleszewski JJ, Leduc C, Heins CN, Schaff HV. Late durability of decellularized allografts for aortic valve replacement: A word of caution. The Journal of thoracic and cardiovascular surgery. 2016 Oct:152(4):1197-9. doi: 10.1016/j.jtcvs.2016.03.050. Epub 2016 Mar 31 [PubMed PMID: 27131847]
Sibilitz KL, Berg SK, Rasmussen TB, Risom SS, Thygesen LC, Tang L, Hansen TB, Johansen PP, Gluud C, Lindschou J, Schmid JP, Hassager C, Køber L, Taylor RS, Zwisler AD. Cardiac rehabilitation increases physical capacity but not mental health after heart valve surgery: a randomised clinical trial. Heart (British Cardiac Society). 2016 Dec 15:102(24):1995-2003. doi: 10.1136/heartjnl-2016-309414. Epub 2016 Aug 4 [PubMed PMID: 27492941]
Level 1 (high-level) evidence