Introduction
Following the first report of pediatric anti-NMDAR encephalitis in China in 2010, anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis is now recognized more frequently and commonly in the pediatric population. It is the second most common etiology for acute demyelinating encephalitis after mixed disturbance encephalitis surpassing all viral etiologies for encephalitis.[1][2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Anti-NMDAR encephalitis is an immune-mediated neuroinflammatory disease characterized by autoantibodies against the GluN1 subunit 2B (NR2B)/NMDA subunit 2A (NR2A) subunits of the NDMA receptor in the hippocampus causing the symptoms. Initially, it was diagnosed as a paraneoplastic disease most commonly associated with ovarian teratoma.[3][4][5] However, published case reports show evidence of this association in only 31% of children <18 years and 9% of children under the age of 14 years.[5][6]
Another confirmed association has been seen with herpes simplex encephalitis.[7] In a case series comprising of 99 patients, it was found out that 27% (64% NMDAR antibody positive) of the patients developed autoimmune encephalitis 2-16 weeks after herpes simplex encephalitis.[8] Two studies suggest an association with HLA-I allele B*07:02 and HLA-II allele DRB1*16:02, however more evidential proof needs to be researched.[9][10]
In the California encephalitis project, it was found out that anti-NMDAR antibodies were produced in children post-infection with mycoplasma.[11] It was attributed to the body expressing a nonspecific immune response to the pathogens leading to antibodies directed against NMDAR. Therefore, it becomes necessary to screen for concomitant autoimmune encephalitis in an unusual presentation of viral encephalitis.[12][13]
Epidemiology
Despite being recognized only 13 years ago and having an incidence of only 1.5 per million population per year, more than 1000 cases have been reported until now. It affects most age groups and both genders, with more incidence seen in females (75% cases).[4][5][14][15][16][17] Literature shows that non-White race individuals have a higher risk of ovarian teratoma, hence the lifetime risk of getting anti-NMDAR encephalitis increases in these people.[14] This association has been documented in the literature in individuals as young as three months of age.[18][19][20]
Pathophysiology
NMDAR is an excitatory glutamate receptor that, when activated, allows the passage of sodium and calcium ions through the channel. Activation occurs by the removal of magnesium plug, leading to glutamate and glycine binding to their respective sites.[21]
Evidence suggests that these antibodies are produced in the CNS by antibody-producing cells that cross the blood-brain barrier.[22] When antibodies bind to NMDAR, it causes internalization of these receptors from the cell surface leading to receptor hypofunction. The hypoactive receptors failed to cause tonic inhibition on the dopaminergic mesolimbic pathway resulting in psychosis.[21]
Iizuka et al. postulated that NMDAR is present in high density in the frontotemporal area, which atrophies in anti-NMDAR encephalitis leading to changes in behavior, learning, and memory.[23] Research utilizing a super-resolution microscopy model and Monte Carlo simulation found the importance of the interaction between NMDAR with other proteins (e.g., with EphB2R [ephrin-type B receptor 2] and other unknown interacting proteins).[24] Disruption of these interactions reproduced the observed clinical symptoms seen in patients. Drugs such as phencyclidine can mimic the symptoms of anti-NMDAR encephalitis as they are an antagonist at the receptor.
History and Physical
Anti-NMDAR encephalitis has four clinical stages.[25]
- Prodromal phase (phase 1): this stage is characterized by fever, headache, nausea, vomiting, and upper respiratory tract infection-like symptoms.[3][26][27]
- Illness phase (phase 2): During this stage, MRI abnormalities or pleocytosis can be seen, which decreases over several weeks without any visible changes in the symptoms.[6] These findings are strikingly different from the cytotoxic CD8 + T cell-mediated encephalitis, where there is an extensive neuronal loss.[28]
- Psychiatric phase- This phase lasts for 1 to 2 weeks. Children present with behavioral changes, irritability, tantrums, coma, manic symptoms, behavioral outbursts, sleep dysfunction, and hyperactivity.
- Neurological phase- This phase lasts for weeks to months. Children present with seizures (focal, motor, complex partial), dystonia, or status epilepticus.[29] Within weeks, changes in speech and language (mutism and decreased responsiveness) can be seen. Catatonia, cardiac arrhythmia, autonomic instability, hypoventilation, and uncoordinated respiration occur, which requires ICU admission. Speech dysfunction is much more commonly seen in children than autonomic dysfunction and hyperventilation.[26][30] Motor dysfunction, in addition to the typical seizures, can develop as dyskinetic movements such as orofacial dyskinesia. Young children can have ataxia or difficulty walking and even lose the ability to walk.
- Recovery phase (phase 3): Recovery has been described in the reverse order of the presentation of symptoms.[6] The slowest to improve are the cognitive and psychiatric functions.[25] With appropriate immunotherapy and multidisciplinary care, the patients can enter the recovery phase after a few months of treatment. The presence of inflammation on MRI and CSF is minimal. Antibodies can persist even after complete recovery.[31]
- Late phase (phase 4): The majority of the patients make a full recovery of cognition and behavioral abnormality at the time of hospital discharge.
Evaluation
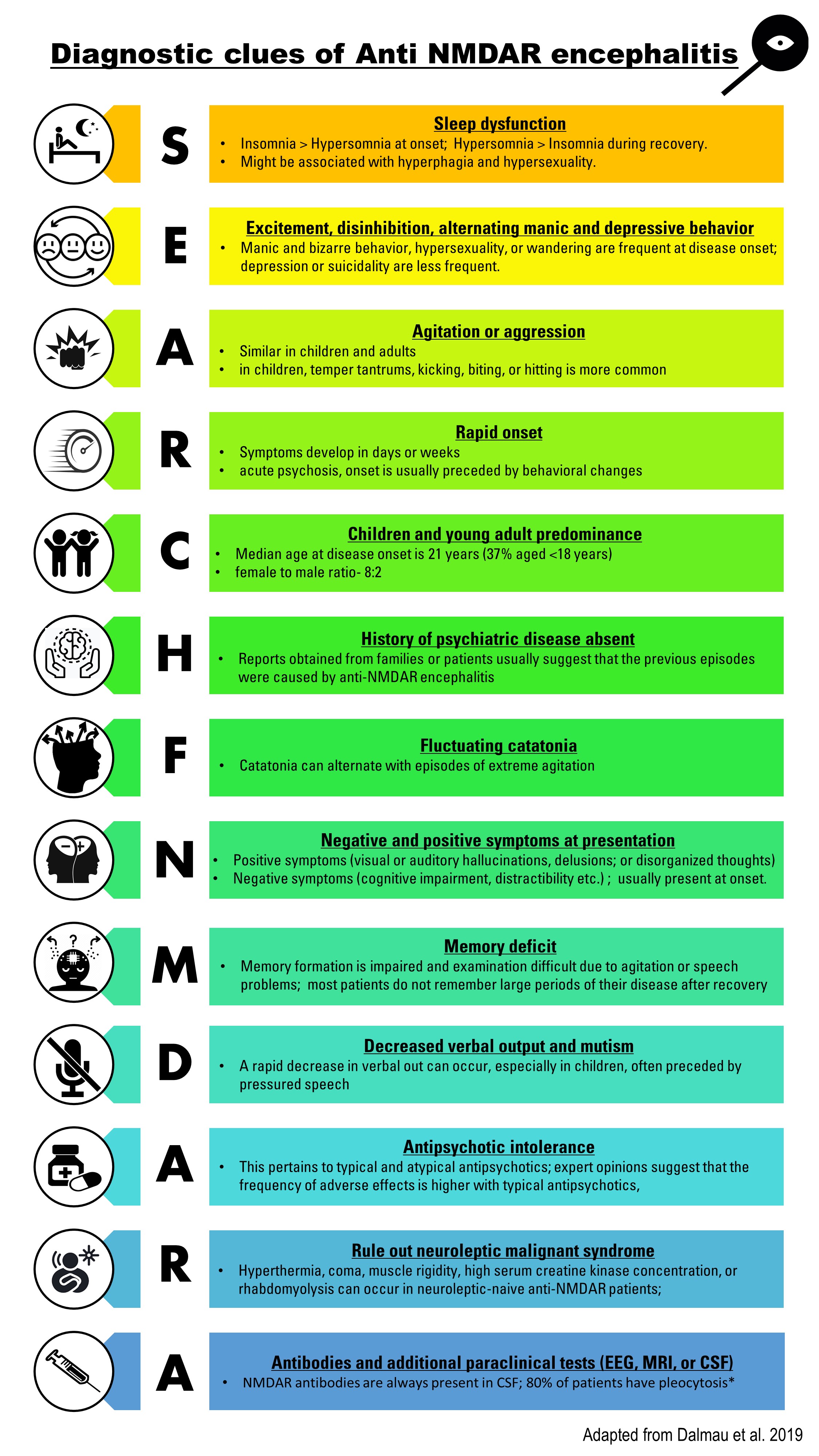
Every child should be tested for herpes simplex virus (HSV) and other viral etiology testing can be based on time of year and geographical location. Diagnostic clues for diagnosing anti- NMDAR encephalitis are shown in figure 1. Confirmation of the clinical diagnosis requires demonstration of IgG antibodies against the GluN1 subunit of NMDAR in the serum or CSF sample.[15][32] CSF sample also demonstrates lymphocytic pleocytosis, the elevation of proteins, and CSF-specific oligoclonal bands.[33][3]
Brain MRI demonstrates bilateral T2 or FLAIR signal hyperintensities in hippocampi, frontal cortex, medial temporal lobe, cerebellar cortex, spinal cord, and medulla oblongata.[34] However, these findings were evident in only 30% of the patients in the study conducted by Titulaer et al.[35] Most commonly, the lesions present in the hippocampus, and therefore it is the main predictor of poor prognosis in patients.
Electroencephalography shows a slow continuous rhythmic and disorganized activity in delta and theta range superimposed seizures, as 90% of patients have a slowing of EEG at some point during the illness.[5][35]
Treatment / Management
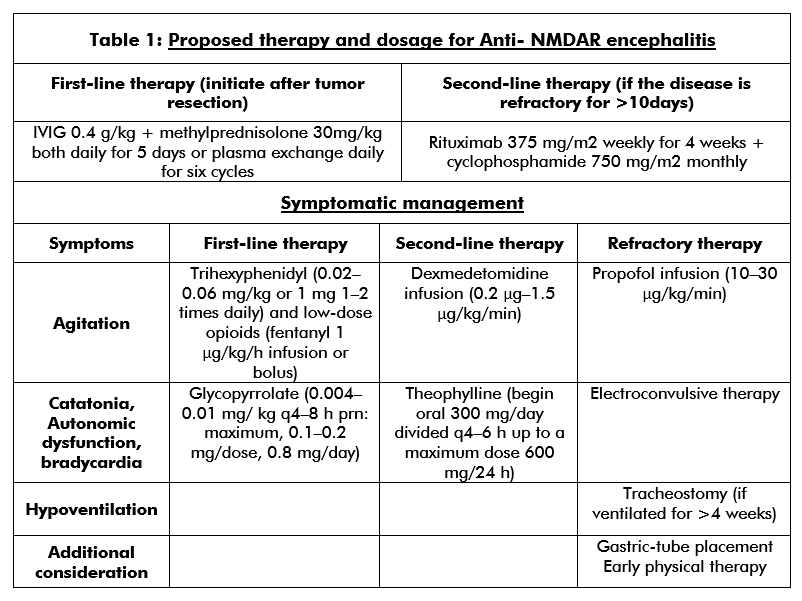
Autonomic dysfunction, hyperventilation, cardiac arrhythmia, or hyperkinetic crisis can occur in children presenting with neurological symptoms. These children should be managed in the ICU. First-line treatment involves teratoma resection (if present), immunotherapy comprising of corticosteroids, intravenous immunoglobulins or plasma exchange, and supportive care.[25][32] Second-line treatment (table 1) using rituximab or cyclophosphamide is most often necessary when the patient does not have an underlying tumor.[4][3][5][36] Relapse is more common in patients without a tumor; therefore, continued immunosuppression is recommended for at least one year using drugs such as mycophenolate mofetil or azathioprine.[5][14][32] (B2)
Clonidine, trazodone, and benzodiazepines help to reverse sleep disturbances. Phenobarbital, trihexyphenidyl, and opioids can treat extreme agitation.[37][38][39][40] Methotrimeprazine or dexmedetomidine infusion prove beneficial for children with refractory agitation. Benzodiazepines manage the catatonic symptoms. A daily dose of up to 20-30 milligrams of lorazepam can be given to patients. In the absence of a response to benzodiazepines, electroconvulsive therapy can be a treatment option.[41][42] In animal models, electroconvulsive therapy has been shown to upregulate the NMDR.[43](B3)
Psychotic and behavioral symptoms are managed using typical or atypical antipsychotics. Severe dopamine blockage can exacerbate dyskinetic movements. The development of neuroleptic malignant syndrome may occur, which can complicate and misdiagnose anti NMDAR encephalitis. Quetiapine is the drug of choice for treating psychosis. Valproate works as an excellent mood stabilizer and also offers seizure prophylaxis. Gabapentin and lithium can also be used for mood dysregulation.[38][39][44](B3)
If children show no improvement with any of the above treatment modalities, mechanical ventilation, and ketamine or propofol infusion can be considered. Children under the age of 5 years are at a higher risk of precipitating propofol infusion syndrome. Vigilant laboratory surveillance is needed in children receiving propofol for longer than 24 hours.[45][46][47] Noise reduction can help to avoid agitation. Muscle relaxants concomitant to corticosteroid therapy should be avoided in children in order to prevent myopathies.[48](B3)
Resting tachycardia is one of the most common autonomic and hemodynamic instability seen in the pediatric population suffering from anti-NMDAR encephalitis.[3][5][29] Bradycardia is associated with seizures in the pediatric age group; hence round the clock, EEG is essential. Medications such as glycopyrrolate or theophylline have been shown to prevent severe bradycardia.[49] Autonomic dysfunction can manifest as fluctuating temperature and blood pressure dysregulation. Movement disorders exacerbate during a febrile state; it is essential to maintain and aggressive temperature control using antipyretics and cooling blankets.(B2)
In the third week, placement of tracheostomy and gastrostomy tubes have shown to improve patient safety and also allows to switch to lesser sedation. Once feasible, it becomes of the utmost importance, that a comprehensive rehabilitation program is begun.[50][51][52] Rehabilitation should include physical therapy, occupational therapy, and speech therapy.(B3)
Differential Diagnosis
The condition can be presented to either a neurologist or a psychiatrist. The following should be considered as differentials: Drug abuse, post-infectious syndromes (viral, Mycoplasma, pediatric autoimmune neuropsychiatric disorders associated with Streptococcal infections). Acquired reversible autistic syndrome in acute encephalopathic illness in children, immune-mediated chorea encephalopathy syndrome in childhood, acute diffuse lymphocytic meningoencephalitis, acute reversible limbic encephalitis, acute juvenile non-herpetic encephalitis, acute disseminated encephalomyelitis (ADEM), inborn errors of metabolism (including urea cycle disorders), environmental toxins and medication overdose, rheumatological conditions such as neuropsychiatric lupus and primary psychiatric conditions such as schizophrenia. Due to the extensive differential diagnosis, the patient’s diagnostic workup should be individualized.
Prognosis
In the research, Dalmau showed that 75% of the patients with NMDAR encephalitis recover entirely or with mild sequelae while the other 25% have severe CNS deficits or eventually die.[3] Some other studies have identified a similar history and symptoms in the preceding months of diagnosis, indicating that there is a lifetime risk of 12% to 24% for relapse.[53][54]
In a study using the data obtained from 382 patients aged between 1 to 85 years, Balu et al. devised a five-point predictions score, Anti-NMDAR encephalitis 1-year functioning status (NEOS) score.[22] This score uses five variables, which account for one point each. The variables are ICU admission, treatment delay of greater than four weeks, lack of clinical improvement within four weeks, abnormal MRI, and CSF WBC count higher than 20 microlitres. A score of zero or one was associated with a 3% chance of having poor functional status at one year.[22] A score of 4 or 5 was associated with 69% chances of having a poor functional condition at the end of 1 year.[22] 1-year functional status is of great importance to physicians and healthcare providers when they are discussing the prognosis with family members.
Complications
Early identification and treatment have been shown to cause less damage to the hippocampus, yet the optimal time window between symptom onset and treatment initiation is still not identified.[55] Dalmau has reported in his research that recovery develops as a multistage process that occurs in the reverse order of the appearance of symptoms.[32]
Deterrence and Patient Education
A careful look at the symptoms and identification of IgG antibodies in CSF is vital to prevent misdiagnosis in patients presenting with anti-NMDAR encephalitis. No specific biomarker exists to predict the outcomes for this encephalitis. Therefore, the dissemination of knowledge about this disorder is required for better patient outcomes.
Enhancing Healthcare Team Outcomes
Child neurologists must work in a timely manner to facilitate early screening and diagnosis. Management of patients in ICU requires a multidisciplinary approach from a team comprising of neurologists, psychiatrists, nutritionists, and physical therapists. Arrangements should be made for social workers, pastoral care, and child life experts to be actively involved in care as children and families find that helpful.
Anti NMDAR encephalitis can be easily diagnosed using serum or CSF sample testing. An index of suspicion should be raised in children, presenting with personality changes, abnormal movements or postures, seizures, autonomic instability, or hypoventilation. It poses considerable difficulty in early diagnosis and treatment. Management may also prove to be clinically challenging as it involves treating both the cause and the symptoms.
The recovery is slow; hence, long-term monitoring is required by an interprofessional team consisting of doctors, and social workers would help decreasing morbidity tremendously. A continuous follow-up by the rehabilitation and neurology team is essential. A psychiatry consultation might be needed for both patients and family members in order to cope during the recovery. Interdisciplinary communication and collaboration are quintessential to good patient outcomes with this condition.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Xu CL, Liu L, Zhao WQ, Li JM, Wang RJ, Wang SH, Wang DX, Liu MY, Qiao SS, Wang JW. Anti-N-methyl-D-aspartate receptor encephalitis with serum anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC neurology. 2011 Nov 29:11():149. doi: 10.1186/1471-2377-11-149. Epub 2011 Nov 29 [PubMed PMID: 22126669]
Level 3 (low-level) evidenceGable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Apr:54(7):899-904. doi: 10.1093/cid/cir1038. Epub 2012 Jan 26 [PubMed PMID: 22281844]
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. The Lancet. Neurology. 2008 Dec:7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2. Epub 2008 Oct 11 [PubMed PMID: 18851928]
Level 3 (low-level) evidenceDalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Annals of neurology. 2007 Jan:61(1):25-36 [PubMed PMID: 17262855]
Level 3 (low-level) evidenceFlorance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, Glaser CA, Lynch DR, Rosenfeld MR, Dalmau J. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Annals of neurology. 2009 Jul:66(1):11-8. doi: 10.1002/ana.21756. Epub [PubMed PMID: 19670433]
Level 2 (mid-level) evidenceFlorance-Ryan N, Dalmau J. Update on anti-N-methyl-D-aspartate receptor encephalitis in children and adolescents. Current opinion in pediatrics. 2010 Dec:22(6):739-44. doi: 10.1097/MOP.0b013e3283402d2f. Epub [PubMed PMID: 21045695]
Level 3 (low-level) evidenceNosadini M, Mohammad SS, Corazza F, Ruga EM, Kothur K, Perilongo G, Frigo AC, Toldo I, Dale RC, Sartori S. Herpes simplex virus-induced anti-N-methyl-d-aspartate receptor encephalitis: a systematic literature review with analysis of 43 cases. Developmental medicine and child neurology. 2017 Aug:59(8):796-805. doi: 10.1111/dmcn.13448. Epub 2017 Apr 25 [PubMed PMID: 28439890]
Level 3 (low-level) evidenceArmangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, Llufriu S, Muchart J, Erro ME, Abraira L, Moris G, Monros-Giménez L, Corral-Corral Í, Montejo C, Toledo M, Bataller L, Secondi G, Ariño H, Martínez-Hernández E, Juan M, Marcos MA, Alsina L, Saiz A, Rosenfeld MR, Graus F, Dalmau J, Spanish Herpes Simplex Encephalitis Study Group. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. The Lancet. Neurology. 2018 Sep:17(9):760-772. doi: 10.1016/S1474-4422(18)30244-8. Epub 2018 Jul 23 [PubMed PMID: 30049614]
Level 2 (mid-level) evidenceMueller SH, Färber A, Prüss H, Melzer N, Golombeck KS, Kümpfel T, Thaler F, Elisak M, Lewerenz J, Kaufmann M, Sühs KW, Ringelstein M, Kellinghaus C, Bien CG, Kraft A, Zettl UK, Ehrlich S, Handreka R, Rostásy K, Then Bergh F, Faiss JH, Lieb W, Franke A, Kuhlenbäumer G, Wandinger KP, Leypoldt F, German Network for Research on Autoimmune Encephalitis (GENERATE). Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Annals of neurology. 2018 Apr:83(4):863-869. doi: 10.1002/ana.25216. Epub [PubMed PMID: 29572931]
Shu Y, Qiu W, Zheng J, Sun X, Yin J, Yang X, Yue X, Chen C, Deng Z, Li S, Yang Y, Peng F, Lu Z, Hu X, Petersen F, Yu X. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. Journal of neurology, neurosurgery, and psychiatry. 2019 Jun:90(6):652-658. doi: 10.1136/jnnp-2018-319714. Epub 2019 Jan 13 [PubMed PMID: 30636700]
Level 2 (mid-level) evidenceDuBray K, Anglemyer A, LaBeaud AD, Flori H, Bloch K, Joaquin KS, Messenger S, Preas C, Sheriff H, Glaser C. Epidemiology, outcomes and predictors of recovery in childhood encephalitis: a hospital-based study. The Pediatric infectious disease journal. 2013 Aug:32(8):839-44. doi: 10.1097/INF.0b013e318290614f. Epub [PubMed PMID: 23518823]
Level 2 (mid-level) evidenceBorlot F, Santos ML, Bandeira M, Liberalesso PB, Kok F, Löhr A Jr, Reed UC. Anti-N-methyl D-aspartate receptor encephalitis in childhood. Jornal de pediatria. 2012 May:88(3):275-8. doi: 10.2223/JPED.2172. Epub 2012 Mar 28 [PubMed PMID: 22456689]
Level 3 (low-level) evidenceShruthi TK, Shuba S, Rajakumar PS, Chitrambalam S. Anti-NMDA receptor encephalitis in an adolescent. Indian pediatrics. 2014 May:51(5):405-6 [PubMed PMID: 24953589]
Level 3 (low-level) evidenceMann A, Machado NM, Liu N, Mazin AH, Silver K, Afzal KI. A multidisciplinary approach to the treatment of anti-NMDA-receptor antibody encephalitis: a case and review of the literature. The Journal of neuropsychiatry and clinical neurosciences. 2012 Spring:24(2):247-54. doi: 10.1176/appi.neuropsych.11070151. Epub [PubMed PMID: 22772674]
Level 3 (low-level) evidenceIrani SR, Vincent A. NMDA receptor antibody encephalitis. Current neurology and neuroscience reports. 2011 Jun:11(3):298-304. doi: 10.1007/s11910-011-0186-y. Epub [PubMed PMID: 21331529]
Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS, UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. The Lancet. Infectious diseases. 2010 Dec:10(12):835-44. doi: 10.1016/S1473-3099(10)70222-X. Epub 2010 Oct 15 [PubMed PMID: 20952256]
Peery HE, Day GS, Doja A, Xia C, Fritzler MJ, Foster WG. Anti-NMDA receptor encephalitis in children: the disorder, its diagnosis, and treatment. Handbook of clinical neurology. 2013:112():1229-33. doi: 10.1016/B978-0-444-52910-7.00045-3. Epub [PubMed PMID: 23622333]
Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA neurology. 2014 Mar:71(3):344-6. doi: 10.1001/jamaneurol.2013.4580. Epub [PubMed PMID: 24473671]
Level 3 (low-level) evidenceIrani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain : a journal of neurology. 2010 Jun:133(Pt 6):1655-67. doi: 10.1093/brain/awq113. Epub [PubMed PMID: 20511282]
Dale RC, Irani SR, Brilot F, Pillai S, Webster R, Gill D, Lang B, Vincent A. N-methyl-D-aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica. Annals of neurology. 2009 Nov:66(5):704-9. doi: 10.1002/ana.21807. Epub [PubMed PMID: 19938173]
Lakhan SE, Caro M, Hadzimichalis N. NMDA Receptor Activity in Neuropsychiatric Disorders. Frontiers in psychiatry. 2013:4():52. doi: 10.3389/fpsyt.2013.00052. Epub 2013 Jun 10 [PubMed PMID: 23772215]
Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019 Jan 15:92(3):e244-e252. doi: 10.1212/WNL.0000000000006783. Epub 2018 Dec 21 [PubMed PMID: 30578370]
Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hata T, Dalmau J. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008 Feb 12:70(7):504-11 [PubMed PMID: 17898324]
Level 3 (low-level) evidenceLadépêche L, Planagumà J, Thakur S, Suárez I, Hara M, Borbely JS, Sandoval A, Laparra-Cuervo L, Dalmau J, Lakadamyali M. NMDA Receptor Autoantibodies in Autoimmune Encephalitis Cause a Subunit-Specific Nanoscale Redistribution of NMDA Receptors. Cell reports. 2018 Jun 26:23(13):3759-3768. doi: 10.1016/j.celrep.2018.05.096. Epub [PubMed PMID: 29949761]
Kayser MS, Dalmau J. Anti-NMDA Receptor Encephalitis in Psychiatry. Current psychiatry reviews. 2011:7(3):189-193 [PubMed PMID: 24729779]
Gable MS, Gavali S, Radner A, Tilley DH, Lee B, Dyner L, Collins A, Dengel A, Dalmau J, Glaser CA. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2009 Dec:28(12):1421-9. doi: 10.1007/s10096-009-0799-0. Epub 2009 Aug 29 [PubMed PMID: 19718525]
Level 3 (low-level) evidenceLuca N, Daengsuwan T, Dalmau J, Jones K, deVeber G, Kobayashi J, Laxer RM, Benseler SM. Anti-N-methyl-D-aspartate receptor encephalitis: a newly recognized inflammatory brain disease in children. Arthritis and rheumatism. 2011 Aug:63(8):2516-22. doi: 10.1002/art.30437. Epub [PubMed PMID: 21547896]
Level 3 (low-level) evidenceDalmau J, Graus F. Antibody-Mediated Encephalitis. The New England journal of medicine. 2018 Mar 1:378(9):840-851. doi: 10.1056/NEJMra1708712. Epub [PubMed PMID: 29490181]
Armangue T, Titulaer MJ, Málaga I, Bataller L, Gabilondo I, Graus F, Dalmau J, Spanish Anti-N-methyl-D-Aspartate Receptor (NMDAR) Encephalitis Work Group. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. The Journal of pediatrics. 2013 Apr:162(4):850-856.e2. doi: 10.1016/j.jpeds.2012.10.011. Epub 2012 Nov 16 [PubMed PMID: 23164315]
Moscato EH, Jain A, Peng X, Hughes EG, Dalmau J, Balice-Gordon RJ. Mechanisms underlying autoimmune synaptic encephalitis leading to disorders of memory, behavior and cognition: insights from molecular, cellular and synaptic studies. The European journal of neuroscience. 2010 Jul:32(2):298-309. doi: 10.1111/j.1460-9568.2010.07349.x. Epub 2010 Jul 14 [PubMed PMID: 20646055]
Level 3 (low-level) evidenceDalmau J, Geis C, Graus F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiological reviews. 2017 Apr:97(2):839-887. doi: 10.1152/physrev.00010.2016. Epub [PubMed PMID: 28298428]
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. The Lancet. Neurology. 2011 Jan:10(1):63-74. doi: 10.1016/S1474-4422(10)70253-2. Epub [PubMed PMID: 21163445]
Barry H, Hardiman O, Healy DG, Keogan M, Moroney J, Molnar PP, Cotter DR, Murphy KC. Anti-NMDA receptor encephalitis: an important differential diagnosis in psychosis. The British journal of psychiatry : the journal of mental science. 2011 Dec:199(6):508-9. doi: 10.1192/bjp.bp.111.092197. Epub 2011 Oct 7 [PubMed PMID: 21984802]
Level 3 (low-level) evidenceRosenfeld MR, Dalmau J. Paraneoplastic Neurologic Syndromes. Neurologic clinics. 2018 Aug:36(3):675-685. doi: 10.1016/j.ncl.2018.04.015. Epub 2018 Jun 18 [PubMed PMID: 30072076]
Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. The Lancet. Neurology. 2013 Feb:12(2):157-65. doi: 10.1016/S1474-4422(12)70310-1. Epub 2013 Jan 3 [PubMed PMID: 23290630]
Level 2 (mid-level) evidenceKashyape P, Taylor E, Ng J, Krishnakumar D, Kirkham F, Whitney A. Successful treatment of two paediatric cases of anti-NMDA receptor encephalitis with cyclophosphamide: the need for early aggressive immunotherapy in tumour negative paediatric patients. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2012 Jan:16(1):74-8. doi: 10.1016/j.ejpn.2011.07.005. Epub 2011 Aug 9 [PubMed PMID: 21831679]
Level 3 (low-level) evidenceRaha S, Gadgil P, Sankhla C, Udani V. Nonparaneoplastic anti-N-methyl-D-aspartate receptor encephalitis: a case series of four children. Pediatric neurology. 2012 Apr:46(4):246-9. doi: 10.1016/j.pediatrneurol.2012.01.012. Epub [PubMed PMID: 22490772]
Level 3 (low-level) evidenceHung TY, Foo NH, Lai MC. Anti-N-methyl-d-aspartate receptor encephalitis. Pediatrics and neonatology. 2011 Dec:52(6):361-4. doi: 10.1016/j.pedneo.2011.08.012. Epub 2011 Nov 6 [PubMed PMID: 22192267]
Level 3 (low-level) evidenceKuo YL, Tsai HF, Lai MC, Lin CH, Yang YK. Anti-NMDA receptor encephalitis with the initial presentation of psychotic mania. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2012 Jun:19(6):896-8. doi: 10.1016/j.jocn.2011.10.006. Epub 2012 Feb 12 [PubMed PMID: 22330692]
Level 3 (low-level) evidenceJones KC, Schwartz AC, Hermida AP, Kahn DA. A Case of Anti-NMDA Receptor Encephalitis Treated with ECT. Journal of psychiatric practice. 2015 Sep:21(5):374-80. doi: 10.1097/PRA.0000000000000100. Epub [PubMed PMID: 26348805]
Level 3 (low-level) evidenceDhossche D, Fink M, Shorter E, Wachtel LE. Anti-NMDA receptor encephalitis versus pediatric catatonia. The American journal of psychiatry. 2011 Jul:168(7):749-50; author reply 750. doi: 10.1176/appi.ajp.2011.11030471. Epub [PubMed PMID: 21724675]
Level 3 (low-level) evidenceDhossche DM, Wachtel LE. Catatonia is hidden in plain sight among different pediatric disorders: a review article. Pediatric neurology. 2010 Nov:43(5):307-15. doi: 10.1016/j.pediatrneurol.2010.07.001. Epub [PubMed PMID: 20933172]
Watkins CJ, Pei Q, Newberry NR. Differential effects of electroconvulsive shock on the glutamate receptor mRNAs for NR2A, NR2B and mGluR5b. Brain research. Molecular brain research. 1998 Oct 30:61(1-2):108-13 [PubMed PMID: 9795172]
Level 3 (low-level) evidenceKuppuswamy PS, Takala CR, Sola CL. Management of psychiatric symptoms in anti-NMDAR encephalitis: a case series, literature review and future directions. General hospital psychiatry. 2014 Jul-Aug:36(4):388-91. doi: 10.1016/j.genhosppsych.2014.02.010. Epub 2014 Mar 5 [PubMed PMID: 24731834]
Level 3 (low-level) evidenceReed MD, Yamashita TS, Marx CM, Myers CM, Blumer JL. A pharmacokinetically based propofol dosing strategy for sedation of the critically ill, mechanically ventilated pediatric patient. Critical care medicine. 1996 Sep:24(9):1473-81 [PubMed PMID: 8797618]
Bray R. Propofol infusion in children. Critical care medicine. 2000 Jun:28(6):2177-8 [PubMed PMID: 10890707]
Level 3 (low-level) evidenceBray RJ. Propofol infusion for ICU sedation in children. Anaesthesia. 2002 May:57(5):521 [PubMed PMID: 12004826]
Level 3 (low-level) evidenceDeem S. Intensive-care-unit-acquired muscle weakness. Respiratory care. 2006 Sep:51(9):1042-52; discussion 1052-3 [PubMed PMID: 16934167]
Sadaka F, Naydenov SK, Ponzillo JJ. Theophylline for bradycardia secondary to cervical spinal cord injury. Neurocritical care. 2010 Dec:13(3):389-92. doi: 10.1007/s12028-010-9454-y. Epub [PubMed PMID: 20878263]
Level 3 (low-level) evidenceBradley L. Rehabilitation following anti-NMDA encephalitis. Brain injury. 2015:29(6):785-8. doi: 10.3109/02699052.2015.1004741. Epub 2015 Jan 27 [PubMed PMID: 25626115]
Level 3 (low-level) evidenceHoutrow AJ, Bhandal M, Pratini NR, Davidson L, Neufeld JA. The rehabilitation of children with anti-N-methyl-D-aspartate-receptor encephalitis: a case series. American journal of physical medicine & rehabilitation. 2012 May:91(5):435-41. doi: 10.1097/PHM.0b013e3182465da6. Epub [PubMed PMID: 22415341]
Level 3 (low-level) evidenceRemy KE, Custer JW, Cappell J, Foster CB, Garber NA, Walker LK, Simon L, Bagdure D. Pediatric Anti-N-Methyl-d-Aspartate Receptor Encephalitis: A Review with Pooled Analysis and Critical Care Emphasis. Frontiers in pediatrics. 2017:5():250. doi: 10.3389/fped.2017.00250. Epub 2017 Nov 24 [PubMed PMID: 29226117]
Chapman MR, Vause HE. Anti-NMDA receptor encephalitis: diagnosis, psychiatric presentation, and treatment. The American journal of psychiatry. 2011 Mar:168(3):245-51. doi: 10.1176/appi.ajp.2010.10020181. Epub [PubMed PMID: 21368306]
Gabilondo I, Saiz A, Galán L, González V, Jadraque R, Sabater L, Sans A, Sempere A, Vela A, Villalobos F, Viñals M, Villoslada P, Graus F. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011 Sep 6:77(10):996-9. doi: 10.1212/WNL.0b013e31822cfc6b. Epub 2011 Aug 24 [PubMed PMID: 21865579]
Level 2 (mid-level) evidenceFinke C, Kopp UA, Prüss H, Dalmau J, Wandinger KP, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. Journal of neurology, neurosurgery, and psychiatry. 2012 Feb:83(2):195-8. doi: 10.1136/jnnp-2011-300411. Epub 2011 Sep 20 [PubMed PMID: 21933952]