Introduction
Comprehension of the anatomy of the spinal cord and their respective functions is paramount when diagnosing and managing spinal cord infarction (SCI). The spinal cord has 31 pairs of dorsal and ventral roots emanating from the cervical (n=8), thoracic (n=12), lumbar (n=5), and coccygeal (n=1) segments. The anterior two-thirds of the spinal cord contains motor and spinothalamic modalities, and the posterior one third houses the proprioceptive tracts.[1] The lateral region of the spinal cord contains the lateral corticospinal, rubrospinal, and medullary reticulospinal tracts.
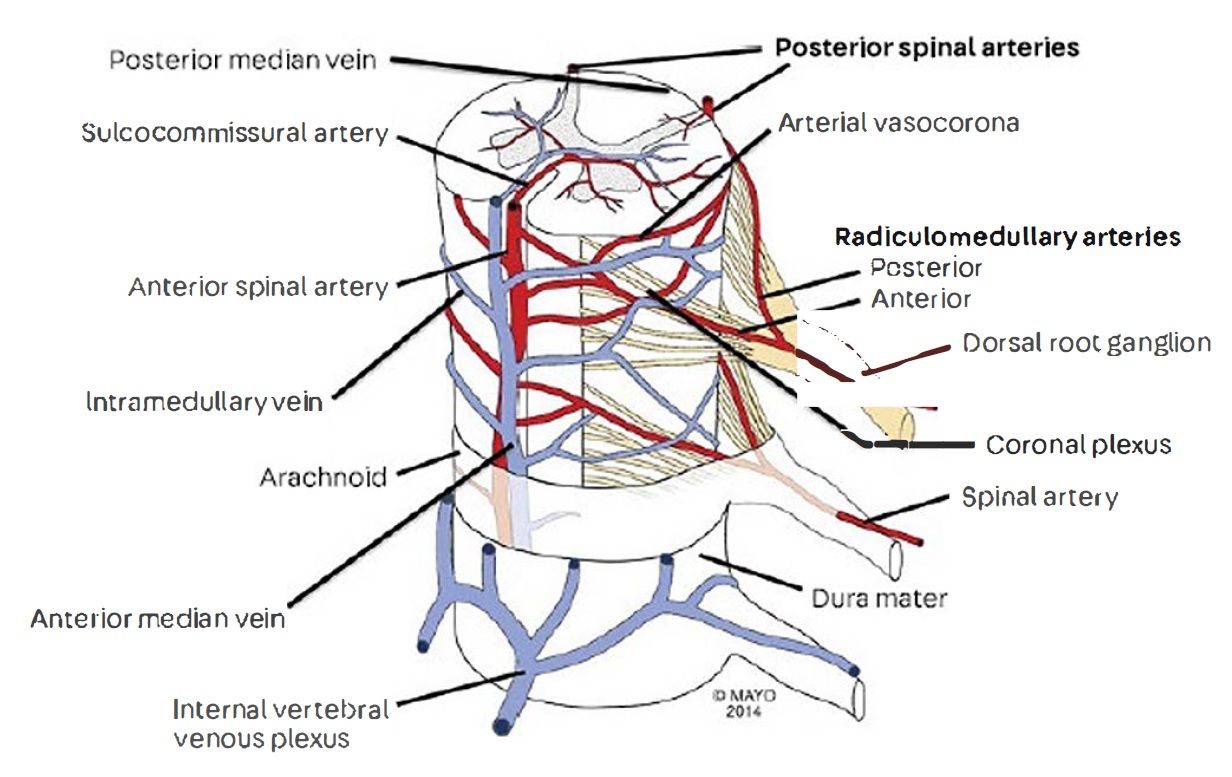
The spinal cord receives its vascular support from one anterior spinal artery (ASA) and two posterior spinal arteries (PSA) that span the length of the cord longitudinally. They originate from the vertebral arteries at the level of the craniocervical junction, they anastomose via the vasocorona and transverse radicular branches forming the pial plexus, then give rise to small perforating branches that enter the spinal cord and supply the different tracts.[2] (Figure 1)
The ASA gives rise to the sulcocommissural artery, which is also responsible for providing blood supply to the anterior of the spinal cord.[3] The PSA can also originate from posterior inferior cerebellar arteries (PICA), it also relies on posterior radicular arteries (originating from a vertebral artery) for vascular supply.[4] Five to eight of the radicular arteries assume a dominant role in supplying the ASA; one of the thoracolumbar arteries emanating from T9 to T12 is said to assume that role in 90% of people and provides vascular perfusion to the lower thoracic and lumbar spinal cord in addition to the conus medullaris.[1][3] It is known as the artery of Adamkiewicz.[1]
The spinal cord bears a high level of collateral circulation thus decreasing its susceptibility to vascular injury;[5][6][7] there is also a degree of variation in circulatory support among different individuals. Perfusion of the anterior two-thirds of the spinal cord and the anterior portion of the posterior column occurs via the anterior spinal artery (ASA), and the posterior one-third region of the spinal cord receives its supply by the two posterior spinal arteries (PSA).[8] The PSA supplies the posterior columns, posterior dorsal horns, portions of the corticospinal and spinothalamic tracts. There are speculations regarding a potential watershed zone between the regions supplied by the ASA and PSA involving the anterior dorsal horns and part of the corticospinal and spinothalamic tracts; however, there has been no clear evidence yet published.[3][4] The lower thoracic spinal cord carries a higher risk of infarction due to its hypovascularity and a lesser degree of collateral circulation.[1][3]
The anterior spinal and medial posterior veins are responsible for providing venous drainage to the spinal cord; together they form a venous network surrounding the cord, similar to the arterial system radicular veins are present in high quantity and channel through to the intervertebral and paravertebral plexi which direct blood flow to the azygos and pelvic venous systems.[9] The spinal venous network is valveless,[10] thus rendering it susceptible to infections.[11]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The age of onset ranges between the first and tenth decade of life. The median age lies between the sixth and seventh decades with the majority (65%) occurring at the thoracolumbar level and others at mid-cervical with the latter carrying a more severe phenotype of autonomic dysfunction and upper extremity involvement.[1][3] Implications of spinal cord infarct differ among children and adults. Cardiovascular abnormalities and trauma lead to SCI in children.[12][13] Aortic disease is the leading culprit in SCI in the adult population; atherosclerosis, aortic surgeries, thoracic aortic aneurysms.[14][15][16] A clinical history of vascular disease also predisposes patients to develop SCI; some of the associated risks include hypertension,[15] smoking, hyperlipidemia, and diabetes mellitus.[17] Other conditions that bring about SCI include adjacent spinal degenerative disease, epidural or spinal anesthesia,[18][19] fibrocartilagenous embolism (FCE) associated with disc herniation, vertebral artery dissection,[4] sympathectomy,[20][21] systemic hypotension,[22] cardiac embolism, coagulopathies, vasculitic disorders, surfer’s myelopathy,[3] and decompression sickness.[1][23] Cocaine use,[24] physical maneuvers such as heavy lifting and Valsalva,[17] concomitant with herniation of nucleus pulposus, chiropractic manipulation, are also implicated and carry an early age of onset.[25] Septic thrombophlebitis can result in spinal venous occlusion and thus lead to ischemic infarction.[11]
Epidemiology
Spinal cord infarction (SCI) is relatively uncommon; this is largely due to its extensive collateral vascular network.[5][6][7] Estimates of its occurrence are 1% to 2% of ischemic strokes and 5% to 8% of all acute myelopathies.[3][26][23] SCI occurs in 8% of patients of multilevel aortic disease.[14] The risk of SCI escalates in patients undergoing thoracoabdominal aortic surgery where the prevalence can be as high as 33%.[27]The rate of SCI was assessed among 15304 patients with ruptured aortic aneurysm or dissection and in 74555 of patients with an unruptured aneurysm; the study revealed the rate of SCI in emergency aortic repair to be one in 130 and one in 600 in non-emergency surgical repairs.[28]
A retrospective analysis assessing the rate of occurrence among 164 patients with acute SCI identified 9 (5.5%) patients with fibrocartilaginous embolism.[25] SCI prevalence studies are scarce, given its rarity.
Pathophysiology
The determination of clinical phenotype is by location and the degree of occlusion. SCI has a wide spectrum of severity; symptoms can range from minor weakness to paraparesis. Symptoms can be abrupt in onset and progress rapidly over minutes to hours.[13] The most frequently encountered clinical presentations are those pertaining to centromedullar dysfunction as a result of anterior spinal artery syndrome.[17][29] PSA infarcts are rare when compared to ASA infarcts.[17] Pain, sensory deficit, and urinary retention can also occur in SCI.[25][1] A large case series analyzing 133 spontaneous SCI patients admitted to one institution reported 96 (72%) of patients presented with severe back/limb pain during symptom onset possibly due to activation of the spinothalamic tract, 83% reported bowel and bladder dysfunction.[17] The extent of the deficits relies on the region of occlusion and the vasculature involved. The phenotypes classify according to the vascular supply that is involved (Table 2).
ASA involvement can manifest abruptly as symmetric motor dysfunction, bilateral spinothalamic sensory deficit below the level of the lesion, autonomic sphincter dysfunction including sphincter flaccidity, atonic bladder, and paralytic ileus, flaccid weakness associated with areflexia, and sudden back pain,[30][1][31] typically followed by upper motor neuron dysfunction including spasticity, and hyperreflexia.[9][13][3] Motor dysfunction occurs at and below the level of the lesion.[29] There are reports of dissociative anesthesia has also been reported.[31] PSA occlusion results in loss of vibration and proprioception below the level of the injury in addition to complete anesthesia at the level of the lesion and weakness in some cases.[13] Symptoms can be bilateral or unilateral; hemiplegia can ensue with corticospinal tract involvement.[32] A thoracic sensory loss could also occur in PSA infarction.[33] Occlusion of the vessels supplying C3 to C5 involved with the phrenic nerve manifests as respiratory dysfunction,[3] cessation of perfusion to the T4 to T9 leads to orthostatic hypotension due to dysfunction of the greater splanchnic nerve.[1] Autonomic dysregulation can occur if sympathetic fibers are affected resulting in constipation, urinary retention, bladder overflow, and sexual dysfunction.[3] Urologic dysfunction can also manifest as a result of detrusor hyperreflexia and sphincter dyssynergia.[34]
Brown-Sequard syndrome
Symptoms include unilateral flaccid weakness ipsilateral to and below the lesion, along with a contralateral absence of spinothalamic sensation with sparing of the posterior column sensory modalities (sulcocommissural artery occlusion).[1]
History and Physical
A thorough history and physical evaluation are crucial in providing rapid detection of SCI. This, together with neuroimaging, can aid in expedited detection of SCI. Suspected patients are managed with hemodynamic support to maximize blood pressure, lumbar drainage to lower the cerebrospinal fluid (CSF) pressure is often indicated to increase spinal perfusion and halt the progression of the injury.[27][3] Maintenance of optimal hemodynamic stability should be at a mean arterial pressure above 90mm Hg, accomplished with volume replacement and vasopressor support, along with proper monitoring for improvement or complications via serial neurological examinations over 12 to 48 hours.[1][27][3]
Evaluation
A review of 49 patients undergoing endovascular repair of descending thoracic aneurysm and thoracoabdominal aortic aneurysms facilitated by continuous intraoperative motor/somatosensory-evoked potential (MEP/SEP), cerebrospinal fluid drainage, and use of iliofemoral conduits allowed for early prediction and management of SCI.[35] Serial neurological evaluations are crucial particularly in the postoperative setting and must be performed immediately after dissipation of anesthesia and the patient awakens with the involvement of complete neurological examination in addition to Glasgow Coma Scale and National Institutes of Health Stroke Scale assessments performed according to previously published guidelines by the American Spinal Injury Association.[36] Transient loss in vibration and proprioception in the lower extremities can commonly occur during the first few hours; postoperative patients possess an elevated susceptibility to epidural hematoma due to anticoagulants during surgery.[1]
Laboratory Evaluation
Laboratory examinations are can differ depending on the clinical presentation they can include copper, zinc, vitamin B12, Lyme disease serology, varicella-zoster viral serology, HIV serology, syphilis serology, human T-lymphotropic virus 1 serology, antinuclear antibody, anti-neutrophil cytoplasmic antibody, anti-cyclic citrullinated peptide, angiotensin-converting enzyme, aquaporin-4-IgG, myelin oligodendrocyte glycoprotein, hypercoagulable profile, and paraneoplastic autoantibody assessment.[17]
CSF Analysis
Lumbar punctures are performed in certain SCI cases. CSF analysis includes red blood cell count, white blood cell count and differential, protein, and glucose. Further analysis may be comprised of IgG index, cytology, flow cytometry, gram stain, oligoclonal bands, bacterial culture, Lyme disease serology and polymerase chain reaction (PCR), cryptococcal antigen, paraneoplastic autoantibodies, venereal diseases, varicella-zoster virus PCR, Epstein-Barr virus PCR, enterovirus PCR, cytomegalovirus PCR, mycobacterium tuberculosis PCR, and cultures.[17] Elevated protein levels and white blood cells may occasionally occur, although not specific, arising from embolism, celiac plexus block, and vasculitis.[3][37][9]
Neuroimaging
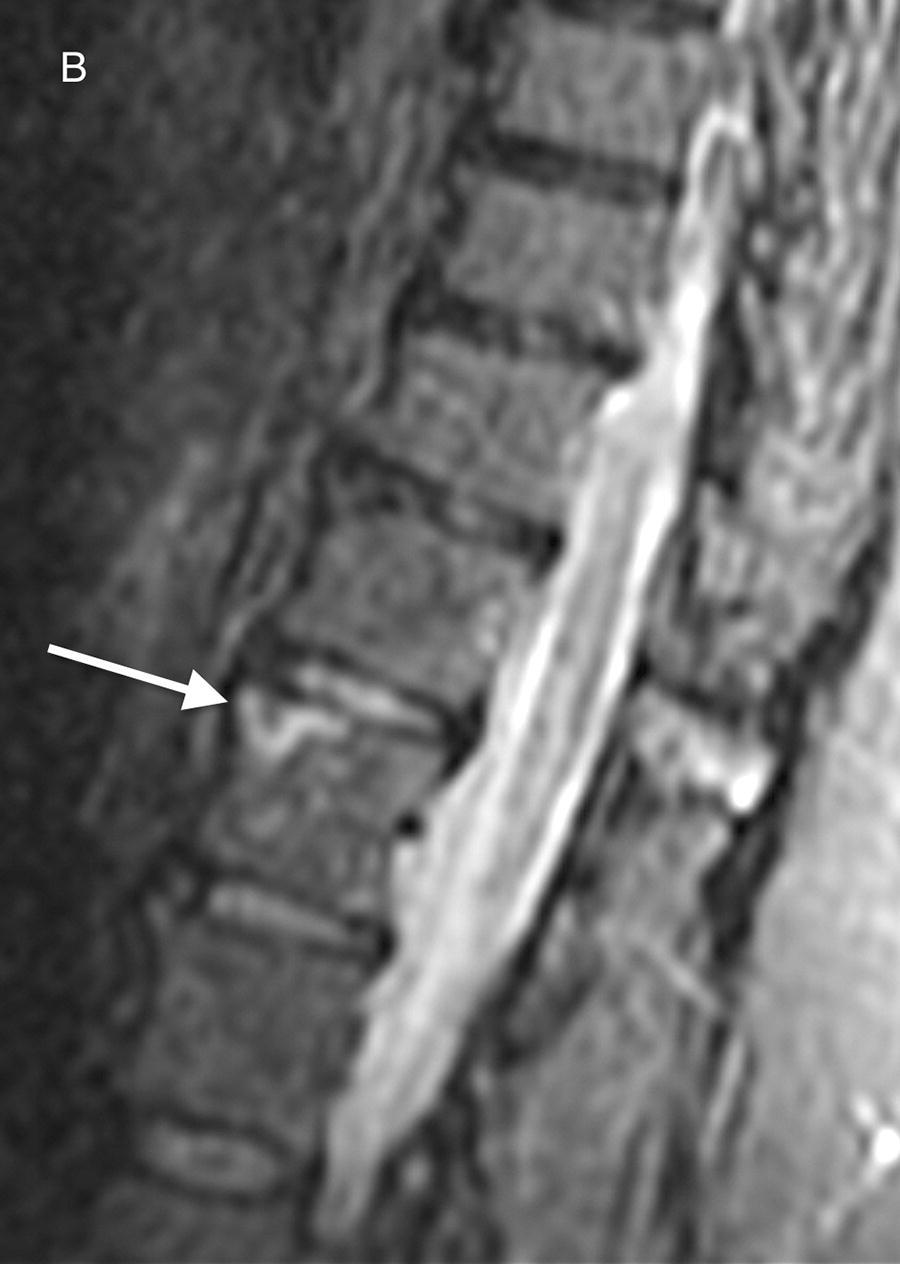
While radiography can be equivocal in certain cases, it remains to be a fundamental aspect of SCI diagnosis. Magnetic resonance imaging (MRI) at 1.5 Tesla (T) including sagittal and axial T1 and T2-weighted sequences and diffusion-weighted imaging (DWI) with contrast are often employed to identify and localize SCI.[13][7] DWI has become increasingly sensitive in SCI diagnosis, particularly during the acute phase (less than 24 hours).[38][31] Initial MRI findings can be unremarkable during the early stages; a second MRI days later is necessary.[31][17] Particular signs on imaging have correlations to SCI; they are not highly specific - however, their presence has been correlated to the vasculature that is involved. Spinal cord swelling and “pencil-like” hyperintensities on T2-weighted images (T2WI), enhancement on post-contrast T1-weighted images and hyperintense signal on DWI are among the frequently encountered imaging features of SCI.[7][31] Spinal cord edema will eventually get replaced with atrophy in the chronic phase.[9][13][3] The presence “owl’s eyes pattern” hyperintensities on axial T2WI is associated with ASA infarcts.[17] Vertebral body infarcts also develop and can be visualized on MRI; they are associated with radicular artery occlusion.[22] Computed tomography (CT) can deliver a diagnosis in cases where there are contraindications to MRI as in epidural hematoma or if the patient is clinically unstable. MRI is performed postoperatively in order to rule out spinal cord compression.[3][1] If neuroimaging illustrates structural lesions or abnormalities, an emergent neurosurgical consultation is warranted. Otherwise supportive measures continue to be carried out while exploring other etiologies.
Additional Diagnostic Evaluations
Additional examinations that can confirm SCI and rule out other etiologies include magnetic resonance angiography, computed tomography angiography, full spinal magnetic resonance angiography, thoracoabdominal computed tomography angiography, digital subtraction angiography and electromyography (EMG).[17] The presence of denervation is discernable with the use of EMG to localize the origin of the ischemic deficit.[9]
Treatment / Management
Prompt surgical management is necessary in cases of vascular compression and acute aortic event in addition to maintaining hemostatic equilibrium.[31] In the setting of aortic surgery, lumbar drainage and blood pressure augmentation are often options for management.[17] A review of spinal manifestations of patients with vertebral artery dissection revealed the use of anticoagulation was the sole form of therapy in spinal ischemia; antiplatelet therapy was also administered in conjunction with anticoagulants.[4] Another investigation used antiplatelet therapy in all 36 participants, including aspirin and clopidogrel.[22] Rehabilitation efforts have proven to be successful in achieving good outcomes.[17] Cases of vasculitis implore the use of corticosteroid therapy.[3] Decompression sickness treatment is with hyperbaric oxygen.[39](B2)
Differential Diagnosis
Neoplastic transformation, disk herniation, epidural abscesses, and epidural hematoma share a similar phenotype with SCI.[3] Multiple sclerosis has similar MRI findings; therefore distinguishing the two is crucial. This differentiation necessitates a thorough CSF analysis and paraneoplastic panel evaluation. Spinal cord compression can originate from spinal cord neoplasms and impede circulation, thus mimicking clinical manifestations of SCI. Infectious or autoimmune transverse myelitis and intramedullary hemorrhage or neoplasm can also present similarly to SCI.[40]
Prognosis
Timely diagnosis and management are essential for improving the outcome and prognosis of SCI. SCI is reversible, and a good functional outcome is attainable with prompt recognition and adequate management; the severity of neurological deficits generally determines the prognosis.[41][37] A study investigating the clinical manifestations, evaluations, and treatment of 36 patients with acute spinal stroke demonstrated favorable outcome including complete and incomplete recovery in 23 (64%) patients, no recovery in 12 (33%) patients, gait assistance required in 18 (50%), 11 (31%) were wheelchair-bound upon discharge and the favorable prognosis was associated with unilateral infarcts.[22] A prospective study including patients with SCI enrolled within 10 days of symptom onset, analysis of clinical and MRI findings demonstrated worst outcome in patients with proprioceptive deficits at the onset, this study also reported the development of pain in half of the patients.[37] Long-term outcome was assessed in 30 patients with SCI and compared to those with cerebral infarction; showed lower mortality rate, higher re-employment rate, and worse functional outcome.[42]
Complications
Loss of motor and sensory modalities, including paresthesias and paralysis, may persist in some instances, increasing the risk for cardiovascular and pulmonary diseases. Some SCI patients continue to suffer from chronic pain,[37][42] along with urinary and bowel dysfunction.SCI patients have reported other secondary complications to include: respiratory infections, spasticity, blood pressure fluctuation, and pressure sores.[43]
Deterrence and Patient Education
The National Institute of Neurological Disorders and Stroke (NINDS) provides a wide variety of educational material and information for patients with spinal cord injuries. Patients are encouraged to explore all the resources available, including clinical trials, patient organizations, and publications in this regard. Facilitation of community support and outreach for patients and caregivers come from organizations such as Christopher and Dana Reeve Foundation, Paralyzed Veterans of America, and the United Spinal Association.
Enhancing Healthcare Team Outcomes
Improving overall outcomes and achieving high-quality results requires joint efforts and full participation from patients and healthcare teams. Health care professionals must exercise concerted efforts in early detection of symptoms, triaging, and providing an expedited workup for patients with suspected spinal cord injuries. Proper and effective communication must take place between the interprofessional team, including emergency physicians, neurologists, neuroradiologists, neurosurgeons, other specialists, physiatrists, and specialized nursing staff to achieve higher quality care. Nurses who manage patients with spinal cord infarction should be aware of the potential complications like urinary retention, pressure sores, DVT, and contractures. The dietitian should be involved in the management of nutrition.
Pharmacist involvement comes with the initiation of antiplatelet or anticoagulation therapy. The pharmacist can verify dosing, check for drug interactions, and help change agents where any contraindications exist. They can also assist nursing in the monitoring of pharmaceutical therapy. These activities will be reported to the managing physician, especially in the event of any issues encountered.
The rehabilitation phase involves direct medical care from physiatrists and nursing staff. The nursing staff has a crucial role in monitoring ventilation and lung function, monitor cardiovascular function, stable blood pressure, and prevent infections and other complications from occurring. The patients’ effort during the rehabilitation period has a considerable impact on the recovery process. Only through such an interprofessional team approach can the morbidity of spinal cord infarction be lowered and patient outcomes optimized. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Rubin MN, Rabinstein AA. Vascular diseases of the spinal cord. Neurologic clinics. 2013 Feb:31(1):153-81. doi: 10.1016/j.ncl.2012.09.004. Epub [PubMed PMID: 23186899]
ROMANES GJ. THE ARTERIAL BLOOD SUPPLY OF THE HUMAN SPINAL CORD. Paraplegia. 1965 Mar:2():199-207 [PubMed PMID: 14261502]
Kramer CL. Vascular Disorders of the Spinal Cord. Continuum (Minneapolis, Minn.). 2018 Apr:24(2, Spinal Cord Disorders):407-426. doi: 10.1212/CON.0000000000000595. Epub [PubMed PMID: 29613893]
Crum B, Mokri B, Fulgham J. Spinal manifestations of vertebral artery dissection. Neurology. 2000 Jul 25:55(2):304-6 [PubMed PMID: 10908913]
Level 3 (low-level) evidenceTurnbull IM, Brieg A, Hassler O. Blood supply of cervical spinal cord in man. A microangiographic cadaver study. Journal of neurosurgery. 1966 Jun:24(6):951-65 [PubMed PMID: 5936484]
Martirosyan NL, Feuerstein JS, Theodore N, Cavalcanti DD, Spetzler RF, Preul MC. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. Journal of neurosurgery. Spine. 2011 Sep:15(3):238-51. doi: 10.3171/2011.4.SPINE10543. Epub 2011 Jun 10 [PubMed PMID: 21663407]
Level 3 (low-level) evidenceWeidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. 2015 Mar:57(3):241-57. doi: 10.1007/s00234-014-1464-6. Epub 2014 Nov 16 [PubMed PMID: 25398656]
Chakravorty BG. Arterial supply of the cervical spinal cord and its relation to the cervical myelopathy in spondylosis. Annals of the Royal College of Surgeons of England. 1969 Oct:45(4):232-51 [PubMed PMID: 4980920]
Level 3 (low-level) evidenceCheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996 Aug:47(2):321-30 [PubMed PMID: 8757000]
Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A. Vascular anatomy of the spinal cord. Journal of neurointerventional surgery. 2012 Jan 1:4(1):67-74. doi: 10.1136/neurintsurg-2011-010018. Epub 2011 May 2 [PubMed PMID: 21990489]
Level 3 (low-level) evidenceDarouiche RO. Spinal epidural abscess. The New England journal of medicine. 2006 Nov 9:355(19):2012-20 [PubMed PMID: 17093252]
Ahmann PA, Smith SA, Schwartz JF, Clark DB. Spinal cord infarction due to minor trauma in children. Neurology. 1975 Apr:25(4):301-7 [PubMed PMID: 1168316]
Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, Lovblad KO, Dietemann JL. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR. American journal of neuroradiology. 2015 May:36(5):825-30. doi: 10.3174/ajnr.A4118. Epub 2014 Oct 16 [PubMed PMID: 25324492]
Piffaretti G, Bonardelli S, Bellosta R, Mariscalco G, Lomazzi C, Tolenaar JL, Zanotti C, Guadrini C, Sarcina A, Castelli P, Trimarchi S. Spinal cord ischemia after simultaneous and sequential treatment of multilevel aortic disease. The Journal of thoracic and cardiovascular surgery. 2014 Oct:148(4):1435-1442.e1. doi: 10.1016/j.jtcvs.2014.02.062. Epub 2014 Feb 26 [PubMed PMID: 24698563]
Level 2 (mid-level) evidenceLynch DR, Dawson TM, Raps EC, Galetta SL. Risk factors for the neurologic complications associated with aortic aneurysms. Archives of neurology. 1992 Mar:49(3):284-8 [PubMed PMID: 1311168]
Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012 Jan 10:78(2):114-21. doi: 10.1212/WNL.0b013e31823efc93. Epub 2011 Dec 28 [PubMed PMID: 22205760]
Zalewski NL, Rabinstein AA, Krecke KN, Brown RD Jr, Wijdicks EFM, Weinshenker BG, Kaufmann TJ, Morris JM, Aksamit AJ, Bartleson JD, Lanzino G, Blessing MM, Flanagan EP. Characteristics of Spontaneous Spinal Cord Infarction and Proposed Diagnostic Criteria. JAMA neurology. 2019 Jan 1:76(1):56-63. doi: 10.1001/jamaneurol.2018.2734. Epub [PubMed PMID: 30264146]
Phillips OC, Ebner H, Nelson AT, Black MH. Neurologic complications following spinal anesthesia with lidocaine: a prospective review of 10,440 cases. Anesthesiology. 1969 Mar:30(3):284-9 [PubMed PMID: 4305091]
Level 3 (low-level) evidenceKane RE. Neurologic deficits following epidural or spinal anesthesia. Anesthesia and analgesia. 1981 Mar:60(3):150-61 [PubMed PMID: 7011100]
Level 3 (low-level) evidenceMOSBERG WH Jr, VORIS HC, DUFFY J. Paraplegia as a complication of sympathectomy for hypertension. Annals of surgery. 1954 Mar:139(3):330-4 [PubMed PMID: 13149078]
HUGHES JT, MACINTYRE AG. SPINAL CORD INFARCTION OCCURRING DURING THORACO-LUMBAR SYMPATHECTOMY. Journal of neurology, neurosurgery, and psychiatry. 1963 Oct:26(5):418-21 [PubMed PMID: 14066633]
Kumral E, Polat F, Güllüoglu H, Uzunköprü C, Tuncel R, Alpaydın S. Spinal ischaemic stroke: clinical and radiological findings and short-term outcome. European journal of neurology. 2011 Feb:18(2):232-239. doi: 10.1111/j.1468-1331.2010.02994.x. Epub [PubMed PMID: 20402756]
Rigney L, Cappelen-Smith C, Sebire D, Beran RG, Cordato D. Nontraumatic spinal cord ischaemic syndrome. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2015 Oct:22(10):1544-9. doi: 10.1016/j.jocn.2015.03.037. Epub 2015 Jul 4 [PubMed PMID: 26154150]
Qureshi AI, Akbar MS, Czander E, Safdar K, Janssen RS, Frankel MR. Crack cocaine use and stroke in young patients. Neurology. 1997 Feb:48(2):341-5 [PubMed PMID: 9040718]
Level 2 (mid-level) evidenceMateen FJ, Monrad PA, Leep Hunderfund AN, Robertson CE, Sorenson EJ. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. European journal of neurology. 2011 Feb:18(2):218-225. doi: 10.1111/j.1468-1331.2010.03200.x. Epub 2010 Sep 6 [PubMed PMID: 20825469]
Level 2 (mid-level) evidenceNovy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Archives of neurology. 2006 Aug:63(8):1113-20 [PubMed PMID: 16908737]
Level 2 (mid-level) evidenceMcGarvey ML, Cheung AT, Szeto W, Messe SR. Management of neurologic complications of thoracic aortic surgery. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2007 Aug:24(4):336-43 [PubMed PMID: 17938602]
Gialdini G, Parikh NS, Chatterjee A, Lerario MP, Kamel H, Schneider DB, Navi BB, Murthy SB, Iadecola C, Merkler AE. Rates of Spinal Cord Infarction After Repair of Aortic Aneurysm or Dissection. Stroke. 2017 Aug:48(8):2073-2077. doi: 10.1161/STROKEAHA.117.017071. Epub 2017 Jun 27 [PubMed PMID: 28655811]
Santana JA, Dalal K. Ventral Cord Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 31082055]
Foo CL, Swann M. Isolated paralysis of the serratus anterior. A report of 20 cases. The Journal of bone and joint surgery. British volume. 1983 Nov:65(5):552-6 [PubMed PMID: 6643557]
Level 3 (low-level) evidenceYadav N, Pendharkar H, Kulkarni GB. Spinal Cord Infarction: Clinical and Radiological Features. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2018 Oct:27(10):2810-2821. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.008. Epub 2018 Aug 6 [PubMed PMID: 30093205]
Etgen T, Höcherl C. Repeated early thrombolysis in cervical spinal cord ischemia. Journal of thrombosis and thrombolysis. 2016 Jul:42(1):142-5. doi: 10.1007/s11239-015-1332-1. Epub [PubMed PMID: 26762860]
Mascalchi M, Cosottini M, Ferrito G, Salvi F, Nencini P, Quilici N. Posterior spinal artery infarct. AJNR. American journal of neuroradiology. 1998 Feb:19(2):361-3 [PubMed PMID: 9504495]
Level 3 (low-level) evidenceKaplan SA, Chancellor MB, Blaivas JG. Bladder and sphincter behavior in patients with spinal cord lesions. The Journal of urology. 1991 Jul:146(1):113-7 [PubMed PMID: 2056568]
Banga PV, Oderich GS, Reis de Souza L, Hofer J, Cazares Gonzalez ML, Pulido JN, Cha S, Gloviczki P. Neuromonitoring, Cerebrospinal Fluid Drainage, and Selective Use of Iliofemoral Conduits to Minimize Risk of Spinal Cord Injury During Complex Endovascular Aortic Repair. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2016 Feb:23(1):139-49. doi: 10.1177/1526602815620898. Epub 2015 Dec 4 [PubMed PMID: 26637837]
Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal cord. 1997 May:35(5):266-74 [PubMed PMID: 9160449]
Level 1 (high-level) evidenceMasson C, Pruvo JP, Meder JF, Cordonnier C, Touzé E, De La Sayette V, Giroud M, Mas JL, Leys D, Study Group on Spinal Cord Infarction of the French Neurovascular Society. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. Journal of neurology, neurosurgery, and psychiatry. 2004 Oct:75(10):1431-5 [PubMed PMID: 15377691]
Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006 Nov:48(11):795-801 [PubMed PMID: 16977443]
Level 1 (high-level) evidenceGempp E, Blatteau JE. Risk factors and treatment outcome in scuba divers with spinal cord decompression sickness. Journal of critical care. 2010 Jun:25(2):236-42. doi: 10.1016/j.jcrc.2009.05.011. Epub 2009 Aug 13 [PubMed PMID: 19682840]
Level 2 (mid-level) evidenceHamiko M, Endlich M, Krämer C, Probst C, Welz A, Wilhelm K, Schiller W. Dilatation of Vascular Prostheses in Ascending Aortic Position: A Long-Term Follow-Up Computed Tomography Study with Comparison of Different Measurement Methods. The Thoracic and cardiovascular surgeon. 2018 Apr:66(3):206-214. doi: 10.1055/s-0036-1597116. Epub 2016 Dec 13 [PubMed PMID: 27960216]
Iseli E, Cavigelli A, Dietz V, Curt A. Prognosis and recovery in ischaemic and traumatic spinal cord injury: clinical and electrophysiological evaluation. Journal of neurology, neurosurgery, and psychiatry. 1999 Nov:67(5):567-71 [PubMed PMID: 10519858]
Level 2 (mid-level) evidenceHanson SR, Romi F, Rekand T, Naess H. Long-term outcome after spinal cord infarctions. Acta neurologica Scandinavica. 2015 Apr:131(4):253-7. doi: 10.1111/ane.12343. Epub 2014 Oct 27 [PubMed PMID: 25346212]
Piatt JA, Nagata S, Zahl M, Li J, Rosenbluth JP. Problematic secondary health conditions among adults with spinal cord injury and its impact on social participation and daily life. The journal of spinal cord medicine. 2016 Nov:39(6):693-698 [PubMed PMID: 26833021]