Introduction
Axillary lymphadenectomy, or axillary dissection, is a surgical procedure involving the removal of all lymphatic tissue from the axilla. Traditionally, this procedure was performed alongside a modified radical mastectomy and was a critical component in breast cancer management. However, advancements in breast cancer biology, improvements in systemic chemotherapy and radiation techniques, and landmark trials demonstrating the effectiveness of less invasive procedures have significantly reduced the frequency of axillary lymphadenectomy.[1][2][3]
The primary indications for axillary lymphadenectomy currently include clinically involved axillary lymph nodes in breast cancer, axillary node recurrences, and positive lymph nodes in nonmammary malignancies such as melanoma and squamous cell carcinoma.[4][5] Axillary lymphadenectomy is associated with significant morbidity, particularly lymphedema, making a comprehensive understanding of axillary anatomy surgical indications crucial for safe and effective surgical performance.[6][7]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Axillary Anatomy
The anatomy of the axilla is fundamental to understanding the critical lymphatic drainage pathways essential for breast cancer staging and surgical interventions. The axilla accounts for 95% of the breast's lymphatic drainage, highlighting the importance of understanding its anatomical structure for safely performing axillary lymphadenectomy. This region forms a pyramidal space with its apex directed toward the base of the neck. The axillary boundaries include the pectoralis muscles, clavipectoral fascia, and clavicle anteriorly; the subscapularis, scapula, teres major, and latissimus dorsi muscles posteriorly; the humerus laterally; and the chest wall, covered by the serratus anterior muscle, medially. The clavipectoral fascia, originating from the clavicle, extends deep to the pectoralis major and helps form the axillary floor. Please see StatPearls' companion resource, "Anatomy, Shoulder and Upper Limb, Axilla," for more information.
The axilla comprises blood vessels, nerves, muscles, tendons, fat, and lymph nodes, with the amount of adipose tissue varying significantly between individuals. The axillary artery, which is a continuation of the subclavian artery beyond the first rib, transitions into the brachial artery at the inferior border of the teres major muscle. Similarly, the axillary vein begins at the inferior border of the teres major, continuing as the subclavian vein superiorly and the basilic vein inferiorly. The brachial plexus accompanies the axillary artery and vein, all of which are encased within the axillary sheath, a continuation of the neck's prevertebral fascia.
The axilla contains several branches of the brachial plexus and various cutaneous nerves, although a detailed exploration of this anatomy is beyond the scope of this activity. Critical nerves at risk during axillary lymphadenectomy include the thoracodorsal nerve, which originates from the posterior cord of the brachial plexus and runs alongside the thoracodorsal artery and vein to innervate the latissimus dorsi muscle, and the long thoracic nerve, arising from the C5 to C7 nerve roots to innervate the serratus anterior muscle along the medial chest wall. Both nerves must be carefully identified and preserved.
Additionally, the intercostobrachial nerve—a sensory nerve originating from the second intercostal nerve and innervating the medial arm and axillary skin—traverses the axilla from medial to lateral. This nerve is often sacrificed during axillary lymphadenectomy. Please see StatPearls' companion resource, "Anatomy, Shoulder and Upper Limb, Axilla," for more information.
The axillary lymph nodes are embedded within the fatty tissue of the axilla and are classified into the 5 groups as mentioned below (see Image. Anatomy of Axillary Lymph Nodes).
- Anterior (pectoral) lymph nodes: These nodes are located along the inferior border of the pectoralis minor near the lateral thoracic vessels. They receive lymph from the breast, skin, and muscles of the supraumbilical anterolateral body wall. They drain into the central and apical nodes.
- Posterior (subscapular) lymph nodes: These nodes are positioned along the posterior axillary wall near the inferior border of the subscapularis. They receive lymph from the scapular region and posterior thoracic wall, which drains into the central and apical nodes.
- Lateral (humeral) lymph nodes: These nodes are located along the lateral wall of the axilla. They receive lymph from the arm and drain into the central, apical, and deep cervical nodes.
- Central lymph nodes: These nodes are located at the base of the axilla. They receive lymph from the anterior, posterior, and lateral lymph node groups and subsequently drain into the apical nodes.
- Apical (terminal) lymph nodes: These nodes are found deep at the apex of the axilla, and they receive lymph from all other axillary groups and the upper breast. Efferent vessels from the apical group form the subclavian lymphatic trunk, which drains into the thoracic duct on the left and into the right lymphatic duct at the right venous angle on the right. Please see StatPearls' companion resource, "Anatomy, Shoulder and Upper Limb, Axillary Lymph Nodes," for more information.
Lymph Node Levels
Axillary lymph nodes are commonly classified based on their relationship to the pectoralis minor muscle. Level I nodes are located lateral to or below the lower border of the pectoralis minor and typically include the external mammary, axillary vein, and scapular lymph node groups. Level II nodes are situated deep to the pectoralis minor muscle and include the central lymph node group and potentially some subclavicular nodes. Level III nodes are located medial or superior to the upper border of the pectoralis minor and include the subclavicular lymph nodes.
Surgeons also commonly identify Rotter (interpectoral) nodes that are located between the pectoralis major and minor muscles. These Rotter nodes may further drain into the central or subclavicular node groups, potentially serving as a "skip pathway" for tumor cells to metastasize from the breast to level III nodes while bypassing level I or II.[8][9] Additional lymphatic drainage sites include the internal mammary chain, the lateral and medial intramammary regions, the interpectoral region, and the subclavicular lymph node basin.
Most axillary lymphatic tissues are located in level I (60%-70%) and level II (20%-30%), making these the most common sites for axillary metastases. As a result, a standard axillary lymphadenectomy typically involves the removal of lymph nodes from levels I and II. Level III lymph nodes are more likely to be involved in cases of aggressive or extensive breast cancer and are commonly affected in malignant melanoma. In such cases, level III nodes should also be included in the dissection.[10]
Indications
Indications for axillary lymphadenectomy in patients with breast cancer include:
- Clinically positive axillary lymph nodes
- Inflammatory breast cancer
- More than 3 positive sentinel lymph nodes
- Persistently positive lymph nodes after neoadjuvant therapy
- Unsuccessful sentinel lymph node biopsy
- Patients unable to receive radiation therapy
- Axillary lymph node recurrence after prior breast cancer treatment [11][12][13][14][15][16][17]
Additional indications for axillary lymphadenectomy include:
- Melanoma with clinically positive lymph nodes
- Squamous cell carcinoma of the skin with clinically positive lymph nodes
Contraindications
Axillary lymph node dissection has no absolute contraindications apart from distant metastatic disease. However, if less invasive treatment options are available for patients with localized breast cancer, they should be considered.
Equipment
Axillary lymph node dissection does not require specialized equipment; a standard surgical instrument tray with retractors is sufficient for the procedure.
Personnel
The operating room team typically consists of a surgeon, an assistant, a surgical technician or operating room nurse, and a circulating nurse. The procedure may be performed under general or regional anesthesia.
Preparation
Patients undergoing axillary lymphadenectomy should undergo a comprehensive preoperative review at an interprofessional tumor board. The indications for the procedure should be carefully evaluated, and less invasive alternatives should be considered. Prophylactic antibiotics should be administered to patients within 30 minutes before the incision.
Patients are typically positioned supine with the arm extended and the axilla aligned at the edge of the operating table to ensure optimal access to the posterior axilla. The involved arm should be included in the operative field, properly prepped, and draped. For axillary dissections involving melanoma or clinically positive level III lymph nodes, a more extensive dissection is required. A self-retaining retractor, such as an Omni-Tract™ or Thompson retractor, can facilitate the procedure.
Technique or Treatment
The axillary dissection procedure begins with patient preparation and positioning. The operative area is cleaned and draped, and the arm is positioned to ensure optimal exposure. Anatomical landmarks, such as the latissimus dorsi posteriorly and the pectoralis major anteriorly, are identified and marked.
The initial incision is typically made along the inferior axillary hairline or posterior and parallel to the margin of the pectoralis major, measuring 5 to 10 cm depending on the patient’s body habitus. A local anesthetic agent is infiltrated, and electrocautery is used to divide the dermis and subcutaneous tissue layers. The clavipectoral fascia is then identified and incised to access the axilla. Deep retractors, such as Army-Navy or Richardson retractors, are employed to improve visualization.
Key Anatomical Landmarks in Axillary Lymphadenectomy
The key anatomical landmarks identified during axillary lymphadenectomy are mentioned below. The sequence of landmark identification may vary based on surgeon preference or patient anatomy.
Axillary vein: The axillary vein, located superiorly, marks the upper boundary of the lymphadenectomy. Careful dissection with judicious use of electrocautery is essential to prevent accidental injury. All fibrofatty tissue overlying the vein is swept downward to ensure it is included in the specimen. Dissection should not extend above this point, as the brachial plexus and its branches lie superior to the axillary vein. Venous branches encountered from medial to lateral, including the lateral thoracic and thoracodorsal bundles, must be ligated.
Thoracodorsal nerve: The thoracodorsal vein is a key landmark for identifying the thoracodorsal nerve, which innervates the latissimus dorsi and should be preserved whenever possible. If tumor involvement is present, sacrificing the nerve may be necessary. The intercostobrachial nerve, a cutaneous nerve supplying sensation to the medial arm, is commonly encountered during dissection and is typically sacrificed.
Pectoralis major: The fascia over the pectoralis major is incised to define the anterior limit of the dissection. Identifying the pectoralis major along its length facilitates exposure of the underlying pectoralis minor. The fibrofatty tissue located between the pectoralis muscles is included in the specimen. Medial retraction of the pectoralis major using an Army-Navy retractor provides optimal visualization and access during this step.
Long thoracic nerve: The dissection proceeds posteriorly to the surface of the serratus anterior, where the long thoracic nerve of Bell is identified. At this stage, this nerve lies directly on the serratus anterior and should be carefully preserved during the procedure.
After clearing the superior, anterior, and medial boundaries of all fibrofatty tissue and identifying the long thoracic and thoracodorsal nerves, the remaining fibrofatty tissue in the axillary space is removed. With no other critical structures in this area, the remainder of the procedure is relatively straightforward. Care is taken to clip or ligate prominent lymphatics to minimize the risk of lymphocele formation.
If level III lymph nodes need to be removed, exposure to the space superior to the pectoralis minor must be obtained. This can be achieved by retracting the pectoralis minor laterally or dividing it at its origin. Once access is gained, the fibrofatty tissue in this region should be swept down and included with the specimen. Division of the pectoralis minor is typically associated with minimal morbidity.
Upon completion of the axillary dissection, the operative site reveals the surfaces of the pectoralis major, serratus anterior, and latissimus dorsi muscles, as well as the skeletonized axillary vein and preserved nerves. A closed suction drain is typically placed at this stage. The clavipectoral fascia is then closed, followed by the closure of the subcutaneous tissue and skin in layers.[18]
Postoperative Care
Patients may be discharged on the same day or, more commonly, kept overnight for observation. Drain output is closely monitored. Resting the arm in a sling can help reduce pain and prevent seroma formation. Physiotherapy, which has been shown to reduce lymphedema rates, should be initiated early, with most patients encouraged to begin mobilizing the affected extremity within 48 to 72 hours post-surgery. Suction drains are typically left in place until the output is less than 30 mL daily for 2 consecutive days. While prolonged drainage may reduce seroma formation, it can increase the risk of wound infection. Most patients require oral analgesics for pain management. Close postoperative surveillance is essential to monitor for signs of lymphedema, as early symptoms can be subtle. Arm circumference measurement is a simple method for detection. Early identification of lymphedema and prompt interventions are associated with improved patient outcomes.[19]
Complications
Seroma formation is nearly universal following axillary lymphadenectomy, although clinically significant seromas requiring intervention are less common, with reported rates ranging from 10% to 80%. Closed suction wound drainage effectively reduces clinically significant seromas. Temporary immobilization with slings or compressive bandages may lower seroma rates; however, the increased risk of lymphedema outweighs this benefit. Additional strategies, such as applying fibrin sealant during surgery and minimizing electrocautery through meticulous surgical technique, can further reduce seroma formation.[20][21]
Lymphedema, the accumulation of lymphatic fluid in the arm after axillary lymphadenectomy, is a common and potentially debilitating complication, with a reported incidence of approximately 20% in the largest meta-analysis.[22] Lymphedema most commonly develops within the first 2 years following surgery, after which new cases are less likely. Risk factors include extensive lymphadenectomy, obesity, adjuvant chemotherapy, radiotherapy, and mastectomy performed alongside lymphadenectomy. While regular physical activity has also been associated with increased risk, its benefits often outweigh this concern. Diagnosis relies on clinical examination, with a high index of suspicion and routine arm circumference measurements at follow-up visits being essential for early detection.[23][24]
Wound infections after axillary lymphadenectomy occur in 1% to 20% of cases, with most being superficial surgical site infections. Deep infections are less common but may necessitate operative drainage when present.[7]
Nerve injury is a potential complication of axillary lymphadenectomy. Damage to the long thoracic nerve can weaken the serratus anterior muscle, leading to scapular winging, while injury to the thoracodorsal nerve causes latissimus dorsi denervation, resulting in weakened shoulder abduction. The intercostobrachial nerve is the most commonly injured during axillary lymphadenectomy, often being intentionally sacrificed, which results in anesthesia on the medial surface of the arm. Injury to the medial and lateral pectoral nerves can cause atrophy of the pectoralis major, which is particularly problematic for patients undergoing breast reconstruction with implants, resulting in poorer cosmetic outcomes.[25]
Angiosarcoma of the extremity often develops in the context of chronic lymphedema following axillary lymphadenectomy—a condition known as Stewart-Treves syndrome. This malignancy is highly aggressive, with rapid progression and a strong tendency for distant metastasis, often leading to a fatal outcome. The typical latency period for angiosarcoma development after lymphadenectomy is approximately 10 years, with the risk further increased in patients who have undergone adjuvant radiation therapy. Management typically involves a multimodal approach, including surgical resection and systemic chemotherapy, although the prognosis remains poor, with limited long-term survival.[26]
Axillary web syndrome is characterized by the development of fibrotic bands in the axilla, most commonly following axillary lymphadenectomy. However, it can also occur after less invasive procedures, eg, sentinel lymph node biopsy. These fibrous cords, primarily composed of scar tissue, typically appear within the first few weeks after surgery and resolve gradually over time. The bands become more prominent with arm abduction, often causing pain and limiting shoulder movement. The condition is self-limiting.[27]
Clinical Significance
Axillary lymphadenectomy involves the removal of all lymphatic tissue from the axilla. Although, historically, axillary lymphadenectomy was considered the standard procedure for managing cancers involving axillary lymph nodes, its use has become less common. However, this procedure remains a critical component of the surgeon’s armamentarium, particularly for treating patients with clinically node-positive breast cancer and other malignancies such as melanoma and cutaneous squamous cell carcinoma.
A comprehensive understanding of axillary anatomy, meticulous surgical technique, and proper postoperative care and surveillance are crucial for minimizing complications associated with axillary lymphadenectomy, particularly lymphedema. In areas with limited access to advanced radiotherapy and chemotherapy, axillary lymph node dissection remains a standard approach for treating patients with suspected axillary cancer involvement.
Enhancing Healthcare Team Outcomes
Axillary lymphadenectomy is a crucial therapeutic procedure for patients with clinically positive lymph nodes in breast cancer, melanoma, or cutaneous squamous cell carcinoma. Effective management requires an interprofessional approach to ensure optimal, patient-centered care and improved outcomes. Physicians and advanced practitioners must collaborate to assess the necessity of the procedure, evaluate the feasibility of less invasive options, and tailor treatment plans to meet the individual needs of the patient.
Interprofessional healthcare providers, including surgeons, radiologists, oncologists, and survivorship specialists, collaborate to develop comprehensive treatment strategies for patients undergoing the procedure. Nursing staff and physiotherapists support postoperative recovery and help prevent complications. Pharmacists are critical in managing pain, preventing infections, and addressing comorbidities. Effective interprofessional communication and seamless care coordination are vital for ensuring patient safety and team performance. By fostering a collaborative, patient-centered model that emphasizes shared decision-making, respect for autonomy, and the integration of interprofessional expertise, healthcare teams can improve outcomes and minimize the risks associated with axillary lymphadenectomy.
Media
(Click Image to Enlarge)
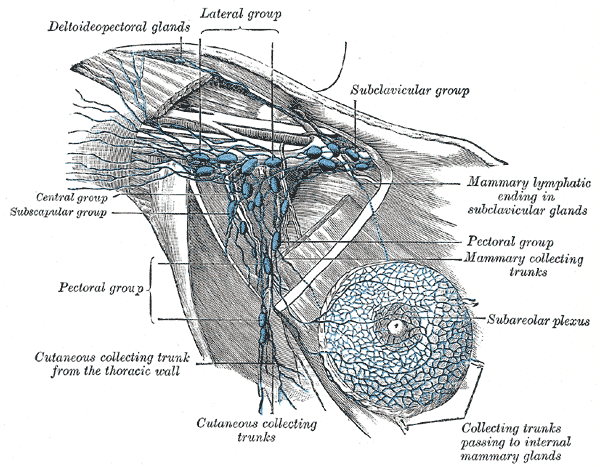
Anatomy of Axillary Lymph Nodes. Illustration of axillary lymph node anatomy, depicting the deltopectoral glands; lateral, subclavicular, central, subscapular, and pectoral groups; cutaneous and mammary collecting trunks; subareolar plexus; and mammary lymphatic ending in the subclavicular glands.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017 Sep 12:318(10):918-926. doi: 10.1001/jama.2017.11470. Epub [PubMed PMID: 28898379]
Level 1 (high-level) evidenceGalimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U, International Breast Cancer Study Group Trial 23-01 investigators. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. The Lancet. Oncology. 2013 Apr:14(4):297-305. doi: 10.1016/S1470-2045(13)70035-4. Epub 2013 Mar 11 [PubMed PMID: 23491275]
Level 1 (high-level) evidenceMontagna G, Mrdutt MM, Sun SX, Hlavin C, Diego EJ, Wong SM, Barrio AV, van den Bruele AB, Cabioglu N, Sevilimedu V, Rosenberger LH, Hwang ES, Ingham A, Papassotiropoulos B, Nguyen-Sträuli BD, Kurzeder C, Aybar DD, Vorburger D, Matlac DM, Ostapenko E, Riedel F, Fitzal F, Meani F, Fick F, Sagasser J, Heil J, Karanlik H, Dedes KJ, Romics L, Banys-Paluchowski M, Muslumanoglu M, Perez MDRC, Díaz MC, Heidinger M, Fehr MK, Reinisch M, Tukenmez M, Maggi N, Rocco N, Ditsch N, Gentilini OD, Paulinelli RR, Zarhi SS, Kuemmel S, Bruzas S, di Lascio S, Parissenti TK, Hoskin TL, Güth U, Ovalle V, Tausch C, Kuerer HM, Caudle AS, Boileau JF, Boughey JC, Kühn T, Morrow M, Weber WP. Omission of Axillary Dissection Following Nodal Downstaging With Neoadjuvant Chemotherapy. JAMA oncology. 2024 Jun 1:10(6):793-798. doi: 10.1001/jamaoncol.2024.0578. Epub [PubMed PMID: 38662396]
Bingoel AS, Vogt PM. [Indications for selective lymphadenectomy and systematic axillary, inguinal and iliac lymph node dissection]. Chirurgie (Heidelberg, Germany). 2023 Feb:94(2):114-120. doi: 10.1007/s00104-022-01739-z. Epub 2022 Nov 1 [PubMed PMID: 36319745]
Level 1 (high-level) evidenceThompson JL, Wright GP. Contemporary approaches to the axilla in breast cancer. American journal of surgery. 2023 Mar:225(3):583-587. doi: 10.1016/j.amjsurg.2022.11.036. Epub 2022 Dec 5 [PubMed PMID: 36522219]
Magnoni F, Galimberti V, Corso G, Intra M, Sacchini V, Veronesi P. Axillary surgery in breast cancer: An updated historical perspective. Seminars in oncology. 2020 Dec:47(6):341-352. doi: 10.1053/j.seminoncol.2020.09.001. Epub 2020 Oct 23 [PubMed PMID: 33131896]
Level 3 (low-level) evidenceAl-Hilli Z, Wilkerson A. Breast Surgery: Management of Postoperative Complications Following Operations for Breast Cancer. The Surgical clinics of North America. 2021 Oct:101(5):845-863. doi: 10.1016/j.suc.2021.06.014. Epub 2021 Aug 7 [PubMed PMID: 34537147]
Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Annals of surgery. 2004 Feb:239(2):232-7 [PubMed PMID: 14745331]
Komenaka IK, Bauer VP, Schnabel FR, Joseph KA, Horowitz E, Ditkoff BA, El-Tamer MB. Interpectoral nodes as the initial site of recurrence in breast cancer. Archives of surgery (Chicago, Ill. : 1960). 2004 Feb:139(2):175-8 [PubMed PMID: 14769576]
Level 2 (mid-level) evidenceZaveri S, Lillemoe HA, Teshome M, Reyna CR, Vreeland TJ, Francescatti AB, Zheng L, Hunt KK, Katz MHG, Kilgore LJ. Operative standards for sentinel lymph node biopsy and axillary lymphadenectomy for breast cancer: review of the American College of Surgeons commission on cancer standards 5.3 and 5.4. Surgery. 2023 Sep:174(3):717-721. doi: 10.1016/j.surg.2023.04.007. Epub 2023 May 16 [PubMed PMID: 37202308]
Ling DC, Iarrobino NA, Champ CE, Soran A, Beriwal S. Regional Recurrence Rates With or Without Complete Axillary Dissection for Breast Cancer Patients with Node-Positive Disease on Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy. Advances in radiation oncology. 2020 Mar-Apr:5(2):163-170. doi: 10.1016/j.adro.2019.09.006. Epub 2019 Sep 27 [PubMed PMID: 32280815]
Level 3 (low-level) evidenceWu D, Liu SY, Amina M, Fan ZM. [Normalization in axillary lymph node management after neoadjuvant therapy for breast cancer]. Zhonghua wai ke za zhi [Chinese journal of surgery]. 2019 Feb 1:57(2):97-101. doi: 10.3760/cma.j.issn.0529-5815.2019.02.005. Epub [PubMed PMID: 30704211]
de Barros ACSD, de Andrade DA. Extended Sentinel Node Biopsy in Breast Cancer Patients who Achieve Complete Nodal Response with Neoadjuvant Chemotherapy. European journal of breast health. 2020 Apr:16(2):99-105. doi: 10.5152/ejbh.2020.4730. Epub 2020 Apr 1 [PubMed PMID: 32285030]
Costaz H, Rouffiac M, Boulle D, Arnould L, Beltjens F, Desmoulins I, Peignaux K, Ladoire S, Vincent L, Jankowski C, Coutant C. [Strategies in case of metastatic sentinel lymph node in breast cancer]. Bulletin du cancer. 2020 Jun:107(6):672-685. doi: 10.1016/j.bulcan.2019.09.005. Epub 2019 Nov 4 [PubMed PMID: 31699399]
Level 3 (low-level) evidenceJung J, Kim BH, Kim J, Oh S, Kim SJ, Lim CS, Choi IS, Hwang KT. Validating the ACOSOG Z0011 Trial Result: A Population-Based Study Using the SEER Database. Cancers. 2020 Apr 11:12(4):. doi: 10.3390/cancers12040950. Epub 2020 Apr 11 [PubMed PMID: 32290437]
Cipolla C, Valerio MR, Grassi N, Calamia S, Latteri S, Latteri M, Graceffa G, Vieni S. Axillary Nodal Burden in Breast Cancer Patients With Pre-operative Fine Needle Aspiration-proven Positive Lymph Nodes Compared to Those With Positive Sentinel Nodes. In vivo (Athens, Greece). 2020 Mar-Apr:34(2):729-734. doi: 10.21873/invivo.11831. Epub [PubMed PMID: 32111777]
Dixon JM, Cartlidge CWJ. Twenty-five years of change in the management of the axilla in breast cancer. The breast journal. 2020 Jan:26(1):22-26. doi: 10.1111/tbj.13720. Epub 2019 Dec 19 [PubMed PMID: 31854498]
Thomson DR, Trevatt AE, Furniss D. When should axillary drains be removed? A meta-analysis of time-limited versus volume controlled strategies for timing of drain removal following axillary lymphadenectomy. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2016 Dec:69(12):1614-1620. doi: 10.1016/j.bjps.2016.09.027. Epub 2016 Oct 5 [PubMed PMID: 27777176]
Level 1 (high-level) evidenceGillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland surgery. 2018 Aug:7(4):379-403. doi: 10.21037/gs.2017.11.04. Epub [PubMed PMID: 30175055]
Gasparri ML, Kuehn T, Ruscito I, Zuber V, Di Micco R, Galiano I, Navarro Quinones SC, Santurro L, Di Vittorio F, Meani F, Bassi V, Ditsch N, Mueller MD, Bellati F, Caserta D, Papadia A, Gentilini OD. Fibrin Sealants and Axillary Lymphatic Morbidity: A Systematic Review and Meta-Analysis of 23 Clinical Randomized Trials. Cancers. 2021 Apr 24:13(9):. doi: 10.3390/cancers13092056. Epub 2021 Apr 24 [PubMed PMID: 33923153]
Level 1 (high-level) evidenceLópez Gordo S, Ruiz-Edo N, Fernández-Planas MT, Viscaya-Martín S, Serra-Serra C, Breast Cancer Research Group. Seroma control in axillary lymphadenectomy with Glubran 2® without drain. Multicenter, prospective, randomized, clinical trial. GALA-ND study (Glubran, Axillary Lymphadenectomy, Ambulatory, No Drain). Trials. 2024 Feb 22:25(1):142. doi: 10.1186/s13063-023-07840-w. Epub 2024 Feb 22 [PubMed PMID: 38388444]
Level 1 (high-level) evidenceChe Bakri NA, Kwasnicki RM, Khan N, Ghandour O, Lee A, Grant Y, Dawidziuk A, Darzi A, Ashrafian H, Leff DR. Impact of Axillary Lymph Node Dissection and Sentinel Lymph Node Biopsy on Upper Limb Morbidity in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Annals of surgery. 2023 Apr 1:277(4):572-580. doi: 10.1097/SLA.0000000000005671. Epub 2022 Aug 10 [PubMed PMID: 35946806]
Level 1 (high-level) evidenceRafn BS, Christensen J, Larsen A, Bloomquist K. Prospective Surveillance for Breast Cancer-Related Arm Lymphedema: A Systematic Review and Meta-Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022 Mar 20:40(9):1009-1026. doi: 10.1200/JCO.21.01681. Epub 2022 Jan 25 [PubMed PMID: 35077194]
Level 1 (high-level) evidenceHara Y, Otsubo R, Shinohara S, Morita M, Kuba S, Matsumoto M, Yamanouchi K, Yano H, Eguchi S, Nagayasu T. Lymphedema After Axillary Lymph Node Dissection in Breast Cancer: Prevalence and Risk Factors-A Single-Center Retrospective Study. Lymphatic research and biology. 2022 Dec:20(6):600-606. doi: 10.1089/lrb.2021.0033. Epub 2022 Mar 29 [PubMed PMID: 35357959]
Level 2 (mid-level) evidenceLudolph I, Arkudas A, Müller-Seubert W, Cai A, Horch RE. [Complications and their management following axillary, inguinal and iliac lymph node dissection]. Chirurgie (Heidelberg, Germany). 2023 Feb:94(2):130-137. doi: 10.1007/s00104-022-01736-2. Epub 2022 Oct 18 [PubMed PMID: 36255475]
Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves Syndrome. JAMA dermatology. 2021 Jun 1:157(6):721. doi: 10.1001/jamadermatol.2021.0341. Epub [PubMed PMID: 33909015]
Dinas K, Kalder M, Zepiridis L, Mavromatidis G, Pratilas G. Axillary web syndrome: Incidence, pathogenesis, and management. Current problems in cancer. 2019 Dec:43(6):100470. doi: 10.1016/j.currproblcancer.2019.02.002. Epub 2019 Mar 15 [PubMed PMID: 30898366]