Introduction
Atypical ductal hyperplasia (ADH) is a pathologic finding in breast tissue. Atypical ductal hyperplasia is usually identified incidentally on specimens obtained by needle biopsy prompted by abnormal findings on mammography. Atypical ductal hyperplasia correlates with an increased risk of breast cancer and therefore classified as a "high risk" lesion but is not a "precursor" lesion - the distinction being the breast cancer associated with ADH can occur anywhere in the breasts and not only in the area of the ADH. Because most ADH is found incidentally, the actual incidence is unknown. It is known, once identified, to increase the risk of breast cancer approximately fivefold.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of atypical ductal hyperplasia is unknown; however, ADH is more prevalent in patients with a strong family history of breast cancer.[2] Hoogerbrugge et al. found that nearly 50% of women undergoing prophylactic mastectomies due to high family risk of breast cancer uncovered high-risk lesions, 39% of which were ADH; this suggests that there is a hereditary component involved, which requires further study.
Additionally, a study from 2009 demonstrated that rates of ADH have declined with the loss of favor of post-menopausal hormonal therapies, suggesting this also may have been a contributing factor.[3]
Epidemiology
Atypical ductal hyperplasia, due to its lack of imaging findings, is, by definition, an incidental pathology finding. Most frequently, this is found on core needle biopsy; however, it can also be discovered on excisional biopsies, breast oncologic surgeries, cosmetic breast reductions, or any other breast surgery resulting in submitting breast tissue to pathology. The prevalence of atypical ductal hyperplasia in biopsies has been in the 3.5 to 5% range and most frequently found on core needle biopsy.[4]
Rates of ADH in a large study from 2009 reviewing nearly 31,000 biopsies demonstrated initially increasing rate of diagnosis with the increase in breast cancer awareness, screening mammographies, prophylactic mastectomies, and use of post-menopausal hormonal therapy - resulting in peak diagnosis of ADH in 1999 (5.5 cases per 10,000 mamograms). Since the loss of favor of post-menopausal hormonal therapy, however, there has been a slight decrease in the diagnosis of ADH over time.[3]
Typical patients are females in their fifth to sixth decade of life, as this is the population most likely to be undergoing breast biopsies. Males are also susceptible to atypical ductal hyperplasia, although diagnosis is less common. One Dutch study analyzing over 5,000 cases of excised breast tissue for gynecomastia in males found a prevalence of 0.4% of atypical ductal hyperplasia.[5]
Pathophysiology
Genetics studies reveal ADH has recurrent alterations involving losses of 16q and 17p and gains of 1q. These genetic abnormalities are similar to those seen in low grade ductal carcinoma in situ (LGDCIS), implying a precursor-product relationship. [6]
Histopathology
Normal breast ducts terminate into a terminal duct lobular unit (TDLU). The duct ends into lobules made up of small glandular structures called acini. There is a bilayer of cells lining the ductal-lobular systems consisting of inner luminal epithelial cells and outer luminal myoepithelial cells. The majority of breast lesions (benign or malignant) develop in the TDLU.[7]
Atypical ductal hyperplasia is an intraductal clonal epithelial cell proliferation. ADH and LGDCIS involve the TDLU or the interlobular ducts. ADH and LGDCIS have the same atypical histological features of small, round, monomorphic, non-overlapping cells that are evenly spaced, with uniform nuclei, rare mitosis, and inconspicuous nucleoli. ADH is differentiated from LGDCIS by size or volume; if the atypical cells involve ≤ 2 mm, ≤ 2 spaces, or a portion of a duct a diagnosis of ADH is made. Although size is helpful there is no universally accepted size criterion for ADH.[8]
ADH has similar architectural features to DCIS, such as arcades, rigid bridges, bars of uniform thickness, a solid growth pattern, and micropapillae (papillae with a broad tip and a slender base and lack fibrovascular cores). ADH commonly grows in the cribriform architecture where cells bridge across and fill the duct, and form pseudo-lumens that appear as punched-out spaces. In ADH the spaces are not as uniform as in DCIS and the bridges may be thin and show streaming cells, versus the polarized cells in DCIS.[9][1]
Benign cell changes may be present such as spindle cells, columnar cell change (some with apical snouts), or cuboidal cells. Benign cellular proliferations like usual ductal hyperplasia can also involve ADH. ADH is often associated with secretory material that fosters the development of microcalcifications, that are commonly identified on mammography. ADH can involve benign lesions such as apocrine metaplasia, papillomas, fibroadenomas, sclerosing adenosis, and gynecomastia. ADH involving a papilloma is termed papilloma with atypia. [10]
Large nuclei with conspicuous nucleoli and frequent mitosis, such as those seen in intermediate or high-grade DCIS, are never present in ADH. If high-grade nuclei are identified, regardless of size, a diagnosis of high-grade DCIS is rendered.
ADH is typically present as a single focus or involves a small area on a core biopsy. However, ADH is occasionally found in close proximity to DCIS when multiple level sections of the core biopsy are performed (however, this is not routinely done). Patients with a diagnosis of ADH on a core biopsy sometimes have worse lesions on surgical excision (DCIS or invasive carcinoma). For this reason, surgical excision is indicated when a diagnosis of ADH is made on a core biopsy.[11]
The immunohistochemistry staining for ADH and LGDCIS is similar. Both lesions stain negatively for Cytokeratin 5/6 (but it is positive in the myoepithelial cells surrounding the duct or lobule due to the in situ nature of the lesions). ADH is typically diffusely and strongly positive for estrogen receptor (ER).[12][13]
Not all pathologies are consistent when diagnosing ADH or LGDCIS. A diagnosis of ADH is applied only to lesions when LGDCIS is highly considered, but the histological features present or the size is not sufficient for it. In true borderline cases, a diagnosis of ADH is made, and the pathologist should relay the complexity of the case to the treating clinician. [8]
History and Physical
Atypical ductal hyperplasia is most often found after biopsy in the setting of calcifications found on mammography or imaging. ADH can also be found incidentally on other breast tissue that is sent to pathology for any number of reasons, including oncologic resections, plastic surgery resections, excisional biopsies, etc. There are no physical findings on the exam, such as a lump, breast discoloration, or breast distortion grossly.
Evaluation
It is important to know the type of specimen in which the ADH is identified because the lesion's management depends on it.
If atypical ductal hyperplasia is found on core needle biopsy, additional tissue is necessary by excisional biopsy. A wire or seed localization technique should be used at the time of the core biopsy to later identify the area potentially requiring excision. The reason for re-excision is that with a more extensive tissue specimen, there is a chance the lesion will be upgraded to carcinoma in situ or invasive carcinoma. Studies suggest that 22 to 65% of ADH found on core needle biopsies were upgraded to carcinoma after subsequent excisional biopsy.[14][15] Most upgrades are to DCIS; however, IDC is also sometimes found.
If, however, ADH alone is diagnosed on an excisional biopsy, no additional surgery is required, even if there are positive margins because ADH is a high-risk lesion, but it is not a pre-cancerous or cancerous lesion.[1][14][16]
Treatment / Management
Once identifying atypical ductal hyperplasia as the diagnosis and ruling out breast carcinoma, it is essential to address risk reduction strategies.
One such measure is treating these patients with tamoxifen, as the vast majority of lesions are ER+. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial, tamoxifen conferred a risk reduction of 86% in women with ADH.[17] Therefore, we recommend the discussion of tamoxifen in patients diagnosed with ADH. Tamoxifen is known to increase the risk of endometrial cancer, stroke, DVT, and PE - particularly in patients over the age of 50. Therefore the risk-benefit discussion starting tamoxifen would need to include consideration of these risks and the decision to begin tamoxifen be patient dependant.(A1)
Additionally, it is important to increase surveillance and awareness in the patient.[18][19](B3)
Differential Diagnosis
If a core biopsy identifies atypical ductal hyperplasia, it is important to take a larger excisional biopsy to ensure a large enough specimen to rule out carcinoma of the breast. It is also important to note that on biopsy, low-grade DCIS and ADH share many morphologic similarities. Therefore the pathology specimen at hand should be carefully examined to rule out DCIS.[1]
Surgical Oncology
Atypical ductal hyperplasia has a history of surgical overtreatment. If diagnosed on core needle biopsy, a more extensive excisional biopsy is required to rule out breast carcinoma.[20] If, however, ADH and only ADH is found on excisional biopsy, the patient is surgically complete; this includes cases where margins are positive. As ADH is not cancer, there is no need for node sampling nor any role for mastectomy.
Radiation Oncology
There is no role at this time for radiation therapy in a patient diagnosed only with atypical ductal hyperplasia.
Complications
Complications of atypical ductal hyperplasia result from both over and undertreating the diagnosis. There is a risk of missing a breast carcinoma with undertreatment of ADH and not proceeding with additional tissue sampling.[21][20] There is also a risk of overtreating ADH with aggressive surgeries (e.g., mastectomy or excessively large biopsies) in the setting of no malignancy.[18] There is toxicity risk from chemotherapy agents if ADH is misrecognized as a cancerous or precancerous lesion rather than a high-risk lesion. As discussed above, there are known and well-established complications as a result of tamoxifen that should also merit consideration before starting treatment.
Deterrence and Patient Education
Patients should receive education on the meaning of the diagnosis as well as the actual risk conferred by the diagnosis of atypical ductal hyperplasia. Patients diagnosed with ADH should be followed closely by a clinician, given a higher risk of breast carcinoma in the future.[18] Additionally, standard cancer risk-reducing guidelines are recommended, such as normalizing BMI and smoking cessation.
Enhancing Healthcare Team Outcomes
Atypical ductal hyperplasia is a pathology finding, usually found incidentally on biopsy of the breast. The diagnosis by itself is not a precancerous or cancerous lesion. It is, however, a high-risk lesion, indicating the presence of ADH on pathology flags the patient as one who is fivefold more likely to develop breast carcinoma - in any area of the breasts - in the future. For this reason, the nuance of the cancer risk of the finding of ADH on pathology is more challenging to communicate with the patient.
Regardless of lesion excision, there is still an increased risk to the patient of developing breast carcinoma that will require continued monitoring and screening. The patient may also benefit from tamoxifen therapy as a breast cancer prevention agent, depending on if ER+ and if the patient is suitable for tamoxifen given the side effect profile. (Level I)
It is also crucial that surgeons know the appropriate surgical management of this finding. ADH on core needle biopsy will require needle or seed localized excisional biopsy to rule out nearby breast carcinoma. However, if ADH is found on excisional biopsy to be ADH only - even if margins are positive - the patient is surgically complete. [Level 2]
Media
(Click Image to Enlarge)
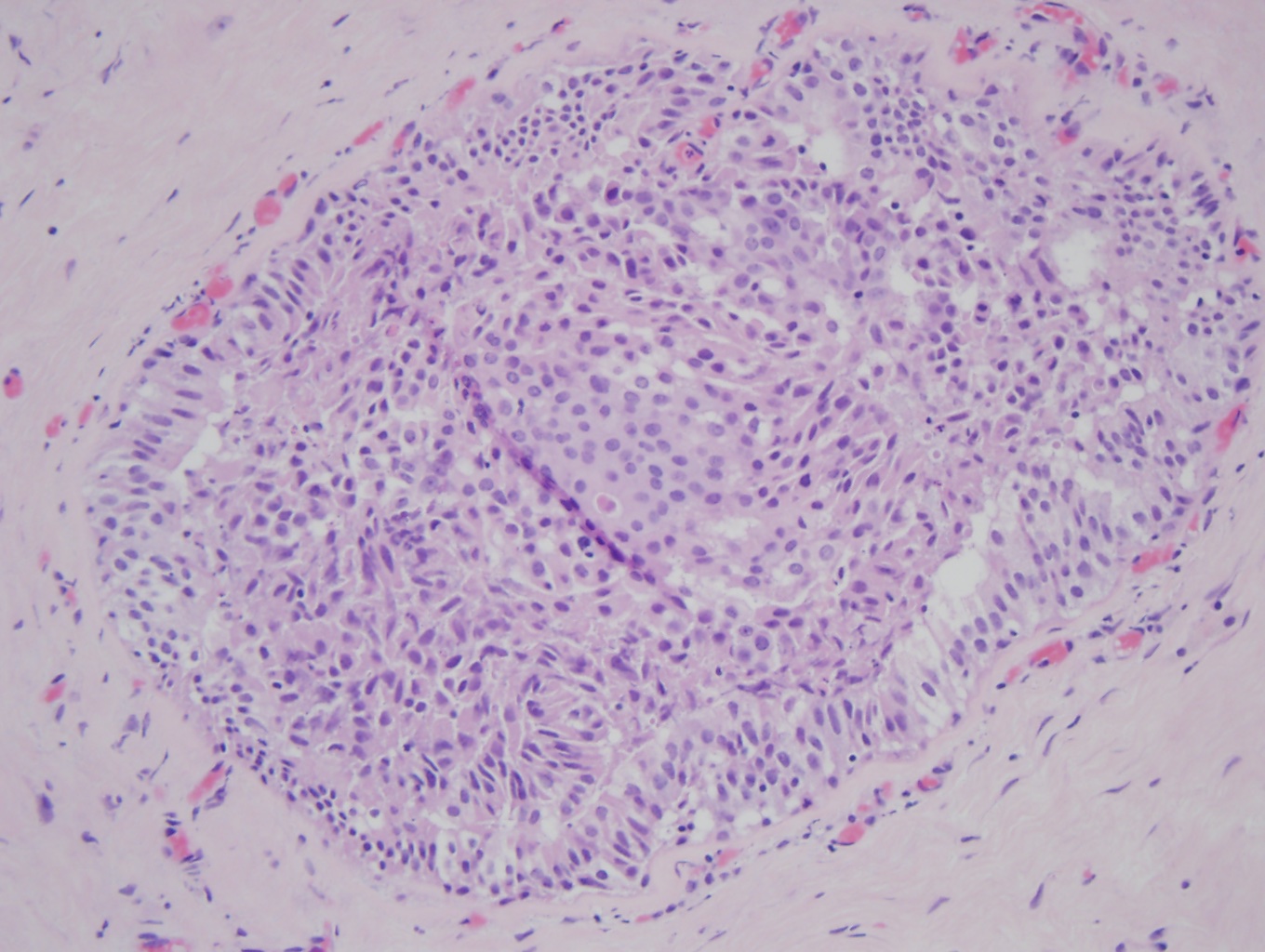
Atypical ductal hyperplasia (ADH) (x20). In the middle of the duct is a proliferation of organized atypical epithelial cells with round, monomorphic, and uniform nuclei, consistent with ADH. The remainder of the duct is filled with a haphazard organization of epithelial cells with overlapping nuclei that are variable in shape and size, consistent with usual ductal hyperplasia (UDH), a benign epithelial cellular proliferation. Contributed by Myra Khan DO
References
Kader T, Hill P, Rakha EA, Campbell IG, Gorringe KL. Atypical ductal hyperplasia: update on diagnosis, management, and molecular landscape. Breast cancer research : BCR. 2018 May 2:20(1):39. doi: 10.1186/s13058-018-0967-1. Epub 2018 May 2 [PubMed PMID: 29720211]
Hoogerbrugge N, Bult P, de Widt-Levert LM, Beex LV, Kiemeney LA, Ligtenberg MJ, Massuger LF, Boetes C, Manders P, Brunner HG. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Jan 1:21(1):41-5 [PubMed PMID: 12506168]
Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti DL. Rates of atypical ductal hyperplasia have declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Nov:18(11):2822-8. doi: 10.1158/1055-9965.EPI-09-0745. Epub [PubMed PMID: 19900937]
Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast--risk assessment and management options. The New England journal of medicine. 2015 Jan 1:372(1):78-89. doi: 10.1056/NEJMsr1407164. Epub [PubMed PMID: 25551530]
Lapid O, Jolink F, Meijer SL. Pathological findings in gynecomastia: analysis of 5113 breasts. Annals of plastic surgery. 2015 Feb:74(2):163-6. doi: 10.1097/SAP.0b013e3182920aed. Epub [PubMed PMID: 23788148]
Level 2 (mid-level) evidenceLarson PS, de las Morenas A, Cerda SR, Bennett SR, Cupples LA, Rosenberg CL. Quantitative analysis of allele imbalance supports atypical ductal hyperplasia lesions as direct breast cancer precursors. The Journal of pathology. 2006 Jul:209(3):307-16 [PubMed PMID: 16604511]
Figueroa JD, Pfeiffer RM, Patel DA, Linville L, Brinton LA, Gierach GL, Yang XR, Papathomas D, Visscher D, Mies C, Degnim AC, Anderson WF, Hewitt S, Khodr ZG, Clare SE, Storniolo AM, Sherman ME. Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. Journal of the National Cancer Institute. 2014 Oct:106(10):. pii: dju286. doi: 10.1093/jnci/dju286. Epub 2014 Oct 1 [PubMed PMID: 25274491]
Lewin AA, Mercado CL. Atypical Ductal Hyperplasia and Lobular Neoplasia: Update and Easing of Guidelines. AJR. American journal of roentgenology. 2020 Feb:214(2):265-275. doi: 10.2214/AJR.19.21991. Epub 2019 Dec 11 [PubMed PMID: 31825261]
Zhang C, Wang EY, Liu F, Ruhul Quddus M, James Sung C. Type of Architecture, Presence of Punctate Necrosis, and Extent of Involvement in Atypical Ductal Hyperplasia Can Predict the Diagnosis of Breast Carcinoma on Excision: A Clinicopathologic Study of 143 Cases. International journal of surgical pathology. 2021 Oct:29(7):716-721. doi: 10.1177/10668969211010954. Epub 2021 Apr 21 [PubMed PMID: 33881947]
Level 3 (low-level) evidenceEast EG, Carter CS, Kleer CG. Atypical Ductal Lesions of the Breast: Criteria, Significance, and Laboratory Updates. Archives of pathology & laboratory medicine. 2018 Oct:142(10):1182-1185. doi: 10.5858/arpa.2018-0221-RA. Epub [PubMed PMID: 30281370]
Pawloski KR, Christian N, Knezevic A, Wen HY, Van Zee KJ, Morrow M, Tadros AB. Atypical ductal hyperplasia bordering on DCIS on core biopsy is associated with higher risk of upgrade than conventional atypical ductal hyperplasia. Breast cancer research and treatment. 2020 Dec:184(3):873-880. doi: 10.1007/s10549-020-05890-1. Epub 2020 Aug 28 [PubMed PMID: 32857242]
Jain RK, Mehta R, Dimitrov R, Larsson LG, Musto PM, Hodges KB, Ulbright TM, Hattab EM, Agaram N, Idrees MT, Badve S. Atypical ductal hyperplasia: interobserver and intraobserver variability. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011 Jul:24(7):917-23. doi: 10.1038/modpathol.2011.66. Epub 2011 Apr 29 [PubMed PMID: 21532546]
Martinez AP, Cohen C, Hanley KZ, Li XB. Estrogen Receptor and Cytokeratin 5 Are Reliable Markers to Separate Usual Ductal Hyperplasia From Atypical Ductal Hyperplasia and Low-Grade Ductal Carcinoma In Situ. Archives of pathology & laboratory medicine. 2016 Jul:140(7):686-9. doi: 10.5858/arpa.2015-0238-OA. Epub 2016 Apr 26 [PubMed PMID: 27116088]
Rageth CJ, O'Flynn EA, Comstock C, Kurtz C, Kubik R, Madjar H, Lepori D, Kampmann G, Mundinger A, Baege A, Decker T, Hosch S, Tausch C, Delaloye JF, Morris E, Varga Z. First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast cancer research and treatment. 2016 Sep:159(2):203-13. doi: 10.1007/s10549-016-3935-4. Epub 2016 Aug 13 [PubMed PMID: 27522516]
Level 3 (low-level) evidenceAllen A, Cauthen A, Dale P, Jean-Louis C, Lord A, Smith B. Evaluating the frequency of upgrade to malignancy following surgical excision of high-risk breast lesions and ductal carcinoma in situ identified by core needle biopsy. The breast journal. 2019 Jan:25(1):103-106. doi: 10.1111/tbj.13162. Epub 2018 Nov 20 [PubMed PMID: 30461129]
Rageth CJ, O'Flynn EAM, Pinker K, Kubik-Huch RA, Mundinger A, Decker T, Tausch C, Dammann F, Baltzer PA, Fallenberg EM, Foschini MP, Dellas S, Knauer M, Malhaire C, Sonnenschein M, Boos A, Morris E, Varga Z. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast cancer research and treatment. 2019 Apr:174(2):279-296. doi: 10.1007/s10549-018-05071-1. Epub 2018 Nov 30 [PubMed PMID: 30506111]
Level 3 (low-level) evidenceFisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005 Nov 16:97(22):1652-62 [PubMed PMID: 16288118]
Level 1 (high-level) evidenceFarshid G, Edwards S, Kollias J, Gill PG. Active surveillance of women diagnosed with atypical ductal hyperplasia on core needle biopsy may spare many women potentially unnecessary surgery, but at the risk of undertreatment for a minority: 10-year surgical outcomes of 114 consecutive cases from a single center. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018 Mar:31(3):395-405. doi: 10.1038/modpathol.2017.114. Epub 2017 Nov 3 [PubMed PMID: 29099502]
Peña A, Shah SS, Fazzio RT, Hoskin TL, Brahmbhatt RD, Hieken TJ, Jakub JW, Boughey JC, Visscher DW, Degnim AC. Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast cancer research and treatment. 2017 Jul:164(2):295-304. doi: 10.1007/s10549-017-4253-1. Epub 2017 May 4 [PubMed PMID: 28474262]
Rageth CJ, Rubenov R, Bronz C, Dietrich D, Tausch C, Rodewald AK, Varga Z. Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens. Breast cancer (Tokyo, Japan). 2019 Jul:26(4):452-458. doi: 10.1007/s12282-018-00943-2. Epub 2018 Dec 27 [PubMed PMID: 30591993]
Co M, Kwong A, Shek T. Factors affecting the under-diagnosis of atypical ductal hyperplasia diagnosed by core needle biopsies - A 10-year retrospective study and review of the literature. International journal of surgery (London, England). 2018 Jan:49():27-31. doi: 10.1016/j.ijsu.2017.11.005. Epub 2017 Nov 13 [PubMed PMID: 29146271]