Introduction
Adenomyosis is a gynecologic condition characterized by ectopic endometrial tissue within the uterine myometrium. First described in 1860 by the German pathologist Carl von Rokitansky, the histopathologic finding was termed “cystosarcoma adenoids uterinum.”[1] Presenting signs and symptoms vary, but most commonly are painful menses and/or heavy menstrual bleeding. Historically, the condition was a histologic diagnosis that required biopsy or more often hysterectomy. Presently, the diagnosis can be made non-invasively using ultrasound or magnetic resonance imaging (MRI). The definitive treatment for women who no longer desire pregnancy is hysterectomy, while a variety of other medical and minimally invasive therapies are available for those who want to preserve fertility or want to avoid more extensive surgery.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
While the histology is well-described, the etiology of adenomyosis is not known definitively. Researchers have postulated several theories. The most commonly accepted theory is that adenomyosis results from a disrupted boundary between the deepest layer of the endometrium (endometrium basalis) and the underlying myometrium. This process leads to a cycle of inappropriate endometrial proliferation into the myometrium with subsequent small vessel angiogenesis as well as adjacent myometrial smooth muscle hypertrophy and hyperplasia. Data demonstrating a higher prevalence of adenomyosis following dilation and curettage and cesarean section support this theory.[2][3]
A second theory proposes an embryologic mechanism whereby pluripotent Mullerian stem cells undergo inappropriate differentiation leading to ectopic endometrial tissue. This theory has support from evidence demonstrating altered expression of specific genetic markers, in addition to case reports of endometrial tissue found in women with Rokitansky-Kuster-Hauser syndrome (Mullerian agenesis).[4][5][6][7]
Other less well-accepted theories propose altered lymphatic drainage pathways and displaced bone marrow stem cells to explain the presence of ectopic endometrial tissue.[8]
Epidemiology
Accurate demographics and disease prevalence are unclear due to previous underreporting and under-diagnosis. Estimates range from 5% to 70% with more recent data suggesting a prevalence of 20% to 35%.[8][9] Classically, adenomyosis is described as a disease of pre-menopausal, multiparous women in their thirties to forties; however, this reflects the inherent bias towards women undergoing a hysterectomy. Improvements in the diagnostic ability of ultrasound and MRI criteria have led to more insight about affected populations.
A rare form of the disease, termed juvenile cystic adenomyosis, is characterized by more extensive hemorrhage within myometrial cysts and is typically seen in women younger than 30. Symptoms are typically refractory to medical therapy and require surgery, either myomectomy or hysterectomy.[10][11]
Risk factors for adenomyosis include conditions leading to increased estrogen exposure (increased parity, early menarche, short menstrual cycles, elevated body mass index, oral contraceptive pill use, tamoxifen use) and prior uterine surgery (dilation and curettage, cesarean section, myomectomy, etc.).[8]
Pathophysiology
Inappropriate endometrial tissue proliferation within the myometrium leads to symptoms through a variety of mechanisms. Normal endometrial tissue is responsible for prostaglandin production which drives the contractions of menstruation. Ectopic foci of adenomyosis lead to increased levels of prostaglandins which result in dysmenorrhea which characterizes the disease. Estrogen also drives endometrial proliferation, which medical therapies aim to reduce.
Heavy menstrual bleeding is thought to be caused by a combination of factors which include increased endometrial surface area, increased vascularization, abnormal uterine contractions, and increased cell signaling molecules such as prostaglandins, eicosanoids, and estrogen.[8][12][13][14]
Histopathology
Histologic diagnosis of adenomyosis is the presence of endometrial stroma and glandular tissue within the smooth muscle of the myometrium. Different definitions of the required depth of invasion exist, some using an absolute measurement between 2.5 to 8 mm while others use a percentage. Additionally, several histologic grading classifications attempt to characterize the burden of disease.[9] Overall, the histologic diagnosis is not entirely standardized and significantly affected by the quality of tissue sampling.
History and Physical
The clinical diagnosis of adenomyosis is difficult as a variety of common gynecologic conditions share the signs and symptoms. The most common symptoms include heavy menstrual bleeding or painful menses. Clinical diagnostic accuracy is neither sensitive nor specific.[9] Other less common symptoms include chronic pelvic pain and dyspareunia.[8] It is also crucial to note that up to 33% of women with adenomyosis may be asymptomatic.[8] While infertility is often considered a potential presenting symptom, the data is inconclusive with some sources reporting an association of 11% to 12% with adenomyosis.[8]
The physical exam classically demonstrates a “boggy” enlarged uterus due to the combined effects of increased vascularization from ectopic endometrial tissue and smooth muscle proliferation. As compared to the enlarged leiomyomatous uterus, a tender uterus is more common.[15]
Evaluation
Laboratory Evaluation
Laboratory testing is useful to rule out other disease entities included in the differential diagnosis, in addition to identifying certain complicating features such as anemia due to heavy menstruation. While some biomarkers do exist, none are specific for adenomyosis.[9]
Radiologic Evaluation
Imaging is the primary means of making the diagnosis. Previously the preferred modality was MRI; however, recent data has shown the transvaginal ultrasound to match the sensitivity and specificity of MRI (89% sensitivity, 86% specificity).[16] The availability of ultrasound and the increased costs of MRI have led to ultrasound becoming the preferred modality for the initial evaluation, reserving MRI for equivocal cases.[17]
Ultrasound
Transvaginal ultrasound is the preferred diagnostic imaging modality for adenomyosis. The characteristic findings reflect the histopathologic changes of the disease process and can be broken down into three categories:
- Endometrial infiltration: Echogenic striations and nodules, myometrial cysts, and “lollipop” diverticula (cystic striations)
- Smooth muscle proliferation: Focal or diffuse myometrial thickening with indistinct borders more commonly involving the posterior fundus and heterogenous echotexture manifesting as “venetian blind” appearance of thin linear shadows
- Vascularity: Color Doppler demonstrating an increased number of tortuous vessels throughout the involved myometrium as opposed to leiomyomas which displace vessels
A number of mimics can have similar findings on the ultrasound exam, including tamoxifen use, prior endometrial ablation, endometriosis, uterine contractions, vascular malformations, leiomyomas, and cancer. Certain techniques such as low-frequency, coronal reconstructions, 3-D ultrasound, cine-clips, color Doppler, and saline infusion sonohysterography (SIS) can be used to differentiate between these entities.
MRI
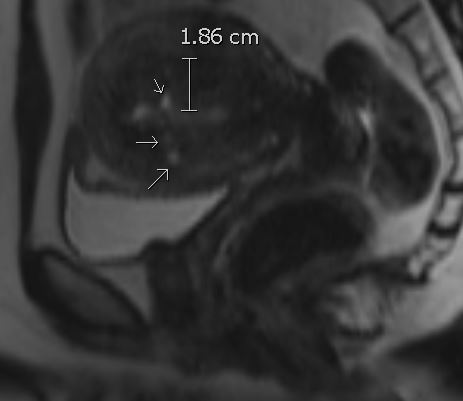
Characteristic findings on MRI parallel the same features seen on ultrasound:[18][19]
- On T2-weighted imaging, uterine enlargement characterized by ill-defined, low-signal-intensity regions within the junctional zone is reflective of smooth muscle hyperplasia (junctional zone thicker than 12 mm is generally accepted as diagnostic)
- T2 hyperintense myometrial cysts reflecting regions of ectopic endometrial tissue (can also have increased intrinsic T1 signal or increased susceptibility in hemorrhagic foci)
- Contrast enhancement is generally not reliable for assessment of vascularity as compared to color Doppler ultrasound
Similar to ultrasound, a variety of mimics ranging from co-existing gynecologic pathologies to physiologic variants exist. Susceptibility weighted imaging, diffusion-weighted imaging, MR spectroscopy, cine MR imaging, and increased 3T field strength are all problem-solving strategies.[18]
It is important to obtain the MRI in the late proliferative or secretory phase (days 7 to 28) due to the decreased signal of normal myometrium during the early proliferative phase (days 1 to 6).[18]
Treatment / Management
The first consideration in treatment selection is the desire for fertility, which will guide treatment considerations. Hysterectomy is the definitive cure. The remaining options target the primary symptoms of heavy, painful menstrual bleeding while preserving the uterus.
Medical Therapies
Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the primary medical therapies. These medications target the cyclooxygenase enzyme which produces the prostaglandins responsible for painful cramping during menstruation.[8][20](A1)
Various hormonal therapies are available which include oral contraceptive pills (OCPs), levonorgestrel intrauterine device (IUD), danazol, and aromatase inhibitors. These therapies aim to reduce the estrogenic effects which lead to endometrial proliferation. Data to support a particular therapy lack well-powered randomly controlled trials (RCTs); however, levonorgestrel IUDs are generally considered to be the primary therapy due to decreased side effect profile and overall success rates.[8][21][22](B3)
Minimally Invasive/Surgical Therapies
Several interventional radiologic procedures exist which may be options for the patient who fails medical therapy but desires future fertility. MRI-guided and ultrasound-guided high-intensity ultrasound thermal ablation can be done to target focal disease.[23][24][25] Uterine artery embolization reduces blood flow to the uterus as a whole, thereby inducing necrosis leading to an overall reduction in uterine size.[26][27][28] These therapies show promise but require additional data on direct treatment comparisons and long-term outcomes.[9] While these therapies aim to preserve fertility, infertility is an understood risk.(B2)
Endometrial ablation may be considered in patients who do not desire future fertility but prefer a less invasive alternative to hysterectomy. Limitations include the inability to target deeper adenomyotic foci due to its superficial approach.
Myomectomy and partial hysterectomy are more invasive options that aim to preserve fertility. These options allow for targeting of deeper foci; however, subsequent scarring may lead to disease recurrence as the endometrial-myometrial interface is disrupted, a risk factor for adenomyosis. Additional considerations include the potential for future pregnancy complications due to altered uterine anatomy with an increased risk of uterine rupture, premature rupture of membranes, premature labor, and spontaneous abortion.[8][9]
Hysterectomy remains the definitive cure for adenomyosis.
Differential Diagnosis
The differential diagnosis of adenomyosis is broad. The signs and symptoms of adenomyosis are similar to other disease entities characterized by abnormal uterine bleeding, dysmenorrhea, or infertility. The Federal International Board of Gynecology (FIGO) classification scheme utilizes the PALM-COEIN mnemonic as a clinical tool for more consistent reporting practices.[9][29] The mnemonic divides the diagnosis of abnormal uterine bleeding into structural and non-structural categories with each letter representing the following entities:
- Polyps
- Adenomyosis
- Leiomyoma
- Malignancy/hyperplasia
- Coagulopathy
- Ovulatory dysfunction
- Endometrial
- Iatrogenic
- Not yet classified
Adenomyosis often co-exists with other entities within the differential diagnosis, most commonly leiomyoma (50%), endometriosis (11%), and endometrial polyps (7%).[8][30]
Prognosis
Adenomyosis is a difficult diagnosis to manage as multiple clinical factors must be considered, including fertility, confidence in diagnosis, side effects of medical management, and risk of invasive procedures. Recent data showing an increased prevalence of adenomyosis in younger populations and asymptomatic patients reflects a spectrum of disease which is incompletely understood. Symptoms seem to correlate with the number of adenomyosis foci and the depth of invasion.[31] The consensus to treatment is a stepwise approach with medical therapy to include anti-inflammatory and hormonal agents with progression to minimally invasive procedures such as endometrial ablation/myomectomy or uterine artery embolization. The definitive cure remains hysterectomy.
Complications
The correlation between adenomyosis and infertility is not clearly defined. While some authors cite an association of 11% to 12%, contradictory data also exists.[8] Due to a multitude of confounders and variable diagnostic criteria for adenomyosis, no clear association has been established.[9]
With more validated diagnostic imaging criteria of ultrasound in recent years, future research will be able to characterize associations between adenomyosis and infertility more confidently.
Deterrence and Patient Education
It is essential that patients understand adenomyosis can be a challenging condition to diagnose and treat. The drivers of treatment options are the burden of disease (correlates to a depth of invasion) and preferences regarding future fertility. Additionally, adenomyosis often co-exists with other gynecologic conditions which further complicates treatment planning. While certain minimally invasive therapies exist and data shows promising results, these procedures (ultra-high frequency ultrasound, uterine artery embolization, etc.) are not validated using well-powered randomly controlled trials with unknown long-term outcomes.
Pearls and Other Issues
Ultrasound is the preferred diagnostic imaging modality for the initial workup of adenomyosis with high sensitivity and specificity. MRI can be helpful in equivocal cases.
Diagnostic imaging utilizing either ultrasound or MRI has some limitations due to numerous mimics and variable physiologic appearance of the uterus.
Treatment options should target primary symptoms. NSAIDs for painful cramping, OCPs or levonorgestrel IUD for heavy bleeding, and hysterectomy for women no longer desiring fertility. Minimally invasive therapies should be a consideration in refractory cases in women who wish to preserve fertility.
Adenomyosis can co-exist with leiomyoma (50%), endometriosis (11%), and endometrial polyps (7%). These associations merit attention in patients who have refractory symptoms.
Up to a third of women with a sonographic diagnosis of adenomyosis may be asymptomatic.[17]
While adenomyosis is classically taught as a disease of premenopausal, multiparous women, there is data showing an increased prevalence in undiagnosed younger populations.
Utilization of the PALM-COEIN mnemonic can be used to broaden the differential diagnosis for abnormal uterine bleeding.
Enhancing Healthcare Team Outcomes
Adenomyosis is a problematic diagnosis due to its variable presentation and shared features with other common gynecologic conditions. The primary care clinician, obstetrician, nurse practitioner, gynecologist, diagnostic radiologist, and interventional radiologist are all possible team members who might diagnose and manage this disease. While the differential diagnosis is broad for women presenting with abnormal uterine bleeding, a transvaginal ultrasound which is the diagnostic imaging of choice for adenomyosis will likely be obtained during the initial workup. It is important to recognize that every test has its limitations to include the ultrasonographer and interpreter. It is vital that the ordering provider does not dismiss the diagnosis of adenomyosis if it is not mentioned in the report, as the diagnostic criteria have been variable for many years until the recent establishment of more standardized imaging features.[17]
For patients who are refractory to the standard medical therapies and desire future fertility, minimally invasive therapies offered by gynecologists or interventional radiologists should be an option. Recent data show promising results, but data demonstrating head-to-head comparisons and long-term outcomes are still lacking.
Similarly, medical therapies beyond the most commonly used levonorgestrel IUD and OCPs have yet to be validated and should only be used in refractory cases and under the supervision of a clinician who understands the hormonal side-effects of these medications.
Regardless of the course of treatment and severity of the disease, an interprofessional approach with collaboration between clinicians, nursing, and pharmacy is the optimal approach to ensure the best patient outcome. [Level 5]
Media
(Click Image to Enlarge)
References
Benagiano G, Brosens I, Lippi D. The history of endometriosis. Gynecologic and obstetric investigation. 2014:78(1):1-9. doi: 10.1159/000358919. Epub 2014 May 16 [PubMed PMID: 24853333]
Taran FA, Stewart EA, Brucker S. Adenomyosis: Epidemiology, Risk Factors, Clinical Phenotype and Surgical and Interventional Alternatives to Hysterectomy. Geburtshilfe und Frauenheilkunde. 2013 Sep:73(9):924-931 [PubMed PMID: 24771944]
Parazzini F, Vercellini P, Panazza S, Chatenoud L, Oldani S, Crosignani PG. Risk factors for adenomyosis. Human reproduction (Oxford, England). 1997 Jun:12(6):1275-9 [PubMed PMID: 9222017]
Garcia L, Isaacson K. Adenomyosis: review of the literature. Journal of minimally invasive gynecology. 2011 Jul-Aug:18(4):428-37. doi: 10.1016/j.jmig.2011.04.004. Epub 2011 May 31 [PubMed PMID: 21622029]
Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertility and sterility. 2011 Jun:95(7):2228-35, 2235.e1. doi: 10.1016/j.fertnstert.2011.02.051. Epub 2011 Mar 27 [PubMed PMID: 21444077]
Level 2 (mid-level) evidenceEnatsu A, Harada T, Yoshida S, Iwabe T, Terakawa N. Adenomyosis in a patient with the Rokitansky-Kuster-Hauser syndrome. Fertility and sterility. 2000 Apr:73(4):862-3 [PubMed PMID: 10731555]
Level 3 (low-level) evidenceMatsumoto Y, Iwasaka T, Yamasaki F, Sugimori H. Apoptosis and Ki-67 expression in adenomyotic lesions and in the corresponding eutopic endometrium. Obstetrics and gynecology. 1999 Jul:94(1):71-7 [PubMed PMID: 10389721]
Struble J, Reid S, Bedaiwy MA. Adenomyosis: A Clinical Review of a Challenging Gynecologic Condition. Journal of minimally invasive gynecology. 2016 Feb 1:23(2):164-85. doi: 10.1016/j.jmig.2015.09.018. Epub 2015 Sep 30 [PubMed PMID: 26427702]
Abbott JA. Adenomyosis and Abnormal Uterine Bleeding (AUB-A)-Pathogenesis, diagnosis, and management. Best practice & research. Clinical obstetrics & gynaecology. 2017 Apr:40():68-81. doi: 10.1016/j.bpobgyn.2016.09.006. Epub 2016 Sep 30 [PubMed PMID: 27810281]
Dogan E, Gode F, Saatli B, Seçil M. Juvenile cystic adenomyosis mimicking uterine malformation: a case report. Archives of gynecology and obstetrics. 2008 Dec:278(6):593-5. doi: 10.1007/s00404-008-0618-3. Epub 2008 Mar 12 [PubMed PMID: 18335227]
Level 3 (low-level) evidenceKriplani A, Mahey R, Agarwal N, Bhatla N, Yadav R, Singh MK. Laparoscopic management of juvenile cystic adenomyoma: four cases. Journal of minimally invasive gynecology. 2011 May-Jun:18(3):343-8. doi: 10.1016/j.jmig.2011.02.001. Epub [PubMed PMID: 21545959]
Level 3 (low-level) evidenceHuang FJ, Kung FT, Chang SY, Hsu TY. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. The Journal of reproductive medicine. 1999 Aug:44(8):741-4 [PubMed PMID: 10483548]
Level 3 (low-level) evidenceAzziz R. Adenomyosis: current perspectives. Obstetrics and gynecology clinics of North America. 1989 Mar:16(1):221-35 [PubMed PMID: 2664619]
Level 3 (low-level) evidenceKoike H, Egawa H, Ohtsuka T, Yamaguchi M, Ikenoue T, Mori N. Correlation between dysmenorrheic severity and prostaglandin production in women with endometriosis. Prostaglandins, leukotrienes, and essential fatty acids. 1992 Jun:46(2):133-7 [PubMed PMID: 1502250]
Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Medical College journal : NMCJ. 2012 Sep:14(3):176-9 [PubMed PMID: 24047010]
Level 2 (mid-level) evidenceReinhold C, McCarthy S, Bret PM, Mehio A, Atri M, Zakarian R, Glaude Y, Liang L, Seymour RJ. Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology. 1996 Apr:199(1):151-8 [PubMed PMID: 8633139]
Cunningham RK, Horrow MM, Smith RJ, Springer J. Adenomyosis: A Sonographic Diagnosis. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018 Sep-Oct:38(5):1576-1589. doi: 10.1148/rg.2018180080. Epub [PubMed PMID: 30207945]
Takeuchi M, Matsuzaki K. Adenomyosis: usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011 Jan-Feb:31(1):99-115. doi: 10.1148/rg.311105110. Epub [PubMed PMID: 21257936]
Novellas S, Chassang M, Delotte J, Toullalan O, Chevallier A, Bouaziz J, Chevallier P. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR. American journal of roentgenology. 2011 May:196(5):1206-13. doi: 10.2214/AJR.10.4877. Epub [PubMed PMID: 21512093]
Marjoribanks J, Proctor ML, Farquhar C. Nonsteroidal anti-inflammatory drugs for primary dysmenorrhoea. The Cochrane database of systematic reviews. 2003:(4):CD001751 [PubMed PMID: 14583938]
Level 1 (high-level) evidenceFarquhar C, Brosens I. Medical and surgical management of adenomyosis. Best practice & research. Clinical obstetrics & gynaecology. 2006 Aug:20(4):603-16 [PubMed PMID: 16563872]
Fong YF, Singh K. Medical treatment of a grossly enlarged adenomyotic uterus with the levonorgestrel-releasing intrauterine system. Contraception. 1999 Sep:60(3):173-5 [PubMed PMID: 10640162]
Level 3 (low-level) evidenceZhou M, Chen JY, Tang LD, Chen WZ, Wang ZB. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertility and sterility. 2011 Mar 1:95(3):900-5. doi: 10.1016/j.fertnstert.2010.10.020. Epub 2010 Nov 10 [PubMed PMID: 21067723]
Liu X, Wang W, Wang Y, Wang Y, Li Q, Tang J. Clinical Predictors of Long-term Success in Ultrasound-guided High-intensity Focused Ultrasound Ablation Treatment for Adenomyosis: A Retrospective Study. Medicine. 2016 Jan:95(3):e2443. doi: 10.1097/MD.0000000000002443. Epub [PubMed PMID: 26817877]
Level 2 (mid-level) evidenceKim KA, Yoon SW, Lee C, Seong SJ, Yoon BS, Park H. Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: symptomatic relief and pain reduction. Fertility and sterility. 2011 Mar 1:95(3):1152-5. doi: 10.1016/j.fertnstert.2010.09.024. Epub [PubMed PMID: 20970127]
Liang E, Brown B, Kirsop R, Stewart P, Stuart A. Efficacy of uterine artery embolisation for treatment of symptomatic fibroids and adenomyosis - an interim report on an Australian experience. The Australian & New Zealand journal of obstetrics & gynaecology. 2012 Apr:52(2):106-12. doi: 10.1111/j.1479-828X.2011.01399.x. Epub 2012 Jan 5 [PubMed PMID: 22221154]
Smeets AJ, Nijenhuis RJ, Boekkooi PF, Vervest HA, van Rooij WJ, Lohle PN. Long-term follow-up of uterine artery embolization for symptomatic adenomyosis. Cardiovascular and interventional radiology. 2012 Aug:35(4):815-9. doi: 10.1007/s00270-011-0203-1. Epub 2011 Jun 30 [PubMed PMID: 21717251]
Level 2 (mid-level) evidenceKim MD, Won JW, Lee DY, Ahn CS. Uterine artery embolization for adenomyosis without fibroids. Clinical radiology. 2004 Jun:59(6):520-6 [PubMed PMID: 15145722]
Level 2 (mid-level) evidenceMarnach ML, Laughlin-Tommaso SK. Evaluation and Management of Abnormal Uterine Bleeding. Mayo Clinic proceedings. 2019 Feb:94(2):326-335. doi: 10.1016/j.mayocp.2018.12.012. Epub [PubMed PMID: 30711128]
McElin TW, Bird CC. Adenomyosis of the uterus. Obstetrics and gynecology annual. 1974:3(0):425-41 [PubMed PMID: 4608783]
Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstetrics and gynecology. 2000 May:95(5):688-91 [PubMed PMID: 10775730]
Level 2 (mid-level) evidence