Introduction
The aorta is the first and largest artery in the body. It is responsible for transporting nutrient-rich blood to the systemic circulation following ejection from the left ventricle of the heart. The aorta extends from the aortic valve of the left ventricle to the proximal iliac bifurcation at the L4 vertebral level. The vessel can be divided into various segments depending on course and location. The thoracic aorta consists of the ascending aorta, aortic arch, and descending aorta. The descending thoracic aorta passes through the diaphragm’s aortic hiatus at the T12 vertebral level at which point it continues as the abdominal aorta. The abdominal aorta terminates as it bifurcates into common iliac arteries, which subsequently provide arterial supply to the pelvis and lower limbs.[1][2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The aorta receives nutrient-rich blood from the left ventricle after blood is ejected through the aortic valve. The aortic valve, a tri-leaflet unidirectional valve, ensures that blood maintains anterograde flow from the heart to the systemic system. From this point, the aorta extends approximately 5.0cm superiorly to the level of the T4 vertebrae and is known as the ascending aorta. The coronary arteries, found at the cusp of the aortic valve, are the first branches of the aorta.[3]
The ascending aorta then forms the aortic arch as it curves posteriorly, towards the left, passing over the root of the left lung and anterior to the trachea. The superior aspect of the aortic arch is commonly found in the Plane of Ludwig, a transverse cross-section between the T4 vertebrae and the second sternocostal articulation, commonly demarcated by the sternal angle. The brachiocephalic, left common carotid, and left subclavian arteries all branch off the aortic arch. The aortic arch continues to curve inferiorly and posteriorly, towards the vertebral column. The aortic arch transitions to the descending aorta, which can be further subdivided into the thoracic and abdominal aorta. The thoracic aorta is the portion of the descending aorta contained within the posterior mediastinal cavity. This portion of the aorta lies just to the left of the vertebral column and drifts medially as it continues to move inferiorly through the thorax until it crosses the diaphragm at the T12 aortic hiatus and continues as the abdominal aorta.[4][5]
The thoracic aorta gives rise to multiple branches. Visceral branches include the pericardial, bronchial, esophageal, and mediastinal branches. The pericardial branches are small vessels that travel to the posterior surface of the pericardium. The left bronchial arteries, typically two in number, arise from the thoracic aorta as well. These arteries supply the bronchial airways, areolar tissue of the lungs, and esophagus. The esophageal arteries arise from the anterior portion of the aorta and travel inferiorly towards the esophagus where they anastomose with several other arteries. The mediastinal branches of the thoracic aorta go on to supply the lymph glands and areolar tissue located within the posterior mediastinum. Parietal branches include the intercostal, subcostal, and superior phrenic branches. There are nine pairs of intercostal arteries in total which arise from the posterior portion of the aorta. These arteries further divide and provide branches for intercostal, lateral cutaneous, mammary, and spinal arteries, to name a few.The superior phrenic arteries also arise from the thoracic aorta, later anastomosing with the pericardiophrenic and musculophrenic arteries. The lowest branching arteries of the thoracic aorta are the subcostal arteries which will later give off a posterior branch.[6][4]
The abdominal aorta gives rise to five major branches: the celiac trunk, superior mesenteric artery, left and right renal arteries, and inferior mesenteric artery. The celiac trunk mainly supplies organs of the foregut, while the superior and inferior mesenteric arteries supply organs of the midgut and hindgut, respectively. Other notable branches of the abdominal aorta include inferior phrenic, middle suprarenal, gonadal, lumbar, and median sacral arteries. It is important to note the superior suprarenal arteries originate from the inferior phrenic arteries while the inferior suprarenal arteries originate from the renal arteries.[5]
The celiac trunk branches into three vessels: splenic, common hepatic, and left gastric arteries. The common hepatic artery further divides into the proper hepatic artery, which further divides into the right gastric and hepatic arteries, and the gastroduodenal artery, which supplies the proximal duodenum and pancreas. The left gastric artery mainly supplies the lesser curvature of the stomach and anastomoses with the right gastric artery. The splenic artery supplies the spleen. It also provides the left gastroepiploic artery which anastomoses with the right gastroepiploic and gastroduodenal arteries. These vessels supply the greater pyloric curvature.[7]
The superior mesenteric arteries branch into several ileal and jejunal arteries as well as the middle colic, right colic, and ileocecal arteries. This aortic branch supplies the bowel from the Ligament of Treitz (duodenal suspensory ligament), found in the third part of the duodenum, to the left splenic flexure of the colon. The superior mesenteric artery anastomoses with branches of the celiac trunk (gastroduodenal-pancreaticoduodenal anastomosis) and inferior mesenteric artery (middle colic-left colic anastomosis).[8]
The inferior mesenteric artery provides circulation to the bowel from the distal 1/3 of the transverse colon to the rectum’s pectinate line. Its branches include the left colic, sigmoidal, and superior rectal arteries, to name a few. The inferior mesenteric artery shares an important portocaval anastomosis with the inferior rectal artery, which becomes notable in cases such as portal hypertension.[9][10]
Embryology
The aorta develops during the third gestational week concurrently with the endocardial tube. During this early developmental stage, the primitive aorta demonstrates dorsal and ventral segments which are continuous through the first aortic arch. The first aortic arch will disappear during a subsequent developmental stage. The ventral segments of the primitive aorta will join and form the aortic sac while the dorsal segments will merge and create the midline descending aorta. Six paired aortic arches will develop between the ventral and dorsal aortae, and are known as the branchial arch arteries. Towards the end of aortic development, regression of the right dorsal aortic root along with the right ductus arteriosus will result in the formation of the physiologically normal left aortic arch.[9][11]
Blood Supply and Lymphatics
The aorta is supplied by a network of small vessels known as the vasa vasorum. The vasa vasorum are most prevalent superior to the renal arteries. As a result, the infrarenal abdominal aorta is predisposed to aneurysm formation as inflammation and oxidation rates in the aortic walls increase as vasa vasorum decrease. Lymphatics drainage closely follows vascular supply. The cisterna chyli, located just inferior to the diaphragm’s aortic hiatus, receives lymphatics from much of the lower limbs and abdomen. Lymph then moves superiorly through the thoracic duct.[9][12]
Nerves
The aorta is innervated primarily by components of the autonomic nervous system. Sympathetic and parasympathetic nerves closely follow the aorta as it descends into the abdomen. These nerves form plexuses such as the celiac, superior mesenteric, and inferior mesenteric.[13]
Muscles
The aorta is primarily composed of vascular smooth muscle and elastin. It is considered both a muscular and elastic artery. Three different layers make up its walls. The first of those layers is the intima, which is the thin inner layer of the aorta. The other two layers are the middle elastic, or media, and the outer fibrous layer, or adventitia.
Physiologic Variants
Variations in structure may occur during gestational growth as the aorta forms in conjunction with the endocardial tube around day 21 of development. Magnetic resonance imaging (MRI) is the currently accepted gold standard for the imaging of the aortic arch. MRI is able to provide imaging of both the structural relationship between the aorta and its branches to the trachea and bronchi as well as the arterial branching pattern. Other imaging modalities that may be used for assessment of the aorta and which provide the ability to accurately assess aortic pathology include transthoracic echocardiography (TTE), computed tomography (CT), chest radiographs (CXR), transesophageal echocardiography (TOE), and invasive catheter angiography. Several anatomical abnormalities or variations are possible.
Coarctation of the aorta is a relatively common cause of congenital heart disease (CHD). This particular anatomical variation makes up 5-7% percent of all CHD cases. Coarctation of the aorta is an abnormality in which there is a narrowing of the aorta resulting in decreased blood flow. Presenting symptoms and time to diagnosis may vary from patient to patient based on the severity of the narrowing. The severity of the obstruction can range from a milder narrowing which often takes longer to diagnose to more severe blockages which will often be diagnosed in early infancy.
Hypoplasia of the ascending aorta results in diminished blood flow and typically occurs alongside hypoplastic left heart syndrome (HLHS). HLHS is severe and if untreated may result in death.
Patent ductus arteriosus is yet another potential pathological variation that may occur. It results when the ductus arteriosus fails to close following birth. The ductus arteriosus allows for the flow of blood between the aorta and pulmonary artery during intrauterine development and typically closes within a few days of delivery. An interrupted aortic arch is also a pathological variation that may occur with the aorta. In this abnormality, a portion of the aorta is missing which results in interrupted blood flow and is therefore of significant concern. This particular variant correlates with high rates of mortality.
Pulmonary sequestration is a rare anomaly that may occur involving the aorta in which a section of lung tissue obtains its blood supply from an abnormal source such as a systemic artery that originated from the aorta or one of its branches.
Aortic ductus diverticulum is an aortic variation in which there is an outpouching of the thoracic aorta. This condition is seen in about 9% of individuals and may be mistaken for an acute injury. Ductus diverticulum is most common at the aortic isthmus, distal to the origin point of the left subclavian artery. This variant may resemble a pseudoaneurysm of the aortic isthmus, and therefore differentiation between the two is of particular importance.[9][14][15][16][17]
Surgical Considerations
Knowledge of the aorta’s anatomy and topography is vital during surgical procedures, especially those that involve the retroperitoneal region as injury to the vessel can lead to massive hemorrhage.[18][19][5]
Clinical Significance
The aorta is known for certain pathology including aortic aneurysms, dissection, coarctation, aortitis, and atherosclerotic disease. Ascending aortic aneurysms and dissections involve surgical management. Descending thoracic and abdominal aneurysms involve medical management until the vessel reaches a size greater than 50% of its baseline diameter at which point they require surgical placement of stents or grafts. Smoking, hypertension, and hyperlipidemia are strong risk factors. Aortic coarctation is often diagnosed within the first few years of life. Blood pressures are often higher in the upper limbs as opposed to the lower limbs. Severe cases can lead to lower limb cyanosis. Collateral flow via the intercostal arteries provides nutrient-rich blood to the abdomen and distal extremities. This condition is usually managed surgically. Aortitis may result from an infectious etiology such as syphilis or an autoimmune origin such as arteritis. Depending on the cause, the aorta may dilate or narrow. Atherosclerotic disease may lead to peripheral artery disease and predispose individuals to dissection or aneurysm. However, plaques may simply decrease the luminal radius, leading to distal ischemia. Leriche syndrome, or aortoiliac occlusive disease, is a notable atherosclerotic disease affecting the distal abdominal aorta and iliac arteries.[20][21][22][23]][24][25]
Media
(Click Image to Enlarge)

Branches of the Aorta. This illustration includes the right common carotid artery, right vertebral artery, right subclavian artery, brachiocephalic artery, ascending aorta, left coronary artery, right coronary artery, left common carotid artery, left vertebral artery, left subclavian artery, left axillary artery, left brachial artery, arch of aorta, and descending aorta.
Contributed by Beckie Palmer
(Click Image to Enlarge)
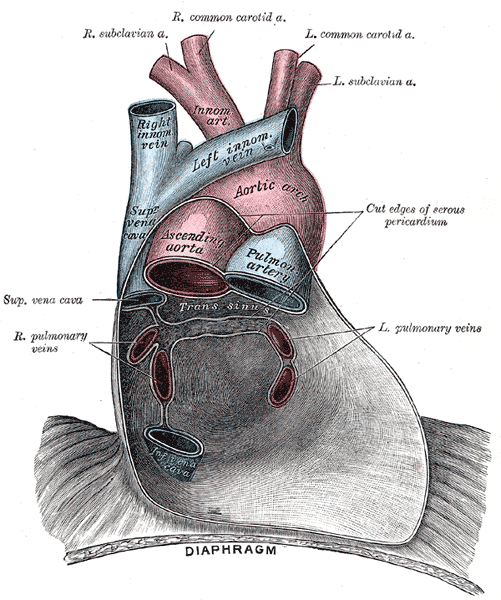
Pericardium anatomy, Right Subclavian Artery, Right Common Carotid Artery, Left Common Carotid Artery, Left Subclavian Artery, Innominate Artery, Right Innominate vein, Left innominate vein, Aortic Arch, Superior Vena cava, Ascending Aorta, Pulmonary Artery, Left and Right Pulmonary veins, Inferior Vena Cava, Diaphragm
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Collins JA, Munoz JV, Patel TR, Loukas M, Tubbs RS. The anatomy of the aging aorta. Clinical anatomy (New York, N.Y.). 2014 Apr:27(3):463-6. doi: 10.1002/ca.22384. Epub 2014 Feb 12 [PubMed PMID: 24523152]
Komutrattananont P, Mahakkanukrauh P, Das S. Morphology of the human aorta and age-related changes: anatomical facts. Anatomy & cell biology. 2019 Jun:52(2):109-114. doi: 10.5115/acb.2019.52.2.109. Epub 2019 Jun 30 [PubMed PMID: 31338225]
Loukas M, Bilinsky E, Bilinsky S, Blaak C, Tubbs RS, Anderson RH. The anatomy of the aortic root. Clinical anatomy (New York, N.Y.). 2014 Jul:27(5):748-56. doi: 10.1002/ca.22295. Epub 2013 Sep 2 [PubMed PMID: 24000000]
Dagenais F. Anatomy of the thoracic aorta and of its branches. Thoracic surgery clinics. 2011 May:21(2):219-27, viii. doi: 10.1016/j.thorsurg.2010.12.004. Epub [PubMed PMID: 21477772]
FELLER I, WOODBURNE RT. Surgical anatomy of the abdominal aorta. Annals of surgery. 1961 Dec:154(6)Suppl(Suppl 6):239-52 [PubMed PMID: 13892214]
Peidro J, Boufi M, Loundou AD, Hartung O, Dona B, Vernet F, Bensoussan D, Alimi YS. Aortic Anatomy and Complications of the Proximal Sealing Zone after Endovascular Treatment of the Thoracic Aorta. Annals of vascular surgery. 2018 Apr:48():141-150. doi: 10.1016/j.avsg.2017.09.029. Epub 2017 Dec 8 [PubMed PMID: 29225128]
Santos PVD, Barbosa ABM, Targino VA, Silva NA, Silva YCM, Barbosa F, Oliveira ASB, Assis TO. ANATOMICAL VARIATIONS OF THE CELIAC TRUNK: A SYSTEMATIC REVIEW. Arquivos brasileiros de cirurgia digestiva : ABCD = Brazilian archives of digestive surgery. 2018 Dec 6:31(4):e1403. doi: 10.1590/0102-672020180001e1403. Epub 2018 Dec 6 [PubMed PMID: 30539978]
Level 1 (high-level) evidenceNegoi I, Beuran M, Hostiuc S, Negoi RI, Inoue Y. Surgical Anatomy of the Superior Mesenteric Vessels Related to Colon and Pancreatic Surgery: A Systematic Review and Meta-Analysis. Scientific reports. 2018 Mar 8:8(1):4184. doi: 10.1038/s41598-018-22641-x. Epub 2018 Mar 8 [PubMed PMID: 29520096]
Level 1 (high-level) evidenceKau T, Sinzig M, Gasser J, Lesnik G, Rabitsch E, Celedin S, Eicher W, Illiasch H, Hausegger KA. Aortic development and anomalies. Seminars in interventional radiology. 2007 Jun:24(2):141-52. doi: 10.1055/s-2007-980040. Epub [PubMed PMID: 21326792]
Murono K, Kawai K, Kazama S, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T. Anatomy of the inferior mesenteric artery evaluated using 3-dimensional CT angiography. Diseases of the colon and rectum. 2015 Feb:58(2):214-9. doi: 10.1097/DCR.0000000000000285. Epub [PubMed PMID: 25585080]
Level 2 (mid-level) evidenceSchleich JM. Images in cardiology. Development of the human heart: days 15-21. Heart (British Cardiac Society). 2002 May:87(5):487 [PubMed PMID: 11997429]
Sano M, Unno N, Sasaki T, Baba S, Sugisawa R, Tanaka H, Inuzuka K, Yamamoto N, Sato K, Konno H. Topologic distributions of vasa vasorum and lymphatic vasa vasorum in the aortic adventitia--Implications for the prevalence of aortic diseases. Atherosclerosis. 2016 Apr:247():127-34. doi: 10.1016/j.atherosclerosis.2016.02.007. Epub 2016 Feb 12 [PubMed PMID: 26897260]
Beveridge TS, Johnson M, Power A, Power NE, Allman BL. Anatomy of the nerves and ganglia of the aortic plexus in males. Journal of anatomy. 2015 Jan:226(1):93-103. doi: 10.1111/joa.12251. Epub 2014 Nov 9 [PubMed PMID: 25382240]
Weinberg PM. Aortic arch anomalies. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2006:8(4):633-43 [PubMed PMID: 16869315]
Fox EB, Latham GJ, Ross FJ, Joffe D. Perioperative and Anesthetic Management of Coarctation of the Aorta. Seminars in cardiothoracic and vascular anesthesia. 2019 Jun:23(2):212-224. doi: 10.1177/1089253218821953. Epub 2019 Jan 7 [PubMed PMID: 30614372]
Tchervenkov CI, Jacobs JP, Sharma K, Ungerleider RM. Interrupted aortic arch: surgical decision making. Seminars in thoracic and cardiovascular surgery. Pediatric cardiac surgery annual. 2005:():92-102 [PubMed PMID: 15818364]
Holloway BJ, Rosewarne D, Jones RG. Imaging of thoracic aortic disease. The British journal of radiology. 2011 Dec:84 Spec No 3(Spec Iss 3):S338-54. doi: 10.1259/bjr/30655825. Epub [PubMed PMID: 22723539]
DE BAKEY ME, COOLEY DA, CREECH O Jr. Surgical considerations of dissecting aneurysm of the aorta. Annals of surgery. 1955 Oct:142(4):586-610; discussion, 611-2 [PubMed PMID: 13259422]
Carpenter SW, Kodolitsch YV, Debus ES, Wipper S, Tsilimparis N, Larena-Avellaneda A, Diener H, Kölbel T. Acute aortic syndromes: definition, prognosis and treatment options. The Journal of cardiovascular surgery. 2014 Apr:55(2 Suppl 1):133-44 [PubMed PMID: 24796906]
Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart (British Cardiac Society). 2017 Aug:103(15):1148-1155. doi: 10.1136/heartjnl-2017-311173. Epub 2017 Apr 4 [PubMed PMID: 28377475]
González-Salvado V, Bazal P, Alonso-González R. Aortic Coarctation With Extensive Collateral Circulation. Circulation. Cardiovascular imaging. 2018 Aug:11(8):e007918. doi: 10.1161/CIRCIMAGING.118.007918. Epub [PubMed PMID: 30354495]
Wahl L, Tubbs RS. A review of the clinical anatomy of hypertension. Clinical anatomy (New York, N.Y.). 2019 Jul:32(5):678-681. doi: 10.1002/ca.23369. Epub 2019 Apr 3 [PubMed PMID: 30873636]
Bossone E, Pluchinotta FR, Andreas M, Blanc P, Citro R, Limongelli G, Della Corte A, Parikh A, Frigiola A, Lerakis S, Ehrlich M, Aboyans V. Aortitis. Vascular pharmacology. 2016 May:80():1-10. doi: 10.1016/j.vph.2015.11.084. Epub 2015 Dec 22 [PubMed PMID: 26721213]
Ernst CB. Abdominal aortic aneurysm. The New England journal of medicine. 1993 Apr 22:328(16):1167-72 [PubMed PMID: 8455684]
Wooten C, Hayat M, du Plessis M, Cesmebasi A, Koesterer M, Daly KP, Matusz P, Tubbs RS, Loukas M. Anatomical significance in aortoiliac occlusive disease. Clinical anatomy (New York, N.Y.). 2014 Nov:27(8):1264-74. doi: 10.1002/ca.22444. Epub 2014 Jul 25 [PubMed PMID: 25065617]