Introduction
Blood product safety has been an improving area of focus for many countries over recent decades (see Figure. Blood Product Safety). The World Health Organization promotes efforts for improving access to safe transfusion and safe blood products across the world. Safety of blood products begins with blood donor recruitment and includes:[1]
- Emphasis on pre-donation information;
- Blood component collection, preparation, and testing;
- Post-donation information collection;
- Labeling of the blood products for distribution;
- Handling of blood products in the hospital inventory;
- Blood transfusion into the patient by hospital staff; and
- Culminating with hemovigilance and clinical quality improvement to ensure patient safety and to reduce the morbidity and mortality associated with blood transfusion.
Several methods have been employed to reduce the risk of blood transfusions and improve blood product safety. There have been interventions in every step of the process from before blood donation collection through post-procedure follow-up of blood product recipients. There have been improvements made in the collection, storage, management, distribution, utilization, and monitoring of transfusions.
The measures for improved safety begin even before blood collection. It is important to have government and professional organization commitment to improving blood product safety. Other important aspects of improving safety include proper training of staff, along with a dedicated and diligent blood transfusion organization overseeing donation, testing, and eventual transfusion. Blood donor recruitment is the next important piece in securing blood product safety. Voluntary unpaid donors have the lowest rates of transfusion-associated infections and are the ideal population from which to recruit donors. 'Replacement' and 'family' donors are falling out of favor. Donor screening questions add a layer of defense to improve blood product safety. These screening questions are responsible for the single most significant reduction in transfusion-transmitted infections. Donors are deferred based on criteria developed to enhance safety for both the donor and the potential recipient. Donors can be deferred for a period (as in the case of anemia) or indefinitely (as in the case of a donor screened positive for HIV and confirmed to have HIV). Laboratory testing of blood donations also adds a layer of protection against transfusion-associated adverse events. Blood group compatibility testing along with additional antibody testing and molecular testing for known transfusion-transmitted infections occurs. Guidelines to ensure the diligent and appropriate use of blood transfusion improve safety and reduces adverse outcomes for patients receiving a transfusion. Close clinical correlation and monitoring of symptomatic responsive also improve patient outcomes. Monitoring of transfusion has improved over time, with more emphasis placed on accurate detection and evaluation of transfusion reactions. Transfusion alternatives and extended shelf life options have expanded the scope of transfusion and have the potential to improve the blood supply. A summary of the strategies for reducing the risks associated with blood and blood products is shown in Figure 1.
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
Whole blood is often divided into component parts for ease of storage and administration. These typically include Red Blood Cells (RBC), Platelets (thrombocytes), and Plasma. Plasma can be further fractionated into Cryoprecipitate (i.e. cryoprecipitated antihemophilic factor) and many other clotting factor concentrates of variable purity.
The indications for whole blood and blood component transfusion consist of increasing hemoglobin and oxygenation of tissues, maintaining adequate blood volume to avoid ischemia and hypovolemic shock, and to reconstitute platelets, coagulation factors, and other plasma proteins to a functional status. Hemoglobin and hematocrit along with clinical symptoms of anemia have been the traditional markers used to determine the threshold for transfusion of red blood cells. Hemoglobin of less than 10 g/dL or a hematocrit of less than 30% has traditionally been the benchmark as the laboratory indication for transfusion in the right clinical context. Recently, there has been increasing evidence that a lower threshold is associated with better outcomes and conservation of precious blood resources, even in critically ill populations.[2] There is some evidence that the transfusion "trigger" level may be slightly higher in certain conditions such as sepsis with inadequate oxygen delivery, acute coronary syndrome with ischemia, and surgical hemorrhage or in specific populations such as the elderly. However, due controlled trials and meta-analysis data there has been a decrease in the use of liberal transfusion protocols.[3] Restrictive transfusion, even in patients with cardiovascular disease, acute illness, and traumatic brain injury using a threshold of hemoglobin of 7 to 8 g/dL has shown no increased risk of morbidity and mortality.[4][5] There is still variability globally in transfusion thresholds for particular patient populations.[6][7] Refer to specific articles on Indications for Red Blood Cell Transfusion for more information.
Platelets are typically given when patients have a low platelet count (thrombocytopenia) or have platelets that are dysfunctional, due to medications or other acquired or inherited lesions. Refer to specific articles on Indications for Platelet Transfusion for more information.
Plasma can be used to replace coagulation factors in specific coagulopathies (such as in liver disease when bleeding is present), reversal of warfarin effect (when coagulation concentrates are not available) and for treatment of diseases such thrombotic thrombocytopenia purpura. It is sometimes used as a replacement fluid in plasmapheresis and can be used for coagulation factor deficiencies where specific concentrates are not available. Refer to articles on Indications for Plasma Transfusion for more information.
Cryoprecipitate is a blood product derived from plasma. It can be used in emergencies as an alternative for supplying coagulation factors in inherited deficiencies such as von Willebrand disease, and hemophilia A (but only when specific concentrates are unavailable). It can also be used to replenish fibrinogen in acquired coagulopathies such as disseminated intravascular coagulation (DIC) and during trauma or childbirth.
Contraindications
Articles on Patient Blood Management outline the decision making process required before transfusing a patient. Significant contraindications that merit consideration include the risk of transfusion in a patient who is volume overloaded or who has previously had a reaction to blood.[8] Another important consideration is to avoid transfusion of blood without prior compatibility testing and antibody screening unless it is an emergency.
Equipment
Per the American Association of Blood Banks, all blood bags and needles are sterile, used only once, and then discarded to avoid contamination from donor to donor that could later affect multiple recipients of transfusion. The equipment used for blood donation collection such as blood pressure monitors, scales, blood collection monitors/mixers, blood bag tube sealers, transportation boxes, and refrigerators are calibrated, cleaned, and serviced routinely. The chairs and couches used in the area of blood donation are to be of cleanable surfaces such as vinyl. Transport supplies and containers are also cleanable according to the World Health Organization guidelines. All blood bags should undergo routine inspection for sterility, expiration date, appearance, and any evidence of leakage or defects at the time of donation collection.
Personnel
Phlebotomy and collection should be via qualified personnel trained in blood donation. Donation monitoring is under the overall responsibility of a medical practitioner or authorized personnel who can manage blood donor adverse reactions. Blood transfusion into patients is also performed with adequate oversight and under the purview of a medical practitioner or other qualified health care professional. Real-time monitoring of patients during transfusion ensures that accurate information is available for the detection and work-up of suspected transfusion reactions. Laboratory technologists are trained and educated on the importance of appropriate testing for possible infectious agents before transfusion, blood group and compatibility, potential antibodies, and post-transfusion evaluations for hemolysis. Transfusion service personnel who are committed to following accepted guidelines and to determining and auditing thresholds for transfusion have significantly influenced blood product safety.
Preparation
To assure blood product safety, several measures require implementation during product collection, manufacturing, and storage. The World Health Organization has supported a global initiative to improve access to safe and sufficient blood supply. Once collected, the blood is tested for donor blood type and screened for any clinically significant donor antibodies. The collecting facility typically holds the blood until the appropriate preparation and routine screening for potential transfusion-transmitted infections is complete. When all legal and industry standards have been met and the product is ready for transfusion, then it is "labelled" (i.e. identified as ready for use).
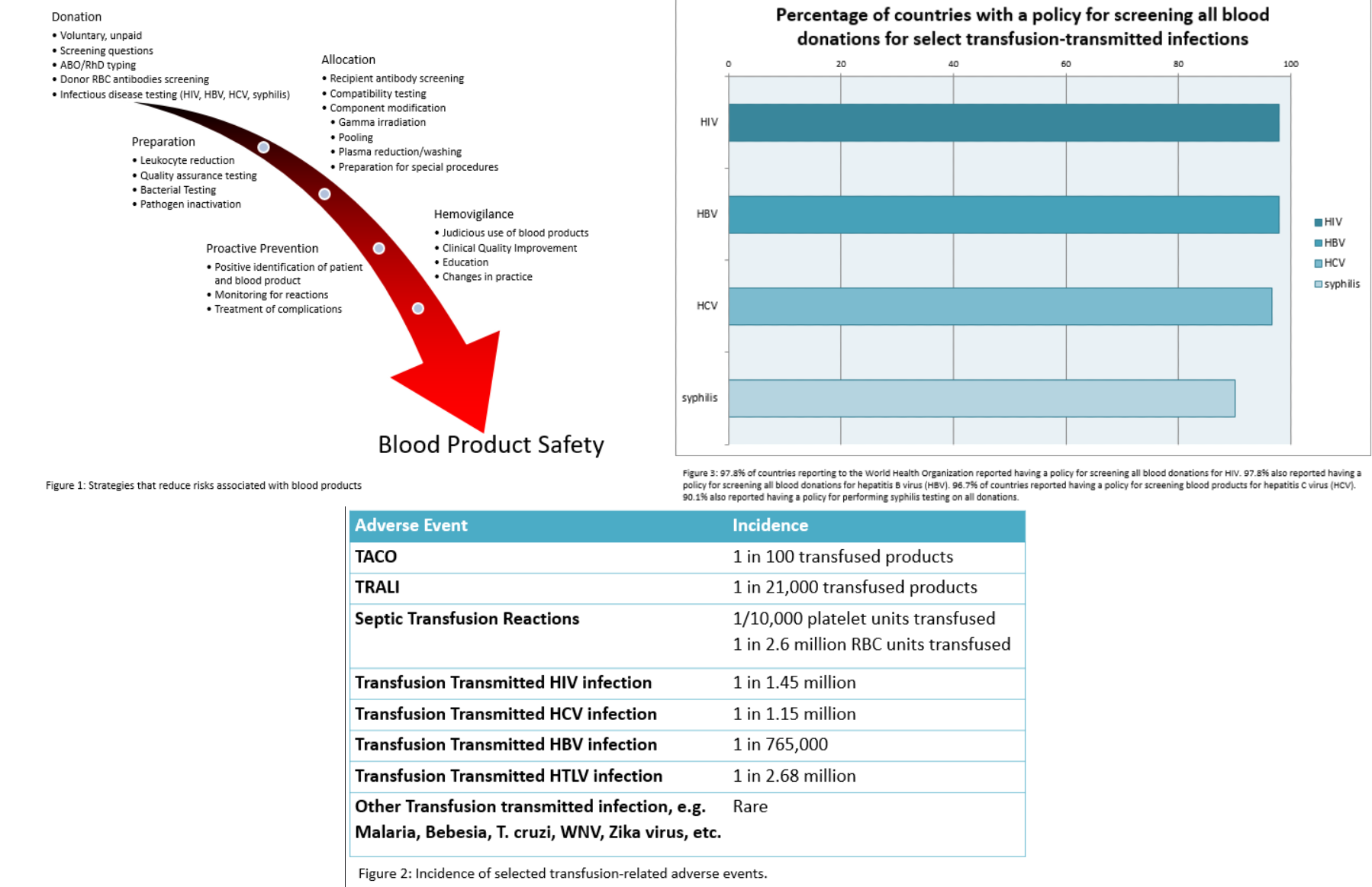
Widespread prioritization of testing for transfusion-transmitted infections has improved blood product safety worldwide. There is a summary of information on countries who responded to questionnaires about their particular policies and guidelines surrounding testing of donor blood in Figure 2 according to the World Health Organization 2016 Global Status Report on Blood Safety and Availability report. The survey found that the majority of responding countries had policies for testing the most common and clinically relevant transfusion-transmitted infections including HIV, hepatitis C, hepatitis B, and syphilis. Eighteen nations in Latin America reported having a policy for testing all blood donations for Trypanosoma cruzi along with twelve countries implementing selective testing for T cruzi in donors who have traveled to high-risk areas or have defined risk factors. Thirty-seven nations reported having a policy of testing all blood donations for antibody to human T-lymphotropic virus (HTLV-I/II) along with seven countries reporting selective additional testing for new donors.
Following collection of a blood donation, several procedures can take place during the preparation of blood for transfusion. Leukodepletion is a procedure to reduce the number of white blood cells in a blood product to reduce the risk of febrile reactions, HLA sensitization, and CMV transmission. Bacterial contamination testing of platelets can be performed prior to transfusion to avoid septic transfusion reactions. Plasma fractionation provides the opportunity to derive specific factors concentrates and intravenous immune globulin. Gamma irradiation of blood products can be performed to reduce the risk of transfusion-associated graft-versus-host disease, which is nearly always fatal.[9] Plasma reduction or washing of blood products limits the amount of plasma within a cellular blood product, which reduces the risk of allergic transfusion reactions or the effects of incompatible ABO antibodies. Volume reduction can also be used to reduce excess potassium and cytokines which can cause electrolyte imbalance and febrile non-hemolytic transfusion reactions, respectively.[10][11] Blood typing and screening for donor and recipient alloantibodies as well as compatibility testing are also important aspects of preparation for transfusion. Screening the donated blood for alloantibodies is essential in the prevention of hemolytic transfusion reactions in recipients.
The new frontier in blood product safety is pathogen reduction (pathogen inactivation) which is a broad term for various methodologies applied to blood products post-collection to reduce the risk of transmission of infectious agents.[12] Many of these technologies confer protection across different classes of infectious agents including viruses, bacteria, and parasites. Another potential benefit is that some of these technologies also inactivate donor white blood cells, which has allowed some to gain approval for the prevention of transfusion-associated graft-versus-host disease (as an alternative to irradiation). Pathogen reduction procedures are currently approved in some countries for platelets and plasma.[13] These novel technologies can increase the shelf life of platelets and decrease the incidence of adverse transfusion reactions and bacterial contamination. These approaches are increasingly common in practice and should help improve blood product safety profiles.[14]
Technique or Treatment
An important aspect of the donation process is the donor-screening questionnaire. Donor recruitment represents an essential front line mechanism for ensuring blood safety. The highest rates of transfusion-transmitted infections are present among donors receiving monetary compensation, and conversely, the lowest rates of infection are among unpaid volunteer donors.[15][16] "Replacement" and "family" donors are relied on in some countries, but these are not considered as safe as true altruistic unpaid volunteers. A great reduction in the risk of transfusion-transmitted HIV, HCV, HBV, and syphilis infections has transpired with the initial donor screening questions and improved testing, including serology and nucleic acid amplification testing.[17][18] According to the United States Food and Drug Administration, highly sensitive donor screening questionnaires designed to defer high-risk donors for infection transmission exclude an estimated 90% of potentially infectious donors from blood donation. Donors that have incentives to donate (such as monetary gain or wanting to help a friend) may not be completely truthful during screening.
Individual blood service organizations may have subtle variations in collection procedures, but the World Health Organization provides guidelines on the proper technique for venipuncture for blood donation. These standardize the process and are in place to prevent transfusion-transmitted infections. A safe collection is paramount to ensure that blood products remain safe through collection, storage, and transfusion.
Bacterial contaminants typically come from normal skin flora; therefore, proper antiseptic technique before the collection is required. The recommended procedure by the World Health Organization includes application of combination 2% chlorhexidine gluconate and 70% isopropyl alcohol for 30 seconds followed by 30 seconds drying time. A closed collection system (not open to the air) is used to ensure sterility. This procedure means that the anticoagulant-containing collection bag has intrinsically attached tube and needle. The first 15 to 20 mL of blood is collected in a diversion bag so that, in the case of possible skin contamination, the initial blood collected is used for laboratory testing and not transfused. This diverted blood is the most likely to be contaminated by skin flora and the skin plug (created by the needle), therefore removing this from the transfusion reduces contamination risk.[19] Blood volumes collected vary by the technique used. According to the World Health Organization, generally for whole blood transfusion, 350 milliliters of blood is collected, and for double or triple bags to make packed red cells, fresh frozen plasma, and platelet concentrations, a volume of 450 milliliters is necessary. The volume is selected to prevent donor transfusion-associated anemia and other adverse events.
Blood donations can be separated into four main components (red blood cells, platelets, plasma, and cryoprecipitate) or left as whole blood. Once the blood has undergone processing, it is stored at appropriate temperatures (often +2 C to +6 C).[20][21] Platelets and fresh frozen plasma (FFP) require preparation within 8 hours of collection. Platelets are stored at room temperature and with agitation typically for five days, unless additional shelf life extending mechanisms are employed.[22] Depending on the national regulations, fresh frozen plasma can remain stored at −18 C for one year, −25 C for 36 months, or at −65 C for seven years.[20] Many countries are moving toward making "plasma" instead of FFP, which gives them up to 24 hours after collection before processing and freezing is required. The temperature and duration of storage depend on blood service guidelines and storage capabilities of individual institutions. Sterility is maintained during processing and storage steps to avoid contamination. Blood units are unavailable for transfusion until undergoing appropriate testing, including ABO and Rh blood group typing and antibody screening, as well as serologic testing for transfusion-transmitted infections.
Complications
According to the World Health Organization Guidelines on Drawing Blood: Best Practices in Phlebotomy, complications of blood donation include (from most likely to least likely) a hematoma, vasovagal reaction or syncope, delayed syncope, arterial puncture, and nerve damage. Complications of blood transfusion include transfusion reactions and transfusion-transmitted infections.[23][8] The risk of transfusion-transmitted infection has fallen very low in high income countries, with a risk of less than 1 in 1 million for most of the concerning pathogens such as HIV, HCV, and HBV.[17] Donor screening techniques to select blood from only low-risk donors has had the most significant impact on the reduction of pathogens transmitted through transfusion. Exclusion criteria include medical history, high-risk behaviors, physical examination findings, geographic, and travel exclusions. There are also serologic and nucleic acid tests that have reduced the risk of infectious agent transmission. It should be noted that laboratory testing can never detect all pathogens due to a very small number of false negative tests. Therefore, screening questionnaires remain vital to eliminate high-risk donors. Donating blood should never be seen as an easy way to find out about the infectious disease status of at-risk persons.
Although the risk of transfusion-transmitted infection has decreased substantially, bacterial contamination in platelets stored at room temperature is still a concern for potential septic transfusion reactions. Bacterial contamination is typically from commensal bacteria normally found on the skin of donors or from transient bacteremia in donors. Another increasing concern is transfusion-transmitted emerging pathogens such as Zika virus and babesiosis. As the risk of infectious complications has decreased, noninfectious transfusion complications have become the primary area of concern and focus for improvement. The efforts focused on detection, recognition, and reporting of transfusion-related adverse events has improved the safety of transfusion of blood and blood components. Transfusion reactions that can occur with the transfusion of blood range from life-threatening reactions to circumstances in which transfusion can continue, once the cause of the reaction is determined (e.g. simple allergic reaction). The most common reactions include the following:[23]
- Transfusion-associated circulatory overload (TACO)
- Transfusion-related acute lung injury (TRALI)
- Transfusion-associated dyspnea (TAD)
- Simple allergic reaction
- Anaphylactic reaction
- Hypotensive transfusion reaction
- Febrile non-hemolytic transfusion reaction (FNHTR)
- Acute hemolytic transfusion reaction (AHTR)
- Delayed hemolytic transfusion reaction (DHTR)
- Delayed serologic transfusion reaction (DSTR)
- Transfusion-associated graft vs. host disease (TAGVHD)
- Post-transfusion purpura (PTP)
- Transfusion-transmitted infection (TTI)
With efforts to reduce TRALI, transfusion-associated circulatory overload (TACO) has been an emerging concern for morbidity and mortality in patients receiving blood transfusions.[24] Improved awareness around transfusion reactions and their etiology has improved blood product safety.[25][26][27][28] Estimated incidence rates for several of the complications of transfusion and specific transfusion-related adverse events are shown in Figure 3.[29][30][31][32][8]
Clinical Significance
Blood transfusion is one of the most common clinical procedures. Blood product safety is an essential aspect of quality health care received throughout the world. Approximately 112 million units of blood are collected globally every year according to the World Health Organization. Nearly 14 million units of blood (whole blood and packed red blood cells) are transfused annually worldwide.[33] The American Red Cross estimates that 36,000 units of blood are necessary every day in the United States of America. Blood transfusion is a lifesaving procedure that occurs every day in hospitals and clinics throughout the world.
There has been a push to move away from blood transfusion and to require more stringent guidelines for blood and blood product use. Per the National Blood Collection and Utilization Survey (NBCUS), there has been a steady decline in the rate of transfusions in the USA without a significant adverse effect on morbidity and mortality.[34] This finding is likely due to advances in surgical management of patients as well as policies for judicious use of blood products. This may also be due to evidence that liberal usage of blood products has correlated with poor patient outcomes.[35] Several studies have found a correlation between perioperative transfusion and increased morbidity and mortality, as well as a longer length of stay during hospitalizations.[36][37][38] Considering these findings, clinically restrictive transfusion protocols have shaped more recent guidelines in many countries.[39][40] However, there remains a delicate balance between treating symptomatic anemia and preventing transfusion-related adverse events.[41][42]
Transfusion reactions continue to be a significant cause of increased hospital stays, and adverse patient outcomes. Transfusion reactions occur in up to 1 in every 100 transfusions, making transfusion reactions an important clinical consideration of which to be aware for patients receiving transfusions.[43] Considering the number of transfusions that take place worldwide, blood product safety is an important part of improving patient care. The education of clinical staff surrounding signs and symptoms of a transfusion reaction, as well as efforts aimed at the judicious use of blood products, has improved the safety of transfusion.[33][23] Blood product transfusion is a life-saving procedure that has significantly improve patient outcomes. New efforts toward restricting the use of blood products to patients with real need, improving collection and storage of blood products, and developing alternatives to transfusion, promise increased safety of this lifesaving procedure.
Enhancing Healthcare Team Outcomes
Nurses, trained technical staff, and phlebotomists are involved in collecting, screening, modifying blood, and monitoring blood donors throughout the donation process in a safe and standardized manner. Laboratory personnel is crucial to ensure safe testing and handling of blood products. Nurses and laboratory technologists play a pivotal role in verifying patient information and ensuring that the correct blood product gets to the right patient at the right time. Nurses who monitor and document patient vital signs and symptoms during blood transfusions play a critical part in patient safety and in the work-up of possible transfusion reactions. Pharmacists and clinicians assist in premedication for patients with known allergic reactions or febrile non-hemolytic transfusion reactions during blood transfusions, and in the case of mild allergic transfusion reactions help with medication so that the transfusion can resume. Physicians work closely with the blood bank from their institution to coordinate care and provide blood in a time-sensitive manner. Communication on multiple levels of the an interprofessional team caring for patients is critical for this life-saving procedure to be effective and safe for patients. Transfusion safety officers and related professionals play a role in monitoring blood transfusion criteria and physician ordering of blood products to contribute to transfusion mindfulness and hemovigilance efforts [44][45] (Level V evidence).
Media
References
Wood EM, Ang AL, Bisht A, Bolton-Maggs PH, Bokhorst AG, Flesland O, Land K, Wiersum-Osselton JC, Schipperus MR, Tiberghien P, Whitaker BI. International haemovigilance: what have we learned and what do we need to do next? Transfusion medicine (Oxford, England). 2019 Aug:29(4):221-230. doi: 10.1111/tme.12582. Epub 2019 Feb 6 [PubMed PMID: 30729612]
Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. American family physician. 2011 Mar 15:83(6):719-24 [PubMed PMID: 21404983]
Simon GI, Craswell A, Thom O, Fung YL. Outcomes of restrictive versus liberal transfusion strategies in older adults from nine randomised controlled trials: a systematic review and meta-analysis. The Lancet. Haematology. 2017 Oct:4(10):e465-e474. doi: 10.1016/S2352-3026(17)30141-2. Epub 2017 Sep 11 [PubMed PMID: 28919087]
Level 1 (high-level) evidenceCarson JL, Stanworth SJ, Alexander JH, Roubinian N, Fergusson DA, Triulzi DJ, Goodman SG, Rao SV, Doree C, Hebert PC. Clinical trials evaluating red blood cell transfusion thresholds: An updated systematic review and with additional focus on patients with cardiovascular disease. American heart journal. 2018 Jun:200():96-101. doi: 10.1016/j.ahj.2018.04.007. Epub 2018 Apr 7 [PubMed PMID: 29898855]
Level 1 (high-level) evidenceNgwenya LB, Suen CG, Tarapore PE, Manley GT, Huang MC. Safety and cost efficiency of a restrictive transfusion protocol in patients with traumatic brain injury. Journal of neurosurgery. 2018 May:128(5):1530-1537. doi: 10.3171/2017.1.JNS162234. Epub 2017 Jun 23 [PubMed PMID: 28644101]
East JM, Viau-Lapointe J, McCredie VA. Transfusion practices in traumatic brain injury. Current opinion in anaesthesiology. 2018 Apr:31(2):219-226. doi: 10.1097/ACO.0000000000000566. Epub [PubMed PMID: 29369066]
Level 3 (low-level) evidenceVincent JL, Jaschinski U, Wittebole X, Lefrant JY, Jakob SM, Almekhlafi GA, Pellis T, Tripathy S, Rubatto Birri PN, Sakr Y, ICON Investigators. Worldwide audit of blood transfusion practice in critically ill patients. Critical care (London, England). 2018 Apr 19:22(1):102. doi: 10.1186/s13054-018-2018-9. Epub 2018 Apr 19 [PubMed PMID: 29673409]
Crookston KP, Koenig SC, Reyes MD. Transfusion reaction identification and management at the bedside. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2015 Mar-Apr:38(2):104-13. doi: 10.1097/NAN.0000000000000097. Epub [PubMed PMID: 25723832]
Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, Keir AK, DenHollander N, Dzik WS, Callum J. A systematic review of transfusion-associated graft-versus-host disease. Blood. 2015 Jul 16:126(3):406-14. doi: 10.1182/blood-2015-01-620872. Epub 2015 Apr 30 [PubMed PMID: 25931584]
Level 1 (high-level) evidenceLu M, Lezzar DL, Vörös E, Shevkoplyas SS. Traditional and emerging technologies for washing and volume reducing blood products. Journal of blood medicine. 2019:10():37-46. doi: 10.2147/JBM.S166316. Epub 2019 Jan 3 [PubMed PMID: 30655711]
Gehrie EA, Dunbar NM. Modifications to Blood Components: When to Use them and What is the Evidence? Hematology/oncology clinics of North America. 2016 Jun:30(3):653-63. doi: 10.1016/j.hoc.2016.01.007. Epub 2016 Mar 31 [PubMed PMID: 27113002]
Jacquot C, Delaney M. Pathogen-inactivated blood products for pediatric patients: blood safety, patient safety, or both? Transfusion. 2018 Sep:58(9):2095-2101. doi: 10.1111/trf.14811. Epub [PubMed PMID: 30204950]
Carson JL, Triulzi DJ, Ness PM. Indications for and Adverse Effects of Red-Cell Transfusion. The New England journal of medicine. 2017 Sep 28:377(13):1261-1272. doi: 10.1056/NEJMra1612789. Epub [PubMed PMID: 28953438]
Epstein JS. Alternative strategies in assuring blood safety: An overview. Biologicals : journal of the International Association of Biological Standardization. 2010 Jan:38(1):31-5. doi: 10.1016/j.biologicals.2009.10.009. Epub 2010 Jan 27 [PubMed PMID: 20110174]
Level 3 (low-level) evidenceGillet P, Neijens E. An Original Approach to Evaluating the Quality of Blood Donor Selection: Checking Donor Questionnaires and Analyzing Donor Deferral Rate. Frontiers in medicine. 2018:5():74. doi: 10.3389/fmed.2018.00074. Epub 2018 Mar 21 [PubMed PMID: 29619370]
Level 2 (mid-level) evidencevan der Poel CL, Seifried E, Schaasberg WP. Paying for blood donations: still a risk? Vox sanguinis. 2002 Nov:83(4):285-93 [PubMed PMID: 12437514]
Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood. 2019 Apr 25:133(17):1854-1864. doi: 10.1182/blood-2018-11-833996. Epub 2019 Feb 26 [PubMed PMID: 30808637]
Zou S, Fujii K, Johnson S, Spencer B, Washington N, Iv EN, Musavi F, Newman B, Cable R, Rios J, Hillyer KL, Hillyer CD, Dodd RY, ARCNET Study Group. Prevalence of selected viral infections among blood donors deferred for potential risk to blood safety. Transfusion. 2006 Nov:46(11):1997-2003 [PubMed PMID: 17076856]
Level 2 (mid-level) evidenceLevy JH, Neal MD, Herman JH. Bacterial contamination of platelets for transfusion: strategies for prevention. Critical care (London, England). 2018 Oct 27:22(1):271. doi: 10.1186/s13054-018-2212-9. Epub 2018 Oct 27 [PubMed PMID: 30367640]
Hess JR. Conventional blood banking and blood component storage regulation: opportunities for improvement. Blood transfusion = Trasfusione del sangue. 2010 Jun:8 Suppl 3(Suppl 3):s9-15. doi: 10.2450/2010.003S. Epub [PubMed PMID: 20606757]
Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016 Nov 15:316(19):2025-2035. doi: 10.1001/jama.2016.9185. Epub [PubMed PMID: 27732721]
Level 1 (high-level) evidenceZhang Q, Lu M, Wu MX. Potentials for prolonging shelf-life of platelets by near infrared low-level light. Journal of biophotonics. 2019 May:12(5):e201800390. doi: 10.1002/jbio.201800390. Epub 2019 Jan 6 [PubMed PMID: 30561165]
Suddock JT, Crookston KP. Transfusion Reactions. StatPearls. 2024 Jan:(): [PubMed PMID: 29489247]
Roubinian NH, Hendrickson JE, Triulzi DJ, Gottschall JL, Michalkiewicz M, Chowdhury D, Kor DJ, Looney MR, Matthay MA, Kleinman SH, Brambilla D, Murphy EL, National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Contemporary Risk Factors and Outcomes of Transfusion-Associated Circulatory Overload. Critical care medicine. 2018 Apr:46(4):577-585. doi: 10.1097/CCM.0000000000002948. Epub [PubMed PMID: 29300236]
Roubinian NH, Hendrickson JE, Triulzi DJ, Gottschall JL, Chowdhury D, Kor DJ, Looney MR, Matthay MA, Kleinman SH, Brambilla D, Murphy EL, NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Incidence and clinical characteristics of transfusion-associated circulatory overload using an active surveillance algorithm. Vox sanguinis. 2017 Jan:112(1):56-63. doi: 10.1111/vox.12466. Epub 2016 Dec 21 [PubMed PMID: 28001313]
Roubinian N. TACO and TRALI: biology, risk factors, and prevention strategies. Hematology. American Society of Hematology. Education Program. 2018 Nov 30:2018(1):585-594. doi: 10.1182/asheducation-2018.1.585. Epub 2018 Dec 14 [PubMed PMID: 30570487]
Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, Lowell CA, Norris PJ, Murphy EL, Weiskopf RB, Wilson G, Koenigsberg M, Lee D, Schuller R, Wu P, Grimes B, Gandhi MJ, Winters JL, Mair D, Hirschler N, Sanchez Rosen R, Matthay MA, TRALI Study Group. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012 Feb 16:119(7):1757-67. doi: 10.1182/blood-2011-08-370932. Epub 2011 Nov 23 [PubMed PMID: 22117051]
Level 2 (mid-level) evidenceHong H, Xiao W, Lazarus HM, Good CE, Maitta RW, Jacobs MR. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood. 2016 Jan 28:127(4):496-502. doi: 10.1182/blood-2015-07-655944. Epub 2015 Nov 23 [PubMed PMID: 26598718]
Lafeuillade B, Eb F, Ounnoughene N, Petermann R, Daurat G, Huyghe G, Vo Mai MP, Caldani C, Rebibo D, Weinbreck P. Residual risk and retrospective analysis of transfusion-transmitted bacterial infection reported by the French National Hemovigilance Network from 2000 to 2008. Transfusion. 2015 Mar:55(3):636-46. doi: 10.1111/trf.12883. Epub 2014 Sep 26 [PubMed PMID: 25257344]
Level 2 (mid-level) evidenceErony SM, Marshall CE, Gehrie EA, Boyd JS, Ness PM, Tobian AAR, Carroll KC, Blagg L, Shifflett L, Bloch EM. The epidemiology of bacterial culture-positive and septic transfusion reactions at a large tertiary academic center: 2009 to 2016. Transfusion. 2018 Aug:58(8):1933-1939. doi: 10.1111/trf.14789. Epub 2018 Aug 28 [PubMed PMID: 30153333]
Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogeneic donations. Transfusion medicine reviews. 2012 Apr:26(2):119-28. doi: 10.1016/j.tmrv.2011.07.007. Epub 2011 Aug 25 [PubMed PMID: 21871776]
Stramer SL, Notari EP, Krysztof DE, Dodd RY. Hepatitis B virus testing by minipool nucleic acid testing: does it improve blood safety? Transfusion. 2013 Oct:53(10 Pt 2):2449-58. doi: 10.1111/trf.12213. Epub 2013 Apr 23 [PubMed PMID: 23607261]
Frazier SK, Higgins J, Bugajski A, Jones AR, Brown MR. Adverse Reactions to Transfusion of Blood Products and Best Practices for Prevention. Critical care nursing clinics of North America. 2017 Sep:29(3):271-290. doi: 10.1016/j.cnc.2017.04.002. Epub 2017 Jun 26 [PubMed PMID: 28778288]
Ellingson KD, Sapiano MRP, Haass KA, Savinkina AA, Baker ML, Chung KW, Henry RA, Berger JJ, Kuehnert MJ, Basavaraju SV. Continued decline in blood collection and transfusion in the United States-2015. Transfusion. 2017 Jun:57 Suppl 2(Suppl 2):1588-1598. doi: 10.1111/trf.14165. Epub [PubMed PMID: 28591469]
Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, Hickner A, Rogers MA. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014 Apr 2:311(13):1317-26. doi: 10.1001/jama.2014.2726. Epub [PubMed PMID: 24691607]
Level 1 (high-level) evidenceElwood NR, Martin AN, Turrentine FE, Jones RS, Zaydfudim VM. The negative effect of perioperative red blood cell transfusion on morbidity and mortality after major abdominal operations. American journal of surgery. 2018 Sep:216(3):487-491. doi: 10.1016/j.amjsurg.2018.02.015. Epub 2018 Feb 17 [PubMed PMID: 29475550]
Leuzinger E, Poblete B, Konrad CJ, Hansen D. How current transfusion practices in geriatric patients with hip fracture still differ from current guidelines and the effects on outcome: A retrospective observational study. European journal of anaesthesiology. 2018 Dec:35(12):972-979. doi: 10.1097/EJA.0000000000000883. Epub [PubMed PMID: 30234668]
Level 2 (mid-level) evidenceMüller S, Oberle D, Drechsel-Bäuerle U, Pavel J, Keller-Stanislawski B, Funk MB. Mortality, Morbidity and Related Outcomes Following Perioperative Blood Transfusion in Patients with Major Orthopaedic Surgery: A Systematic Review. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2018 Oct:45(5):355-367. doi: 10.1159/000481994. Epub 2018 Feb 28 [PubMed PMID: 30498414]
Level 1 (high-level) evidenceOdutayo A, Desborough MJ, Trivella M, Stanley AJ, Dorée C, Collins GS, Hopewell S, Brunskill SJ, Kahan BC, Logan RF, Barkun AN, Murphy MF, Jairath V. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. The lancet. Gastroenterology & hepatology. 2017 May:2(5):354-360. doi: 10.1016/S2468-1253(17)30054-7. Epub 2017 Mar 23 [PubMed PMID: 28397699]
Level 1 (high-level) evidenceSong HK, von Heymann C, Jespersen CM, Karkouti K, Korte W, Levy JH, Ranucci M, Saugstrup T, Sellke FW. Safe application of a restrictive transfusion protocol in moderate-risk patients undergoing cardiac operations. The Annals of thoracic surgery. 2014 May:97(5):1630-5. doi: 10.1016/j.athoracsur.2013.12.025. Epub 2014 Mar 19 [PubMed PMID: 24655469]
Level 1 (high-level) evidenceFowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. The British journal of surgery. 2015 Oct:102(11):1314-24. doi: 10.1002/bjs.9861. Epub [PubMed PMID: 26349842]
Level 1 (high-level) evidenceLilly CM, Badawi O, Liu X, Gill CS, Harris I. Red Blood Cell Product Transfusion Thresholds and Clinical Outcomes. Journal of intensive care medicine. 2020 May:35(5):494-501. doi: 10.1177/0885066618762746. Epub 2018 Mar 18 [PubMed PMID: 29552954]
Level 2 (mid-level) evidenceDelaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, Apelseth TO, Popovsky M, Stanworth SJ, Tinmouth A, Van De Watering L, Waters JH, Yazer M, Ziman A, Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet (London, England). 2016 Dec 3:388(10061):2825-2836. doi: 10.1016/S0140-6736(15)01313-6. Epub 2016 Apr 12 [PubMed PMID: 27083327]
Bolton-Maggs PHB. Conference report: International Haemovigilance Seminar and the SHOT Annual Symposium, 10-12 July 2018. Transfusion medicine (Oxford, England). 2019 Aug:29(4):247-252. doi: 10.1111/tme.12569. Epub 2018 Dec 27 [PubMed PMID: 30592099]
Henneman EA, Andrzejewski C Jr, Gawlinski A, McAfee K, Panaccione T, Dziel K. Transfusion-Associated Circulatory Overload: Evidence-Based Strategies to Prevent, Identify, and Manage a Serious Adverse Event. Critical care nurse. 2017 Oct:37(5):58-65. doi: 10.4037/ccn2017770. Epub [PubMed PMID: 28966196]