Introduction
Maintaining blood in a liquid state is essential for homeostasis, as it ensures the delivery of sufficient oxygen and nutrients to tissues while removing carbon dioxide and other waste products. Conversely, the ability of blood to convert from a liquid to a solid state—that is coagulate—underlies the mechanism that protects the body from life-threatening exsanguination. This process of thrombosis is normally a localized event at the site of vascular injury while the remaining circulating blood remains in a liquid state. Thrombosis is a dynamic process that includes associated thrombolysis to maintain or restore blood flow through vessels once an injury has been sealed. These unique properties of blood are largely determined by a complex and active balance between pro-coagulation factors, anticoagulants, and fibrinolysis. Two major pathological conditions are commonly associated with the disequilibrium of this intricate system—bleeding and vessel thrombosis.
Major bleeding is a serious medical complication that may be caused by external trauma, surgery, invasive procedures, or an underlying medical condition such as aneurysm rupture or peptic ulcer disease. According to the World Health Organization, injuries are responsible for 5.8 million deaths per year worldwide, with the associated bleeding responsible for about 30% to 40% of these deaths.[1] Several congenital disorders, such as Von Willebrand disease and hemophilia A or B, associated with coagulation factor deficiencies may cause significant bleeding even from minor injuries. In addition, prescribed anticoagulants and antiplatelet agents can induce a coagulopathic state, potentially leading to excessive bleeding associated with trauma or medical procedures. Acute blood loss can lead to coagulopathy due to the depletion of coagulation factors. Predictably, trauma-related coagulopathy has been associated with significantly higher mortality.[2] Patients with ongoing or expected major bleeding benefit from an accurate assessment of the functional state of the hemostatic system to provide optimal care, providing cost-effective replacement of only the necessary blood components.
Venous thromboembolism is another common and serious condition associated with abnormal blood coagulation. In these cases, systemic hypercoagulability shifts the body's homeostatic mechanisms toward a prothrombotic state. However, in many cases, a definitive cause for venous thromboembolism may be unclear. Routine coagulation testing has not been shown to predict such events, and even a detailed hypercoagulability investigation fails to identify an underlying disorder. Many individuals take anticoagulants and antiplatelet agents regularly, which impacts the accuracy of the results of many laboratory coagulation studies.[3][4] An accurate and cost-effective method to monitor antithrombotic activity could help maintain an acceptable risk-benefit ratio in such patients. Inadequate anticoagulation or antiplatelet therapy can lead to devastating thromboembolic conditions.
Several commonly used blood tests assess blood coagulation. These tests include prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), platelet count, fibrinogen concentration, D-dimer levels, activated clotting time, and whole blood bleeding time. These tests are typically used to diagnose coagulopathy and a possible prothrombotic state, monitor anticoagulation therapy, and assist in treating bleeding episodes. More specific factor analyses, such as Factor V, proteins C and S, anti-thrombin III, anticardiolipin antibodies, and prothrombin gene mutation, are useful but not as readily available in emergency clinical situations. Despite their effectiveness for specific clinical applications, such as anticoagulation monitoring, traditional diagnostic tests have notable limitations. One of the primary drawbacks in the context of acute major bleeding is their prolonged turnaround time. Moreover, these tests fail to provide a comprehensive assessment of hemostasis, as they do not evaluate certain factors, such as Factor XIII; platelet function; or fibrinolytic activity. Platelet concentration, easily measured as part of a complete blood count, does not necessarily reflect their function, especially in the presence of elements known to affect platelet reactivity, such as nonsteroidal anti-inflammatory drugs, antiplatelet agents, uremia, malignancy, or alcohol intake. Bleeding time has a low sensitivity and high inconsistency in detecting platelet disorders.[5] Delayed or inadequate diagnosis of coagulopathy in a bleeding patient may lead to an excessive and improperly balanced transfusion of scarce blood components with increased morbidity, treatment costs, and mortality.[6]
Thromboelastography (TEG) is a promising diagnostic modality that offers several advantages compared to other tests mentioned above. TEG was developed in 1948 by Dr Hellmut Hartert at the University of Heidelberg.[7] The first reported clinical application of the test occurred during the Vietnam War in an attempt to guide transfusions of blood components in injured soldiers.[8] In the 1980s, TEG was found to be beneficial in liver transplant patients, and in the 1990s, it was demonstrated to be useful in cardiac surgery.[9][10] Since then, TEG has become increasingly used as growing evidence supports its clinical efficacy. A PubMed search using the keywords TEG and thromboelastometry results in approximately 6000 publications, highlighting its expanding role in medical practice.
This article outlines the general principles of TEG, including its methodology, normal values, current evidence, clinical applications, limitations, and future research directions.
Pathophysiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Pathophysiology
TEG is a noninvasive test that quantitatively measures the ability of whole blood to form a clot. The principle of this in vitro test is to detect and quantify dynamic changes in the viscoelastic properties of a blood sample during clotting under low shear stress. The test is performed in a specially designed system called a thromboelastograph. The system consists of 2 chambers simultaneously examining a blood sample in duplicate to reduce the risk of sampling and measurement errors. Each chamber consists of a platform with a disposable cup where a blood sample is placed and a detection pin suspended in its center. The cup oscillates around the detection pin in a limited arc of ±4° 45′ 5″. Induced pin movement is recorded, and changes are measured as a function of time. Initially, there is little movement of the pin since liquid blood possesses minimal viscosity, and the oscillations of the cup are not transmitted to the pin.
As the blood coagulates, it begins to adhere to both the cup and the pin, and movement of the cup induces motion on the pin. These gradually increasing viscoelastic mechanical properties of the blood reflect the developing three-dimensional fibrin mesh and platelet components of the clot. The greater the viscoelasticity of the clot, the higher the amplitude of the pin motion. As fibrinolysis starts, the fibrin-platelet structure begins to dissolve gradually, and the clot loses its contact with the detection pin, which has less induced motion. The thromboelastogram is a graphical image of the recorded amplitude of movement of the pin as a function of time. Analytical software measures and quantifies these changes (see Figure. Theombelastography Tables). Therefore, TEG measures the functional ability of the blood to make a hemostatic plug. A newer version replaces the cup rotation method with a resonance technique wherein the blood sample is subjected to vibration, and the vertical movement of the blood meniscus is measured under light-emitting diode illumination. The system uses pre-measured cartridges that do not require pipetting and allows the simultaneous performance of 4 blood tests.
Specimen Requirements and Procedure
The blood sample is collected through venipuncture in a plastic vial containing 3.2% buffered sodium citrate with a citrate-to-blood ratio of 1:9. The vial is inverted several times to mix the blood and citrate. Maintaining this citrate-blood ratio is crucial for test accuracy. Citrate binds calcium, an important cofactor of coagulation, preventing the blood from clotting before the beginning of the test. A clotted specimen, reflecting a vial overfilled with blood, cannot be used. For TEG testing, the collected non-clotted samples are considered stable and usable for up to 2 hours at room temperature. Non-citrated whole blood (native blood TEG or NATEM) can also be tested, but it must be used immediately. The test and reagents used are at room temperature. A volume of 340 uL of citrated blood is pipetted to the study cup, recalcified by adding 20 uL of 0.2 M calcium chloride and then activated with a kaolin-cephalin reagent. Cephalins or phosphatidylethanolamines, a class of phospholipids commonly present in membranes of human cells, are an essential cofactor of the coagulation cascade, enabling the assembly of tenase and prothrombinase complexes on the surface of platelets that are critical for thrombin generation. Kaolin is a mineral primarily composed of hydrated aluminum silicate, a negatively charged molecule that can initiate the intrinsic coagulation pathway by activating Factor XII. Precise proportioning of the blood and kaolin-cephalin reagent is essential for accurate and reproducible TEG results.[11] Non-activated TEG is also possible, but the lack of activators significantly prolongs clotting time and the testing process, which is undesirable in a clinical emergency.
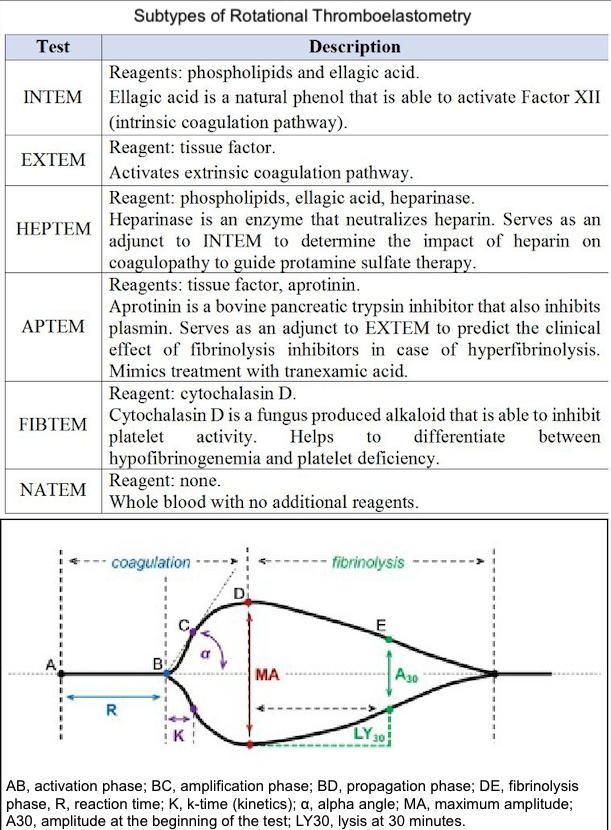
Several modifications of the classic TEG assay have been developed to improve its diagnostic value. Rapid TEG (r-TEG) uses tissue factor instead of the kaolin-cephalin reagent to activate blood coagulation. Because the tissue factor triggers the extrinsic coagulation pathway, which involves a smaller number of coagulation factors, the test can be performed faster compared to conventional TEG. r-TEG can be completed within 15 minutes and thus helps manage massive transfusions in trauma patients.[12][13] The TEG platelet mapping assay was developed to predict the inhibitory effect of antiplatelet agents, such as aspirin and clopidogrel, by evaluating platelet aggregation in the presence of adenosine diphosphate or arachidonic acid. TEG with added heparinase measures the effect of heparin reversal on blood coagulation. Rotational thromboelastography, also known as rotational thromboelastometry (RoTEM), uses an oscillating pin that rotates ±4° 45′ 6″ while maintaining the cup in a stable position. In this assay, different activator reagents are used to investigate specific components of the coagulation pathway (Table 1).
Results, Reporting, and Critical Findings
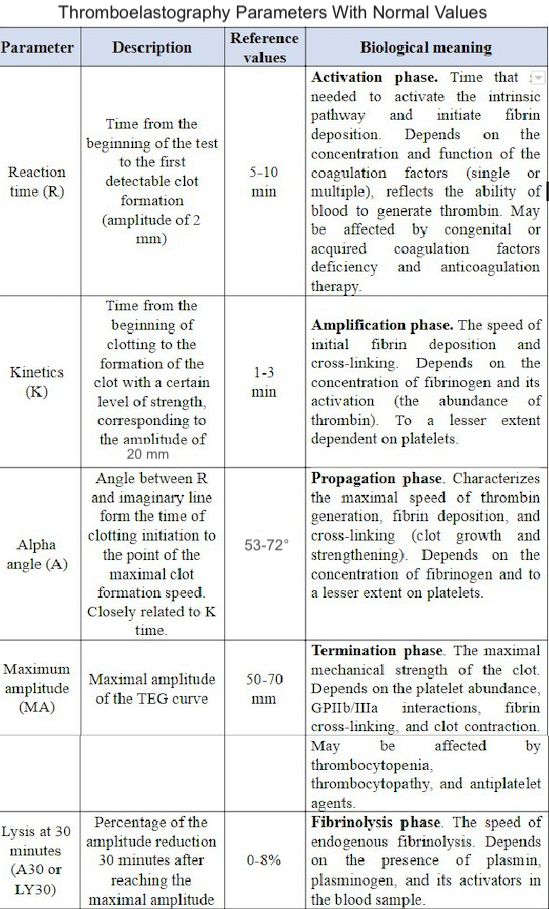
A normal thromboelastogram is schematically represented in Figure 1. Prompt qualitative analysis of the TEG tracing can be performed during the test. The quantitative analysis of TEG includes measuring the 5 parameters listed and described in Table 1. The manufacturer has also suggested a coagulation index to assess the overall coagulation status. The coagulation index (CI) for whole blood may be calculated as follows:
- CI = −0.2454R+ 0.0184K + 0.1655MA − 0.0241a − 5.0220
Normal values of the coagulation index lie within −3.0 and +3.0, which is 3 standard deviations from the mean of zero. A hypercoagulable state is defined as a CI greater than +3.0, and coagulopathy as a CI less than −3.0. Previous studies demonstrated a significantly elevated CI in the postoperative period after general surgery and in cancer patients, suggesting a prothrombotic state.[14][15] However, this index is not widely used, and its clinical usefulness is not yet validated. Other parameters derived from the thromboelastogram include projected maximal amplitude; time to maximal amplitude; the G parameter, such as shear elastic modulus strength or clot strength; and a thrombodynamic potential index. Although these variables provide additional insights, they are rarely utilized in routine clinical practice due to their complexity and limited practical application.
Normal TEG values are summarized in Table 2, although some patient-related factors may affect these values. Elderly patients tend towards more pro-coagulable TEG results, suggesting a need to correct these reference values in older individuals.[16] In some circumstances, such as in patients undergoing cardiac surgery and liver transplantation, specific reference values are less important because the principal application of TEG is to compare the patient's baseline to changes during the intraoperative and postoperative periods. In other clinical scenarios, such as trauma or postoperative bleeding, reference values are important for interpreting the results, as no baseline data are available. There is also some variability in testing results. A study involving 118 healthy volunteers revealed at least one abnormal parameter in 19% of specimens and a coagulopathy (defined as at least 2 abnormal parameters) in 9%, leading to a calculated specificity of 81%.[17] A larger prospective trial encompassing a more diverse group of patients helps establish analyzer- and reagent-specific reference values in selected patient subgroups.
Deviation of each TEG parameter from the reference values suggests specific disturbances of hemostasis and coagulation.
- Prolonged R time suggests a quantitative or qualitative deficiency of coagulation factors that may be corrected by fresh frozen plasma transfusion, prothrombin complex, or anticoagulant reversal.
- Prolonged K time or decreased alpha-angle suggests fibrinogen deficiency, which may be corrected using cryoprecipitate or lyophilized fibrinogen concentrate.
- Low maximum amplitude indicates a quantitative or functional deficiency of platelets and could be corrected by platelet concentrate transfusion or desmopressin.
- Increased LY value implies activated fibrinolysis that may be treated by fibrinolysis inhibitors (aminocaproic or tranexamic acid).
The opposite changes in TEG parameters suggest a prothrombotic state. This interpretive approach represents a convenient but rather simplified perspective on disturbances of blood coagulation. However, it is important to remember that these TEG parameters are interrelated due to the complex nature of hemostasis.
A different nomenclature is used for RoTEM assays to define the same TEG parameters—clotting time (CT) instead of R, clot formation time (CFT) instead of K, maximum clot firmness (MCF) instead of MA, and CL instead of LY. Reference values for RoTEM have been established in a multicenter study involving 500 healthy volunteers.[18] Depending on the specific TEG analyzer (TEG versus RoTEM) and the reagents used, differing results may be obtained from the same blood sample, potentially affecting clinical decision-making.[19][20] Some of these differences remain poorly understood. For example, NATEM may be more sensitive to hyperfibrinolysis compared to INTEM and EXTEM.[21] A systematic review of 4 clinical trials comparing TEG and RoTEM found clinically significant differences between the 2 tests with a lack of comparability of the results.[22] A recent systematic review did not discover a sufficient number of well-designed studies to compare the results of TEG and RoTEM in healthy individuals.[23] Hence, the results of different modifications of TEG and RoTEM cannot be considered interchangeable until head-to-head prospective comparative studies are performed.
Clinical Significance
The main advantage of TEG testing is its potential to deliver immediate goal-oriented and individualized care to a bleeding patient:
- Global assessment of blood coagulability, including coagulation cascade, platelet function, and fibrinolysis
- Rapid real-time bedside test with a simple methodology (point-of-care testing)
- Diagnosis of coagulopathic bleeding
- Guide transfusion therapy and decrease the use of blood products
- Detect dynamic changes in blood coagulation during resuscitation
- Predict the clinical efficacy of therapeutic agents affecting blood coagulability
TEG has convincingly demonstrated its utility in improving outcomes in cardiac surgery. A meta-analysis of 17 randomized controlled trials showed that TEG decreases blood product transfusions and surgical re-exploration due to postoperative bleeding in cardiac surgery patients.[24] These effects were associated with a lower incidence of acute kidney injury and thromboembolic events. Another systematic review of 17 randomized controlled trials involving 1493 patients, mainly elective on-pump cardiac surgery, revealed that TEG/RoTEM decreases transfusion of blood components and reduces overall mortality.[25] However, the quality of the included studies was considered low.[25] A recent randomized controlled trial found that intraoperative correction of coagulopathy guided by EXTEM and FIBTEM can reduce postoperative bleeding, blood transfusions, and duration of critical care in pediatric cardiac surgery patients.[26] TEG is also a more cost-effective method compared to standard coagulation tests in diagnosing coagulopathy in cardiac surgery.[27]
There is conflicting evidence regarding the usefulness of TEGs in trauma patients. A recent Cochrane database systematic review found insufficient data to compare the accuracy of TEG and RoTEM versus PT/INR in diagnosing trauma-induced coagulopathy.[28] The review concluded that these tests are still in the clinical research phase. However, whether PT/INR can be considered a good reference standard to diagnose coagulopathy is questionable. In major trauma, r-TEG is better in predicting the need for transfusion of fresh frozen plasma, red blood cells, and platelets compared to conventional coagulation tests of PT, aPTT, INR, platelet count, and fibrinogen.[29] Based on another large systematic review, although with evidence limited to cohort studies with a moderate-to-high risk of bias, TEG/RoTEM can diagnose coagulopathy and may predict blood components transfusion and mortality in trauma patients.[30] Another review of 13 cohort studies involving only RoTEM in 2835 adult trauma patients reached similar conclusions.[31] However, there was no improvement in patient morbidity or mortality.[30] Another Cochrane database systematic review of 9 randomized controlled trials involving 776 participants, mainly cardiac surgery patients, found a decreased amount of bleeding when TEG or RoTEM was used but also without a decrease in morbidity or mortality.[22] This inability of TEG/RoTEM testing to significantly reduce mortality may become a barrier to widespread clinical use. However, it is essential to realize that overall mortality in hospitalized patients with bleeding is relatively low, necessitating large clinical trials to detect a statistically significant impact of TEG on mortality. Furthermore, these patients are often complex cases, and the overall treatment strategy, rather than diagnostic testing, plays a significant role in affecting overall morbidity and mortality.
There is expanding evidence of using different TEG and RoTEM assays in other additional clinical scenarios. A small randomized controlled trial has demonstrated the ability of TEG to better guide anticoagulation during ECMO compared to aPTT, reducing the dose of heparin.[32] TEG platelet mapping can detect platelet inhibition caused by clopidogrel and aspirin in surgical patients.[33] A novel TEG-based scoring system has been suggested to diagnose disseminated intravascular coagulation.[34] TEG may detect possible coagulopathy in patients with intracranial bleeding and hematoma enlargement.[35] TEG may also be useful for patients with liver disease, as conventional coagulation tests often show abnormalities in these individuals. At the same time, TEG/RoTEM results are normal in many patients despite an abnormal INR or platelet count due to adjustments in the hemostatic system or rebalanced hemostasis.[36] Thus, TEG/RoTEM may provide a better insight into the risk of bleeding in patients with liver disease compared to conventional coagulation tests.[37] EXTEM has been found helpful in detecting intraoperative coagulopathy in liver transplant patients.[38] There are other clinical situations when TEG has demonstrated potential benefits, but listing all of them is beyond the scope of this review.
Clinical Guidelines
The NICE guidelines recommend TEG for detecting, managing, and monitoring hemostasis in cardiac surgery patients. Other clinical guidelines do not strongly recommend TEG for use in other settings due to the lack of high-quality evidence. The recently updated guidelines from the European Society of Anesthesiology recommended viscoelastic hemostatic assays (TEG/RoTEM) to guide the management of perioperative bleeding and severe peripartum hemorrhages, albeit with a low level of evidence.[39]
Limitations
An ideal test on blood coagulation does not yet exist. TEG measures blood coagulation in vitro, with or without an additional activator. A tissue factor, an essential component of the coagulation cascade, cannot be quantified in vitro. Furthermore, blood coagulation is the only component of complex processes such as clinical thrombosis and bleeding. Blood coagulation also depends on the size of the injured vessel, blood flow characteristics, and local vessel wall biology, which determines the quantity and functional activity of the membrane-bound pro- and anticoagulation factors. In other words, there are significant aspects of coagulation that are not components of the blood. An abnormal TEG in a patient without clinically relevant bleeding does not require transfusion of blood components. A single test or patient-related factor rarely guides the decision to transfuse blood components or initiate or correct antithrombotic therapy.
TEG has a sensitivity and specificity that may vary significantly in different populations. Patients on anticoagulants and antiplatelet agents are a major concern in the trauma setting. Warfarin is a commonly prescribed medication associated with increased mortality in trauma patients.[40][41] In about half of patients on warfarin, R-time may be normal in both TEG and r-TEG tests, with a poor correlation between TEG and INR.[11] Such findings are a good example of how TEG may miss a clinically significant coagulopathic state. Hence, INR is still the gold standard for monitoring warfarin therapy. Several important blood tests also cannot be currently replaced by TEG, such as P2Y12 platelet function assay to guide clopidogrel therapy, D-Dimer to exclude venous thromboembolism in low-risk outpatients, and advanced thrombophilia diagnostic tests.
Future Directions: Guiding Anticoagulation and Antiplatelet Therapy
Anticoagulation therapy is a field where TEG may become more applicable pending future clinical studies. Up to one-third of patients on warfarin therapy may have subtherapeutic anticoagulation at some point during treatment.[42] One of the advantages of direct oral anticoagulants is that there is no requirement to monitor anticoagulation therapy. However, they may be problematic in certain groups of patients, such as those with renal failure, liver failure, pregnancy, extremes of body weight, high bleeding risk, thrombosis progression, or recurrence on anticoagulation.[43] High plasma concentration of direct oral anticoagulants has been associated with higher bleeding risk.[44][45] In these situations, TEG may provide the ability to adjust the level of anticoagulation during the same office visit. As TEG activates an intrinsic coagulation pathway with kaolin, it is sensitive to heparin and low-molecular-weight heparin therapy (R time). An interesting case of an emergent heparin reversal under TEG control in a patient with intracranial bleeding has been reported.[46]
Predicting platelet inhibition by antiplatelet agents, such as aspirin, clopidogrel, abciximab, eptifibatide, or tirofiban, is another promising avenue for TEG application. Most antiplatelet agents are used with a standard dose despite several known issues associated with this approach. For example, up to 25% of patients with ST-segment elevation myocardial infarction may be resistant to clopidogrel, increasing the risk of recurrent cardiovascular events.[47] Aspirin resistance has been associated with increased myocardial infarction, stroke, or death in patients with cardiovascular disease.[48] Hence, there is significant variability in individual response to antiplatelet therapy. Available evidence does not support the use of usual laboratory testing to guide the dose of aspirin or clopidogrel.[49] Future studies may determine whether TEG can measure the effect of antiplatelet therapy, detect hyporesponsiveness, and predict the risk of bleeding or thromboembolic complications.
A novel concept of individualized health care applies to anticoagulation and antiplatelet therapy monitoring. Using a standard dose of the same medications to treat patients with different medical conditions and comorbidities may not be ideal. The potential of TEG to improve the quality of antithrombotic therapy is a promising avenue for experimental and clinical research.
Future Directions: Prevention of Venous Thromboembolism
Another potential application of TEG is to improve the diagnosis, prevention, and treatment of patients with venous thromboembolism. The most appropriate evidence-based practice to prevent venous thromboembolism is to stratify patients based on the venous thromboembolism risk using one of the risk prediction models elaborated for surgical and medical patients. These models do not incorporate conventional blood coagulation tests, as they do not predict venous thromboembolism. Several initial reports suggest that TEG may be a useful tool to help with risk stratification. TEG may have a venous thromboembolism predictive value in critically ill patients, gynecological oncology patients, and individuals with prostate cancer.[50][51][52][53] A large prospective cohort involving adult trauma patients revealed a 2-fold higher risk of venous thromboembolism in patients with hypercoagulable TEG parameters on arrival to the trauma bay.[54] However, other reports did not find TEG valuable in predicting venous thromboembolism in selected patients, such as those undergoing orthopedic surgery.[55]
Despite appropriate prophylaxis, venous thromboembolism is still a frequent concern in hospitalized patients. In a study, the R time of TEG was significantly shorter in critically ill patients on low-molecular-weight heparin prophylaxis who develop DVT compared to patients who do not.[56] Thus, TEG may help predict venous thromboembolism that occurs despite standard pharmacological prophylaxis.
Quality Control and Lab Safety
TEG/RoTEM testing requires skilled personnel to ensure accurate results, as test precision can vary significantly depending on the operator's expertise.[57] To address this, regular external quality assessments and proficiency testing are recommended to maintain consistency and reliability.[57] For optimal utilization, users should adhere to the guidelines outlined in the manufacturer's manual.
Enhancing Healthcare Team Outcomes
A team of healthcare professionals, including clinicians, specialists, advanced practice providers, pharmacists, and other healthcare providers, collaboratively participate in patient care. These members should be well-versed in TEG and its applications in diagnosing and guiding interventions. Familiarity with TEG enables these team members to interpret its diagnostic insights effectively and contribute to therapeutic decision-making. By understanding the implications of TEG results, they can provide informed recommendations, optimize care plans, and improve overall patient outcomes.
Enhancing team collaboration around TEG usage ensures that diagnostic information is seamlessly integrated into patient care strategies. For example, clinicians can use TEG to identify coagulopathies, whereas pharmacists can guide appropriate anticoagulant or hemostatic agent selection. Nurses and advanced practice providers can assist in monitoring patient responses and adjusting interventions as needed. Such a collaborative approach reinforces the critical role of interprofessional teams in delivering goal-directed, individualized care, ultimately leading to better clinical outcomes in settings where hemostatic control is crucial.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sobrino J, Shafi S. Timing and causes of death after injuries. Proceedings (Baylor University. Medical Center). 2013 Apr:26(2):120-3 [PubMed PMID: 23543966]
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003 Jun:54(6):1127-30 [PubMed PMID: 12813333]
Level 2 (mid-level) evidenceBarnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. The American journal of medicine. 2015 Dec:128(12):1300-5.e2. doi: 10.1016/j.amjmed.2015.05.044. Epub 2015 Jul 2 [PubMed PMID: 26144101]
Luepker RV, Steffen LM, Duval S, Zantek ND, Zhou X, Hirsch AT. Population Trends in Aspirin Use for Cardiovascular Disease Prevention 1980-2009: The Minnesota Heart Survey. Journal of the American Heart Association. 2015 Dec 23:4(12):. doi: 10.1161/JAHA.115.002320. Epub 2015 Dec 23 [PubMed PMID: 26702085]
Level 3 (low-level) evidenceRodgers RP, Levin J. A critical reappraisal of the bleeding time. Seminars in thrombosis and hemostasis. 1990 Jan:16(1):1-20 [PubMed PMID: 2406907]
Johnson DJ, Scott AV, Barodka VM, Park S, Wasey JO, Ness PM, Gniadek T, Frank SM. Morbidity and Mortality after High-dose Transfusion. Anesthesiology. 2016 Feb:124(2):387-95. doi: 10.1097/ALN.0000000000000945. Epub [PubMed PMID: 26569167]
HARTERT H. [Blood clotting studies with Thrombus stressography; a new Investigation procedure]. Klinische Wochenschrift. 1948 Oct 1:26(37-38):577-83 [PubMed PMID: 18101974]
Walsh M, Fritz S, Hake D, Son M, Greve S, Jbara M, Chitta S, Fritz B, Miller A, Bader MK, McCollester J, Binz S, Liew-Spilger A, Thomas S, Crepinsek A, Shariff F, Ploplis V, Castellino FJ. Targeted Thromboelastographic (TEG) Blood Component and Pharmacologic Hemostatic Therapy in Traumatic and Acquired Coagulopathy. Current drug targets. 2016:17(8):954-70 [PubMed PMID: 26960340]
Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW Jr, Starzl TE, Winter PM. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesthesia and analgesia. 1985 Sep:64(9):888-96 [PubMed PMID: 3896028]
Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesthesia and analgesia. 1999 Feb:88(2):312-9 [PubMed PMID: 9972747]
Level 1 (high-level) evidenceQuarterman C, Shaw M, Johnson I, Agarwal S. Intra- and inter-centre standardisation of thromboelastography (TEG®). Anaesthesia. 2014 Aug:69(8):883-90. doi: 10.1111/anae.12748. Epub 2014 May 20 [PubMed PMID: 24841452]
Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, Kozar RA, Holcomb JB. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. The Journal of trauma. 2011 Aug:71(2):407-14; discussion 414-7. doi: 10.1097/TA.0b013e31821e1bf0. Epub [PubMed PMID: 21825945]
Level 3 (low-level) evidencePezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, Silliman CC. Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery. 2012 Jan:151(1):48-54. doi: 10.1016/j.surg.2011.06.023. Epub 2011 Sep 6 [PubMed PMID: 21899867]
Caprini JA, Zuckerman L, Cohen E, Vagher JP, Lipp V. The identification of accelerated coagulability. Thrombosis research. 1976 Aug:9(2):167-80 [PubMed PMID: 788222]
Caprini JA, Arcelus JI, Laubach M, Size G, Hoffman KN, Coats RW 2nd, Blattner S. Postoperative hypercoagulability and deep-vein thrombosis after laparoscopic cholecystectomy. Surgical endoscopy. 1995 Mar:9(3):304-9 [PubMed PMID: 7597604]
Ng KF. Changes in thrombelastograph variables associated with aging. Anesthesia and analgesia. 2004 Aug:99(2):449-54, table of contents [PubMed PMID: 15271723]
Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, Rizoli SB. Normal range values for thromboelastography in healthy adult volunteers. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2009 Dec:42(12):1210-7 [PubMed PMID: 19882085]
Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2005 Jun:16(4):301-10 [PubMed PMID: 15870552]
Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. Journal of cardiothoracic and vascular anesthesia. 2006 Aug:20(4):548-53 [PubMed PMID: 16884987]
Nielsen VG. A comparison of the Thrombelastograph and the ROTEM. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2007 Apr:18(3):247-52 [PubMed PMID: 17413761]
Durila M. Nonactivated thromboelastometry able to detect fibrinolysis in contrast to activated methods (EXTEM, INTEM) in a bleeding patient. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2016 Oct:27(7):828-830 [PubMed PMID: 26656899]
Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. The Cochrane database of systematic reviews. 2016 Aug 22:2016(8):CD007871. doi: 10.1002/14651858.CD007871.pub3. Epub 2016 Aug 22 [PubMed PMID: 27552162]
Level 1 (high-level) evidenceAdler M, Ivic S, Bodmer NS, Ten Cate H, Bachmann LM, Wuillemin WA, Nagler M. Thromboelastometry and Thrombelastography Analysis under Normal Physiological Conditions - Systematic Review. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2017 Apr:44(2):78-83. doi: 10.1159/000464297. Epub 2017 Mar 8 [PubMed PMID: 28503123]
Level 1 (high-level) evidenceDeppe AC, Weber C, Zimmermann J, Kuhn EW, Slottosch I, Liakopoulos OJ, Choi YH, Wahlers T. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. The Journal of surgical research. 2016 Jun 15:203(2):424-33. doi: 10.1016/j.jss.2016.03.008. Epub 2016 Mar 26 [PubMed PMID: 27363652]
Level 1 (high-level) evidenceWikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2017 Apr:72(4):519-531. doi: 10.1111/anae.13765. Epub 2017 Jan 4 [PubMed PMID: 28052313]
Level 1 (high-level) evidenceNakayama Y, Nakajima Y, Tanaka KA, Sessler DI, Maeda S, Iida J, Ogawa S, Mizobe T. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. British journal of anaesthesia. 2015 Jan:114(1):91-102. doi: 10.1093/bja/aeu339. Epub 2014 Oct 10 [PubMed PMID: 25303988]
Level 1 (high-level) evidenceWhiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, Misso K, Ross J, Severens J, Kleijnen J. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health technology assessment (Winchester, England). 2015 Jul:19(58):1-228, v-vi. doi: 10.3310/hta19580. Epub [PubMed PMID: 26215747]
Level 1 (high-level) evidenceHunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. The Cochrane database of systematic reviews. 2015 Feb 16:2015(2):CD010438. doi: 10.1002/14651858.CD010438.pub2. Epub 2015 Feb 16 [PubMed PMID: 25686465]
Level 2 (mid-level) evidenceHolcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, Adams PR, McCarthy JJ, Cotton BA. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Annals of surgery. 2012 Sep:256(3):476-86. doi: 10.1097/SLA.0b013e3182658180. Epub [PubMed PMID: 22868371]
Level 2 (mid-level) evidenceDa Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Critical care (London, England). 2014 Sep 27:18(5):518. doi: 10.1186/s13054-014-0518-9. Epub 2014 Sep 27 [PubMed PMID: 25261079]
Level 1 (high-level) evidenceVeigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scandinavian journal of trauma, resuscitation and emergency medicine. 2016 Oct 3:24(1):114 [PubMed PMID: 27716278]
Level 1 (high-level) evidencePanigada M, E Iapichino G, Brioni M, Panarello G, Protti A, Grasselli G, Occhipinti G, Novembrino C, Consonni D, Arcadipane A, Gattinoni L, Pesenti A. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: a safety and feasibility pilot study. Annals of intensive care. 2018 Jan 16:8(1):7. doi: 10.1186/s13613-017-0352-8. Epub 2018 Jan 16 [PubMed PMID: 29340875]
Level 2 (mid-level) evidenceCollyer TC, Gray DJ, Sandhu R, Berridge J, Lyons G. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by Thrombelastography Platelet Mapping. British journal of anaesthesia. 2009 Apr:102(4):492-8. doi: 10.1093/bja/aep039. Epub [PubMed PMID: 19286767]
Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. American journal of clinical pathology. 2010 Jul:134(1):97-102. doi: 10.1309/AJCPPZ4J6CAFYDVM. Epub [PubMed PMID: 20551273]
Kawano-Castillo J, Ward E, Elliott A, Wetzel J, Hassler A, McDonald M, Parker SA, Archeval-Lao J, Tremont C, Cai C, Pivalizza E, Rahbar MH, Grotta JC. Thrombelastography detects possible coagulation disturbance in patients with intracerebral hemorrhage with hematoma enlargement. Stroke. 2014 Mar:45(3):683-8. doi: 10.1161/STROKEAHA.113.003826. Epub 2014 Jan 14 [PubMed PMID: 24425123]
Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010 Aug 12:116(6):878-85. doi: 10.1182/blood-2010-02-261891. Epub 2010 Apr 16 [PubMed PMID: 20400681]
Mallett SV. Clinical Utility of Viscoelastic Tests of Coagulation (TEG/ROTEM) in Patients with Liver Disease and during Liver Transplantation. Seminars in thrombosis and hemostasis. 2015 Jul:41(5):527-37. doi: 10.1055/s-0035-1550434. Epub 2015 Jun 6 [PubMed PMID: 26049072]
Roullet S, Pillot J, Freyburger G, Biais M, Quinart A, Rault A, Revel P, Sztark F. Rotation thromboelastometry detects thrombocytopenia and hypofibrinogenaemia during orthotopic liver transplantation. British journal of anaesthesia. 2010 Apr:104(4):422-8. doi: 10.1093/bja/aeq022. Epub 2010 Feb 25 [PubMed PMID: 20185519]
Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Haas T, Jacob M, Lancé MD, Pitarch JVL, Mallett S, Meier J, Molnar ZL, Rahe-Meyer N, Samama CM, Stensballe J, Van der Linden PJF, Wikkelsø AJ, Wouters P, Wyffels P, Zacharowski K. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. European journal of anaesthesiology. 2017 Jun:34(6):332-395. doi: 10.1097/EJA.0000000000000630. Epub [PubMed PMID: 28459785]
Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Archives of internal medicine. 2007 Jul 9:167(13):1414-9 [PubMed PMID: 17620536]
Dossett LA, Riesel JN, Griffin MR, Cotton BA. Prevalence and implications of preinjury warfarin use: an analysis of the National Trauma Databank. Archives of surgery (Chicago, Ill. : 1960). 2011 May:146(5):565-70. doi: 10.1001/archsurg.2010.313. Epub 2011 Jan 17 [PubMed PMID: 21242422]
Level 2 (mid-level) evidenceRose AJ, Ozonoff A, Grant RW, Henault LE, Hylek EM. Epidemiology of subtherapeutic anticoagulation in the United States. Circulation. Cardiovascular quality and outcomes. 2009 Nov:2(6):591-7. doi: 10.1161/CIRCOUTCOMES.109.862763. Epub 2009 Sep 22 [PubMed PMID: 20031897]
Level 2 (mid-level) evidenceFavaloro EJ, Lippi G. Laboratory testing in the era of direct or non-vitamin K antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Seminars in thrombosis and hemostasis. 2015 Mar:41(2):208-27. doi: 10.1055/s-0035-1546827. Epub 2015 Feb 19 [PubMed PMID: 25703514]
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2009 Sep 17:361(12):1139-51. doi: 10.1056/NEJMoa0905561. Epub 2009 Aug 30 [PubMed PMID: 19717844]
Level 1 (high-level) evidenceGiugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2013 Nov 28:369(22):2093-104. doi: 10.1056/NEJMoa1310907. Epub 2013 Nov 19 [PubMed PMID: 24251359]
Level 1 (high-level) evidenceFigueiredo S, Vigué B, Benhamou D, Duranteau J. Emergency reversal of heparin overdose in a neurosurgical patient guided by thromboelastography. British journal of anaesthesia. 2013 Aug:111(2):303-4. doi: 10.1093/bja/aet245. Epub [PubMed PMID: 23858079]
Level 3 (low-level) evidenceMatetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004 Jun 29:109(25):3171-5 [PubMed PMID: 15184279]
Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. Journal of the American College of Cardiology. 2003 Mar 19:41(6):961-5 [PubMed PMID: 12651041]
Michelson AD, Bhatt DL. How I use laboratory monitoring of antiplatelet therapy. Blood. 2017 Aug 10:130(6):713-721. doi: 10.1182/blood-2017-03-742338. Epub 2017 Jun 9 [PubMed PMID: 28600334]
Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, Pezold M, Lawrence J, Biffl WL, Cothren CC, Johnson JL. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009 Oct:146(4):764-72; discussion 772-4. doi: 10.1016/j.surg.2009.06.054. Epub [PubMed PMID: 19789037]
Level 2 (mid-level) evidenceTartamella F, Vassallo MC, Berlot G, Grassi P, Testa F. Thromboelastographic predictors of venous thromboembolic events in critically ill patients: are we missing something? Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2016 Oct:27(7):804-811 [PubMed PMID: 26895213]
Liu J, Wang N, Chen Y, Lu R, Ye X. Thrombelastography coagulation index may be a predictor of venous thromboembolism in gynecological oncology patients. The journal of obstetrics and gynaecology research. 2017 Jan:43(1):202-210. doi: 10.1111/jog.13154. Epub 2016 Oct 20 [PubMed PMID: 27762468]
Toukh M, Siemens DR, Black A, Robb S, Leveridge M, Graham CH, Othman M. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thrombosis research. 2014 Jan:133(1):88-95. doi: 10.1016/j.thromres.2013.10.007. Epub 2013 Oct 12 [PubMed PMID: 24246296]
Level 2 (mid-level) evidenceBrill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. The journal of trauma and acute care surgery. 2017 Sep:83(3):413-419. doi: 10.1097/TA.0000000000001618. Epub [PubMed PMID: 28598908]
Parameswaran A, Krishnamoorthy VP, Oommen AT, Jasper A, Korula RJ, Nair SC, Poonnoose PM. Is pre-operative assessment of coagulation profile with Thrombelastography (TEG) useful in predicting venous thromboembolism (VTE) following orthopaedic surgery? Journal of clinical orthopaedics and trauma. 2016 Oct-Dec:7(Suppl 2):225-229. doi: 10.1016/j.jcot.2016.08.003. Epub 2016 Aug 24 [PubMed PMID: 28053389]
Van PY, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. The Journal of trauma. 2009 Jun:66(6):1509-15; discussion 1515-7. doi: 10.1097/TA.0b013e3181a51e33. Epub [PubMed PMID: 19509608]
Level 3 (low-level) evidenceKitchen DP, Kitchen S, Jennings I, Woods T, Walker I. Quality assurance and quality control of thrombelastography and rotational Thromboelastometry: the UK NEQAS for blood coagulation experience. Seminars in thrombosis and hemostasis. 2010 Oct:36(7):757-63. doi: 10.1055/s-0030-1265292. Epub 2010 Oct 26 [PubMed PMID: 20978996]
Level 2 (mid-level) evidence