Introduction
Imaging plays an essential role in evaluating the lungs, both anatomically and functionally. Whether it is for establishing a diagnosis, monitoring disease severity, or screening, chest imaging serves many goals. In this article, we will discuss the normal anatomy of the lungs, common imaging modalities used to assess the lungs, basic technical aspects of imaging, and the appearance of frequently seen pathologies.
Anatomy
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy
The lungs are paired, conical organs within the thoracic cavity that perform gaseous exchange. They are covered by the visceral and parietal layers of the pleura, separated by the pleural space, which normally contains about 10-20 mL of fluid.[1] The lungs are bordered by the diaphragm inferiorly and the pleura and thoracic cage laterally, with the mediastinum centered in the middle.
The trachea bifurcates into the right and left main bronchi, which enter the respective lungs at the hilum. The right lung is usually divided into the upper, middle, and lower lobes by the major (oblique) and minor (horizontal) fissures. The left is divided into the upper and lower lobes by the major fissure. Each lobe is served by a second-order bronchus arising from the division of the mainstem bronchus. The lobes are divided into bronchopulmonary segments based on tertiary or segmental bronchi. The terminal bronchioles eventually divide into respiratory bronchioles, where alveoli start to appear.
The lungs have a dual blood supply: oxygenated blood from bronchial circulation, with two vessels to the left and one to the right, and deoxygenated blood via pulmonary circulation. Oxygenated blood is transported via four pulmonary veins into the left atrium. The right bronchial vein drains into the azygos vein, and the left into the hemiazygos or the superior intercostal vein.
The unique anatomy of the lungs and their direct contact with the outside environment makes them vulnerable to a variety of pathologies.
Plain Films
Radiographs are usually one of the first investigations performed in suspected pulmonary diseases. They have minimal radiation exposure making repeat imaging relatively safe.
There are various approaches to interpreting a chest radiograph, such as ABCDE (airway, breathing, circulation, disability, everything else) strategy, starting from the outside in strategy, or inside out strategy. After assessing whether a radiograph was taken in the posteroanterior (PA) or anteroposterior (AP) projection, it is important to be aware of the quality of the radiographs. One must check if the patient is rotated by comparing the distance of the medial ends of the clavicles from the midline and if there is adequate inspiratory effort. Ideally, one should be able to see 8-10 posterior ribs. With the use of digital imaging, penetration is not as pertinent anymore. These aspects are not as applicable in portable radiographs where the patients are usually acutely ill and difficult to position. Irrespective of the reading pattern, frequently missed regions on a radiograph need extra attention. These include the apices, retrosternal area, subdiaphragmatic region, hilum, soft tissue, and bones.
Airspace opacities are most commonly thought to reflect pneumonia, especially in the presence of air bronchograms, but can also be caused by atelectasis, hemorrhage, edema, aspiration, or even malignancies such as bronchoalveolar carcinoma. In such cases, the distribution of the pathology, comparison with prior imaging, and clinical presentation play an important role. Prominent interstitial markings on radiographs are seen in patients with interstitial lung diseases, or in patients with congestive heart failure (CHF) causing pulmonary edema. Some of the classic findings of CHF include cardiomegaly, Kerley B lines, cephalization of blood flow, prominent interstitial markings, and pleural effusions. Airfield assessment can also reveal masses, nodules, or suspicious pleural thickening, which warrants further investigation. Air trapping in patients with chronic obstructive diseases manifests as flattening of the diaphragm and retrosternal lucency. Pleural effusions can be seen as homogenous opacities on lateral radiographs with as little as 50mL fluid. On posteroanterior views, at least 200mL is required for diagnosing pleural effusions seen as blunting of the costophrenic angles with a concave meniscus. Effusions can also be loculated, and sometimes hard to distinguish from consolidations, in which case oblique views are helpful. Loculated pleural effusion along the fissures, also known as phantom tumors, can be transient and are commonly seen with congestive heart failure.
A pneumothorax is seen as a translucent area without lung markings beyond the pleural line. A large pleural effusion leads to tension pneumothorax. It is essential to check for signs of tension pneumothorax such as deviation of the trachea and mediastinum to the contralateral side. Pneumothorax can be quantified in different ways by measuring the distance from the chest wall to the pleural margin at the apex or at the level of the hilum. Pneumothoraces less than 2-3 cm are considered small and usually do not need treatment as long as the patient is stable.[2] It is sometimes difficult to differentiate between a pneumothorax and an underlying bullous disease. It is, however, essential to do so to avoid dangerous interventions such as chest tube placement through a bulla. In such cases, one should consider further imaging with computed tomography (CT) of the chest.
Soft tissue assessment includes checking for subcutaneous emphysema. An important component of evaluating a chest radiograph is the evaluation of bony structures. Degenerative changes and vertebral wedge compression can be easily seen on radiographs. While incidental rib fractures can be identified on routine chest films, up to 50% of rib fractures can be missed. Thus additional oblique projections should be obtained if rib fracture assessment is the primary goal of a study.[3] Imaging of the mediastinum is discussed separately. Another important utilization of radiographs is to evaluate the optimal position of various lines and supporting devices. Endotracheal tubes should be beyond the clavicles but at least 3-4 cm above the carina. Nasogastric tubes should cross the GE junction. Other supporting devices include chest tubes, cardiac leads, intravenous (IV) access devices, etc. Often, in the intensive care unit (ICU) setting, chest radiographs are obtained every day for monitoring; however, daily radiographs without clear indications have been shown to have minimal impact on patient care. They should ideally be obtained only if there is a change in the clinical condition of the patient or following any new intervention, like the placement of a new line or tube.[4][5]
Computed Tomography
After chest radiographs, computed tomography (CT) is the most common thoracic imaging modality performed and perhaps the most useful. A conventional CT chest usually refers to a multidetector CT (MDCT) with the acquisition of multiple, thin slices covering the entire chest. Current MDCT techniques have rendered high-resolution CT (HRCT), which was earlier used for evaluating interstitial lung disease, obsolete.
Infections of the lung are very common and can present in different ways. These range from peribronchial nodules and ground-glass opacities to dense consolidation involving the entire lobe. Aspiration pneumonia usually appears in the dependent portions of the lungs. Air bronchograms in a consolidated segment of the lung favors pneumonia. Bronchopneumonia or bronchiolitis manifests as peribronchial nodules, especially in a tree-in-bud pattern. Viral, fungal, and atypical pneumonia may present as ground-glass opacities.
In recent times, infection with the COVID-19 virus has lead to a global pandemic, and CT chest has emerged as one of the most important diagnostic tools in these patients. Studies show the presence of peripheral ground-glass opacities in a subpleural distribution. In more severely affected patients, there are dependent airspace opacities along with the peripheral ground-glass densities.[6] Some patients develop pleural effusions, but this is rare. Mediastinal lymphadenopathy is also seen in a small percentage of cases.[7]
Bronchiectasis and bronchial wall thickening can be seen in many infectious and inflammatory disorders, such as cystic fibrosis, bronchitis, and allergic conditions such as allergic bronchopulmonary aspergillosis. Bronchiectasis can be cylindrical, varicose, or cystic based on the morphology.
One of the most common reasons for patients to get a chest CT is for evaluation of lung masses (defined as more than 3 cm in diameter) or nodules (less than 3 cm in diameter). Low dose lung CT is offered to all patients between the ages of 55-80, with a smoking history of 30 pack-years or more. Lung-RADS is a nomenclature used for standardizing reporting and management of lung nodules seen on low dose screening CTs. This ranges from Lung Rads 1-where no nodule is seen, or there is a less than 1% chance of malignancy to Lung Rads 4- where there is a greater than 15% chance of malignancy in the nodule.
A nodule can have characteristics that favor it to be benign or malignant. Size is an important consideration when stratifying nodules, as is the density of the nodule i.e., solid or ground glass. Irregular non-spherical nodules with spiculated margins are more suspicious of malignancy than smooth nodules. Pleural based nodules are usually benign.[8] Patterns of calcification such as central, diffuse, popcorn type, or lamellated favor a benign nodule.[9] Peripheral lung masses with pleural and extrapleural extension can be associated with rib erosions.[10]
Interstitial lung disease (ILD) refers to a large group of heterogeneous disorders that affect the interstitium. The interstitium consists of the alveolar epithelium, pulmonary capillary endothelium, basement membrane, perivascular and perilymphatic tissues. These can be seen in a variety of pathologies like infections, connective tissue disease, autoimmune conditions, drug toxicities, environmental exposures like asbestos or various allergens, in smokers, or maybe idiopathic. Patterns and distribution help in characterizing ILD into one of many categories, including usual interstitial pneumonia (UIP), non-specific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia, etc.[11] Radiology is often the cornerstone of establishing a diagnosis in these patients, as a biopsy may not always be feasible. Since the treatment and prognosis of these entities varies greatly, it is important to be familiar with them. Honeycombing is characterized by small, thin-walled clustered peripheral cysts of 0.3 cm to 1 cm in size, typically in multiple layers. Ground glass opacity is another commonly used term to describe an area of haziness in the lung parenchyma that allows visualization of the underlying vessels. Traction bronchiectasis refers to bronchiectasis with associated fibrosis. UIP is characterized by symmetric, peripheral lower lobe predominant honeycombing with associated traction bronchiectasis with an apicobasal gradient. NSIP is similar to UIP but is characterized by the presence of ground-glass opacities. It is important to differentiate UIP from other conditions like NSIP or hypersensitivity pneumonitis, as UIP carries a much poorer prognosis and limited treatment options. Hypersensitivity pneumonitis demonstrates upper lobe predominant, often asymmetric ground-glass nodules.[11]
Sarcoidosis, a systemic granulomatous disorder, has characteristic imaging findings of symmetric mediastinal lymphadenopathy, thickened bronchovascular septae, and nodules in a perilymphatic distribution. This is predominantly an upper lobe disease.[12] End-stage sarcoidosis leads to extensive fibrosis.
CT chest is more sensitive for evaluating pleural effusions as compared to plain radiographs. The density of the pleural fluid alludes to the content, such as a hemorrhagic pleural effusion or empyema. CT is also useful for assessing loculated effusions. Malignant pleural effusions are associated with thickened pleural and nodules.[13]
CT chest is also very sensitive for evaluating pneumothorax. This is especially so in patients with underlying cystic or bullous changes. It should be considered prior to interventions like placing chest tubes in a patient with multiple bullae.
Lastly, CT is also more sensitive than radiographs for detecting rib fractures. Abnormalities in the chest wall and soft tissue should not be ignored. It is also important to evaluate lymph nodes, breast tissue, and the subcutaneous tissue for incidental findings, especially in patients with known or suspected malignancies.
CT is also an invaluable tool for planning and performing interventions like biopsies.
Magnetic Resonance
Magnetic Resonance Imaging (MRI) is rapidly becoming a vital modality to evaluate the lungs. Limitations such as a low signal from the lungs and motion artifacts have been overcome by technical advances in this field, including gated imaging and breath-hold techniques.[14][15] It is preferred in cases where ionizing radiation is contraindicated or undesirable, such as pregnant and pediatric patients, in patients requiring very frequent imaging, and in cases where contrast material is contraindicated.
Basic imaging protocols should include breath-hold T1- and T2- T2-weighted images for detecting pathologies like infiltrates and nodules or masses. Since image quality heavily depends on patient compliance with breath-hold instructions, respiration-triggered imaging may also be considered for uncooperative patients. Diffusion-weighted sequences may be added if evaluating masses or nodules. Contrast-enhanced, fat-saturated gradient echo imaging can be used for further evaluation of suspicious lesions. Steady-state free precession sequences (SSFP) do not require breath-holding and are useful in the assessment of respiratory mechanics and for detecting pulmonary embolism.[14][16]
Lung pathologies that cause an increase in tissue density (“plus-pathologies”) are better evaluated on MRI than those that lead to tissue destruction and loss of density (“minus-pathology”). The superior soft-tissue detail obtained on MRI allows the characterization of nodules and masses, adding to the information obtained on a CT and helping in classifying a lesion as benign or malignant.[17] MRI is also indispensable in staging more advanced thoracic masses where there is a concern for chest wall, diaphragm, or mediastinal invasion. In Pancoast tumors, MRI is essential to determine the involvement of the surrounding vasculature and brachial plexus.[18]
MRI is shown to be comparable to MDCT in cystic fibrosis patients over 6 years of age. In addition to morphologic changes like bronchiectasis and mucus plugging, the inflammatory activity can also be easily recognized on MRI.[18] It is a viable alternative to MDCT in patients with known interstitial lung diseases.[19] MRI allows functional lung assessment in chronic obstructive lung disease (COPD) patients along with respiratory motion, dysfunction of the chest wall, and regional ventilatory defects. Its evaluation of perfusion abnormalities in patients with COPD has also shown good concordance with nuclear imaging.[18] Overall, MRI in lung imaging is expected to grow exponentially.
Ultrasonography
Point-of-care ultrasound has an important role to play in emergent and critical care settings and is now an established modality. Easy access, portability, relatively high sensitivity, and specificity, and the ability to arrive at a diagnosis relatively quickly without ionizing radiation exposure are all making lung ultrasound (LUS) an indispensable tool in lung assessment.
Ideally, the choice of the probe is determined by the area of interest. Low frequency (3-5 MHz) convex probes are better suited to assess deeper structures and are used to evaluate effusions and consolidations. Linear probes with higher frequencies (6-13 MHz) are better for the evaluation of more superficial structures like the ribs, soft tissue, and pleura.[20] Curvilinear (convex and micro convex) probes are generally the first choice due to their intermediate frequencies and smaller footprint, which allows them to be placed in intercostal spaces.[21] Both B- and M-modes are used. Doppler has a limited role in lung evaluation.[22]
A well-aerated lung is characterized by the presence of a white band or "pleural line" up to 2 mm seen at the lung/pleura interface, lung sliding caused by the movement of the two layers of pleura relative to each other, and a reverberation artifact called A-lines which are regularly spaced white lines seen in Merlin's space parallel to the pleural line. Merlin's space refers to the space between the pleural line, ribs shadow, and the bottom of the image. On M-mode, a characteristic seashore sign is seen, which is caused by lung sliding.[20][23] Important landmarks, viscera, and vasculature should be identified before proceeding with lung evaluation.
Pneumothorax is characterized by the absence of lung sliding with intact A-lines. This sign has a sensitivity of 100% and specificity of 96.5% for diagnosing pneumothorax.[23] The lung point is a transition point seen on dynamic imaging between an area with lung sliding and an area without lung sliding and is 100% specific for a pneumothorax.[23] On M-mode, the seashore sign is replaced by the stratosphere or barcode sign due to the absence of lung sliding. B-lines, an artifact created at the visceral pleura-lung boundary, and lung pulse – the relative movement of the two layers of the pleural with the cardiac cycle – also disappear.[20] Pleural effusions can be seen as anechoic areas between the two pleural layers, often with internal echogenic structures depending on the nature of the effusion. On M-mode, the sinusoid sign is seen, which is caused by the sinusoidal movements of the visceral pleura. Ultrasound has a 93% sensitivity for detecting pleural effusions.[23][24]
Evaluation of the parenchyma by ultrasound, while limited, can be useful in critically ill patients. Consolidations are seen as hyperechoic areas with dynamic air bronchograms and "hepatization" of the parenchyma. Atelectasis can present similarly, but without dynamic air bronchograms and with decreased or absent lung sliding.[24] B-lines, a vertical artifact beginning at the pleural line and extending to the edge of the screen, are seen in the case of pulmonary edema and are also referred to as "lung rockets" or "comet tails." [24] If these are more regularly spaced, it likely indicates septal edema, as opposed to alveolar edema, in which case they are closer.[20] In non-acute settings, ultrasound can be useful in determining the stage of interstitial lung disease by measuring the distance between B-lines, which is about 3 mm in early stages and progressively increases to up to 7 mm as the septae move further apart with the destruction of parenchyma.[23]
In the case of trauma, subcutaneous emphysema, soft tissue hematomas, and rib fractures are easily picked up on ultrasound examination. Multiple studies have demonstrated the higher sensitivity of ultrasound compared to radiographs in detecting rib fractures.[25]
Additionally, ultrasound has a major role to play in therapeutic procedures, such as thoracentesis and biopsy of superficial thoracic masses. It is also shown to have a high sensitivity and specificity in detecting chest wall invasion by neoplasms.[26] It can also be used to assess diaphragmatic movement and can be used for positive end-expiration pressure (PEEP) titration in ICU patients.[24]
Nuclear Medicine
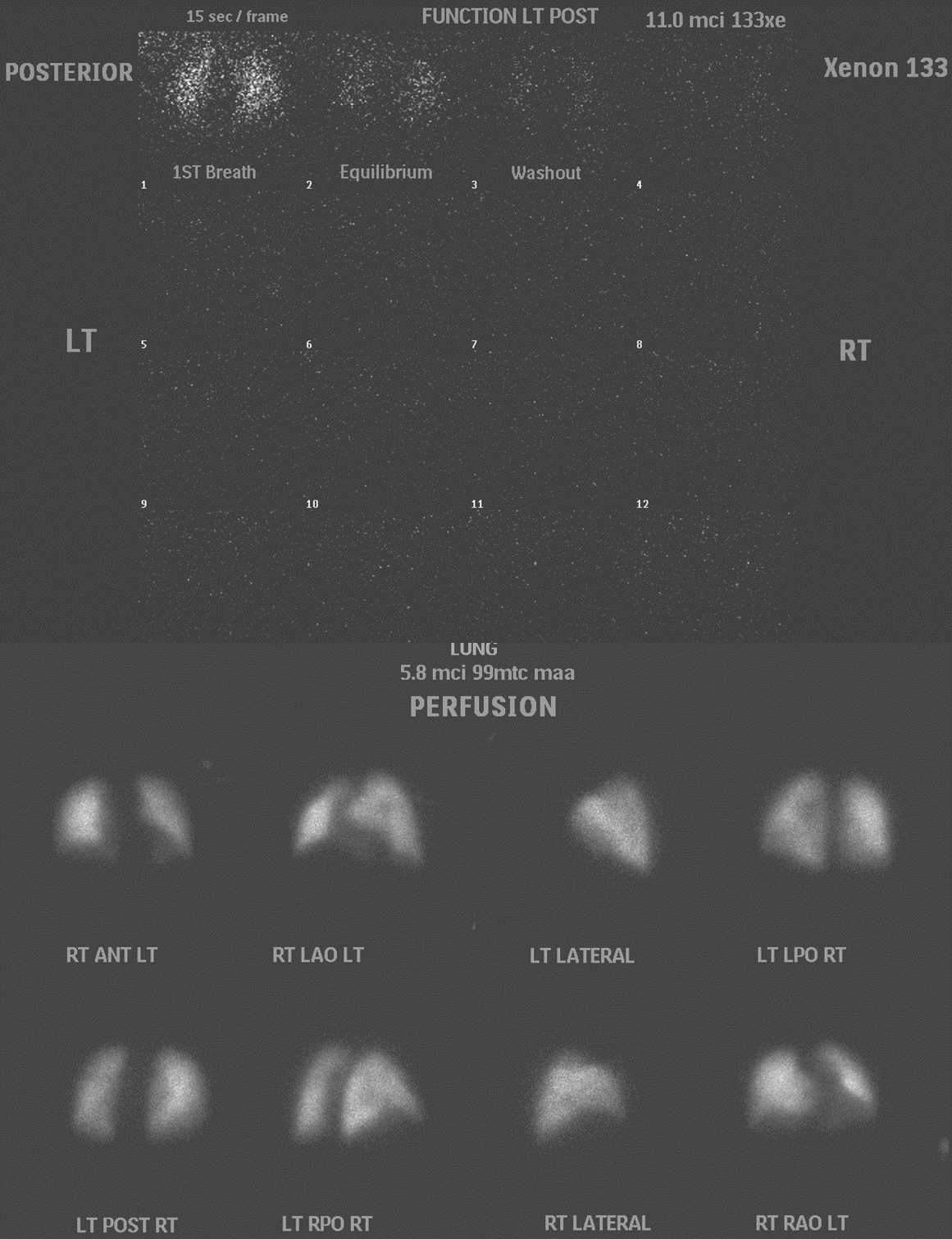
Lung scintigraphy evaluates ventilation, perfusion, or both to diagnose various pulmonary conditions. This is most commonly used to diagnose pulmonary embolism in patients who are unable to tolerate contrast. It is also occasionally used to monitor the resolution of the emboli, evaluate pulmonary hypertension, quantify lung perfusion before surgery or after a transplant, and for other pathologies like bronchopleural fistulas and congenital diseases.[27]
For ventilation studies, technetium-labeled aerosols, such as diethylenetriamine pentaacetate (DTPA) or occasionally Sulphur colloid, are used. However, this cannot provide information about washout. Xenon–133 allows the assessment of single breath, equilibrium, and washout phases and is more commonly used. Activity administered usually ranges from 5-20mCi for adults.[27]
Perfusion is assessed by injecting 1-4 mCi of Tc-99m macro-aggregated albumin (MAA).[27] The patient is supine during the injection and asked to cough and take deep breaths before injection. It is important to note that blood should not be drawn back into the syringe before administering Tc-99m MAA as it can cause the formation of microthrombi. Before scintigraphy studies, recent, preferably concurrent, morphological chest imaging such as a radiograph or CT should be reviewed. Ventilation studies are performed before perfusion studies, but one may also choose to omit ventilation scans if perfusion scans are done first and appear normal.[27]
For scintigraphy, the patients can be scanned standing or sitting, or they may be supine for the ventilation scan, depending on institutional policies. For perfusion scans, the patient is in a supine position for the injection and then preferably imaged standing up, as that minimizes diaphragmatic movement and allows complete expansion of the chest.[27] Planar images are acquired in the anterior, posterior, lateral, and oblique projections. SPECT is usually performed as it allows for the acquisition of 3-dimensional images.[28]
A perfusion defect without a ventilation defect, typically wedge-shaped and peripheral, i.e., a ”mismatch” between ventilation and perfusion, raises concern for a pulmonary embolism. Mismatches may occur in the sub-segmental, segmental, or lobar distributions. However, it is important to remember that other causes of altered blood flow, such as blockage by lymphadenopathy, masses, or radiation changes, can also lead to mismatched defects. Thus, correlation with anatomical studies is essential.[29] There are various guidelines regarding the reporting of ventilation-perfusion (V/Q) scans based on the findings and pretest clinical probability - for example, modified PIOPED II (high likelihood ratio, non-diagnostic, low LR, or normal), perfusion-only modified PIOPED II (pulmonary embolism (PE) present, non-diagnostic, PE absent) and perfusion only PISAPED (PE present, non-diagnostic, PE absent).[27] There is a push to adopt a trinary system of PE present, no PE, and non-diagnostic as it has similar outcomes as the more stratified reporting systems and allows clearer communication.[30]
Many findings confound V/Q scan interpretation. Normal structures like fissures, diaphragm, and mediastinum can give the appearance of perfusion defects. However, if there is any normal perfusion peripheral to the perfusion defect, called the stripe sign, the likelihood of a pulmonary embolism is low.[27][31] In patients with pulmonary venous hypertension, there is often preferential blood flow to the upper lobes, which can also be a confounding factor on VQ scans.[31] Anatomic findings on plain radiographs or CT also need to be kept in mind when interpreting these studies. Sometimes, uptake may be seen in systemic vascular beds, for example, the brain, kidneys, and thyroid, indicating the possibility of a right-to-left shunt. The shunting can also be quantified by comparing the systemic uptake levels with the lungs.[32]
Positron emission tomography combined with CT scans using F18-labeled fluorodeoxyglucose (FDG) is commonly used for assessing suspicious pulmonary nodules and known malignancies. Lung nodules can be characterized as potentially benign or malignant, depending on their FDG uptake. In the case of patients with known malignancies, PET-CT allows for staging by assessing areas of uptake in the remainder of the chest and the entire body. Providing data about the metabolic activity of a lesion is also useful to plan for interventions such as choosing the most optimal lesion for biopsy.
Angiography
CT angiography (CTA) is the imaging test of choice for suspected pulmonary embolism (PE). It is reported to have sensitivity and specificity ranging from 83% to 100% and 89% to 96%, respectively, and a high negative predictive value of 96% to 99%.[29][33] It is a relatively quick and easily available study. Its disadvantages lie in radiation exposure and the requirement for iodinated contrast, restricting its use in certain populations.
The patient is scanned in a supine position, usually in a 16-64 slice MDCT scanner in the craniocaudal direction. Reformatted images are also obtained in the sagittal and coronal planes to help differentiate artifacts from a true finding. PE studies are graded on their quality based on the opacification of the main pulmonary artery, with greater than or equal to 211 HU an excellent study, 100-211 HU adequate, and less than 100 HU suboptimal. Higher intraluminal density is essential, especially when looking for chronic thrombi.[29] Dual-energy CT can be used to generate iodine maps that allow the creation of pulmonary perfusion maps. This has been shown to improve the detection of peripheral clots and correlates well with adverse outcomes and right ventricular strain.[29]
Acute clots appear as central filling defects in a well-opacified vessel giving rise to the polo mint sign. Eccentric clots form an acute angle with the vessel wall. Saddle PEs are large emboli that obstruct the entire main pulmonary artery. Chronic pulmonary emboli can manifest as thin and tapering vessels often completely cut off beyond the clot, eccentric clots forming obtuse angles with the vessel wall, webs, and bands within the vessel and pulmonary hypertension. Acute PEs can also be associated with a pulmonary infarct, which appears as a peripheral wedge-shaped opacity. Areas with chronic PEs may have mosaic attenuation and bronchial dilatation.[29]
Imaging is also important for determining the severity of pulmonary emboli. Right heart strain, seen as straightening or bowing of the septum into the left ventricle and enlargement of the pulmonary artery greater than the aortic diameter, is more important in prognostication than the clot burden itself. There are also many indices, such as the Qanadli and Mastor scores, that can be used to grade the severity of PEs based on CT findings.[33][34]
There has been a lot of research and debate regarding the role of magnetic resonance angiography (MRA), both enhanced and unenhanced, and with or without MR venography (MRV) in the evaluation of pulmonary vasculature. The distinct advantage being the absence of iodinated contrast and ionizing radiation. The Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III) study found CR-MRA technically inadequate for PE evaluation with a low sensitivity of 78% that did increase to 92% with the addition of MRV.[35] There are various limitations in using MRA for PE assessment, most importantly, the time and patient compliance needed for the study, which is not feasible in more unstable patients. There is continuing research in this field, but at present, this is not for routine clinical use.[36]
Patient Positioning
Patient positioning for most studies depends on the clinical condition of the patient.
Chest radiographs for stable patients are taken in the posteroanterior position with the shoulders retracted forward to abduct the scapulae. Lateral projections are also usually obtained with the arms raised overhead. These may be taken in standing or sitting positions. In unstable patients who are unable to move, portable anteroposterior views are obtained. Lateral decubitus views with the patient lying on the side may be obtained to evaluate pleural effusions. Lordotic views are obtained with the patient slightly reclined backward with the arms raised overhead to image the lung apices. Expiratory views can be considered for better evaluation of pneumothoraces. Oblique posteroanterior projections may be obtained in the sitting, supine, or prone positions with one side of the body elevated, usually by 15-20 degrees, which allows for evaluating the bony rib cage.
CT and MRI studies are usually obtained with the patient in the supine position. HRCT is obtained with the patient in supine and prone positions and the inspiratory and expiratory phases. Low radiation dose scan parameters are used for screening for lung cancer. The necessity of contrast depends upon the area of interest or clinical suspicion, eg, evaluation of mediastinal masses, lymph nodes, vasculature, pleural disease, or new lung masses need contrast studies.
For nuclear medicine studies, the position of the patient for the ventilation scan may vary depending on institutional policies. Patients can be scanned standing or sitting, or they may be supine. For perfusion scans, the patient is in a supine position for the injection and then preferably imaged standing up as that minimizes diaphragmatic movement and allows more complete expansion of the chest.[27] Images are acquired in the anterior, posterior, lateral, and oblique projections.
For lung ultrasound examination, the position of the patient depends on the clinical scenario. In a stable patient, a sitting position, often starting at the point of concern, is preferred. In more critical, hospitalized patients, scanning is done in the supine or lateral positions.[21][26] There are various schematics proposed for areas to be scanned, ranging from 8 regions to 28 regions, but again, these also vary depending on the clinical situation. Usually, the chest is divided by the anterior and posterior axillary lines into anterior, lateral, and posterior regions, which are subdivided into upper and lower zones.[20][21] Various protocols allow for different emergency scenarios. The bedside lung ultrasound in emergency (BLUE)-protocol and the fluid administration limited by lung sonography (FALLS)-protocol has been well established in critical care settings for acute respiratory and circulatory failure, respectively. SESAME protocol is used in cardiac arrest.[22][37]
Clinical Significance
Any person who has had an encounter with healthcare has had some sort of chest imaging. Whether it is for screening, establishing a diagnosis, assessing disease severity, or monitoring treatment response, the essentiality and importance of imaging are hard to overstate. In such a situation, one must be conscientious to ensure that studies are ordered based on appropriate indications, optimal for the suspected pathology, and also explained and acceptable to the patient.
While the amount of radiation exposure has now significantly decreased owing to technological advancements and awareness regarding radiation risks, efforts should be made to space out imaging and not expose the patient to ionizing radiation, especially in patients who require repeat imaging. The need and use of intravenous contrast should also be carefully considered. Iodinated contrast should be avoided in patients with a glomerular filtration rate (GFR) of less than 30 mL/min.
There is extensive ongoing research in the realm of radiogenomics, which aims to correlate imaging features with the predicted molecular makeup of a mass, for example, non-small cell lung carcinoma.[38] With the advent of artificial intelligence and enormous amounts of data, algorithms are now available for evaluating pulmonary nodules, facilitating easier comparison, and calculating nodule volumes. Such aids will hopefully serve to streamline the workflow of busy radiologists and help to stratify a multitude of studies based on urgency and severity. With the gradual and thoughtful introduction of artificial intelligence, lung imaging is only expected to become even more efficient and enhanced, allowing for better patient care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
D'Agostino HP, Edens MA. Physiology, Pleural Fluid. StatPearls. 2025 Jan:(): [PubMed PMID: 30020725]
Choi WI. Pneumothorax. Tuberculosis and respiratory diseases. 2014 Mar:76(3):99-104. doi: 10.4046/trd.2014.76.3.99. Epub 2014 Mar 29 [PubMed PMID: 24734096]
Assi AA, Nazal Y. Rib fracture: Different radiographic projections. Polish journal of radiology. 2012 Oct:77(4):13-6 [PubMed PMID: 23269931]
Amorosa JK, Bramwit MP, Mohammed TL, Reddy GP, Brown K, Dyer DS, Ginsburg ME, Heitkamp DE, Jeudy J, Kirsch J, MacMahon H, Ravenel JG, Saleh AG, Shah RD. ACR appropriateness criteria routine chest radiographs in intensive care unit patients. Journal of the American College of Radiology : JACR. 2013 Mar:10(3):170-4. doi: 10.1016/j.jacr.2012.11.013. Epub [PubMed PMID: 23571057]
Keveson B, Clouser RD, Hamlin MP, Stevens P MSN, RN, Stinnett-Donnelly JM, Allen GB. Adding value to daily chest X-rays in the ICU through education, restricted daily orders and indication-based prompting. BMJ open quality. 2017:6(2):e000072. doi: 10.1136/bmjoq-2017-000072. Epub 2017 Nov 25 [PubMed PMID: 29435503]
Level 2 (mid-level) evidenceShi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet. Infectious diseases. 2020 Apr:20(4):425-434. doi: 10.1016/S1473-3099(20)30086-4. Epub 2020 Feb 24 [PubMed PMID: 32105637]
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR. American journal of roentgenology. 2020 Jul:215(1):87-93. doi: 10.2214/AJR.20.23034. Epub 2020 Mar 14 [PubMed PMID: 32174129]
Level 1 (high-level) evidenceXie X, Heuvelmans MA, van Ooijen PM, Oudkerk M, Vliegenthart R. A practical approach to radiological evaluation of CT lung cancer screening examinations. Cancer imaging : the official publication of the International Cancer Imaging Society. 2013 Sep 23:13(3):391-9. doi: 10.1102/1470-7330.2013.9043. Epub 2013 Sep 23 [PubMed PMID: 24061210]
Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: What does it mean? Annals of thoracic medicine. 2010 Apr:5(2):67-79. doi: 10.4103/1817-1737.62469. Epub [PubMed PMID: 20582171]
Sureka B, Thukral BB, Mittal MK, Mittal A, Sinha M. Radiological review of pleural tumors. The Indian journal of radiology & imaging. 2013 Oct:23(4):313-20. doi: 10.4103/0971-3026.125577. Epub [PubMed PMID: 24604935]
Nishino M, Itoh H, Hatabu H. A practical approach to high-resolution CT of diffuse lung disease. European journal of radiology. 2014 Jan:83(1):6-19. doi: 10.1016/j.ejrad.2012.12.028. Epub 2013 Feb 11 [PubMed PMID: 23410907]
Ganeshan D, Menias CO, Lubner MG, Pickhardt PJ, Sandrasegaran K, Bhalla S. Sarcoidosis from Head to Toe: What the Radiologist Needs to Know. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018 Jul-Aug:38(4):1180-1200. doi: 10.1148/rg.2018170157. Epub [PubMed PMID: 29995619]
Dixit R, Agarwal KC, Gokhroo A, Patil CB, Meena M, Shah NS, Arora P. Diagnosis and management options in malignant pleural effusions. Lung India : official organ of Indian Chest Society. 2017 Mar-Apr:34(2):160-166. doi: 10.4103/0970-2113.201305. Epub [PubMed PMID: 28360465]
Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, Van Beek EJ. MRI of the lung (2/3). Why … when … how? Insights into imaging. 2012 Aug:3(4):355-71. doi: 10.1007/s13244-011-0146-8. Epub 2012 Feb 13 [PubMed PMID: 22695944]
Kauczor HU, Kreitner KF. Contrast-enhanced MRI of the lung. European journal of radiology. 2000 Jun:34(3):196-207 [PubMed PMID: 10927161]
Biederer J, Mirsadraee S, Beer M, Molinari F, Hintze C, Bauman G, Both M, Van Beek EJ, Wild J, Puderbach M. MRI of the lung (3/3)-current applications and future perspectives. Insights into imaging. 2012 Aug:3(4):373-86. doi: 10.1007/s13244-011-0142-z. Epub 2012 Jan 15 [PubMed PMID: 22695943]
Level 3 (low-level) evidenceBiederer J, Hintze C, Fabel M. MRI of pulmonary nodules: technique and diagnostic value. Cancer imaging : the official publication of the International Cancer Imaging Society. 2008 May 19:8(1):125-30. doi: 10.1102/1470-7330.2008.0018. Epub 2008 May 19 [PubMed PMID: 18519226]
Wielpütz M, Kauczor HU. MRI of the lung: state of the art. Diagnostic and interventional radiology (Ankara, Turkey). 2012 Jul-Aug:18(4):344-53. doi: 10.4261/1305-3825.DIR.5365-11.0. Epub 2012 Mar 20 [PubMed PMID: 22434450]
Biederer J, Wielpütz MO, Jobst BJ, Dinkel J. [MRI of interstitial lung diseases: what is possible?]. Der Radiologe. 2014 Dec:54(12):1204-12. doi: 10.1007/s00117-014-2738-z. Epub [PubMed PMID: 25503519]
Saraogi A. Lung ultrasound: Present and future. Lung India : official organ of Indian Chest Society. 2015 May-Jun:32(3):250-7. doi: 10.4103/0970-2113.156245. Epub [PubMed PMID: 25983411]
Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovascular ultrasound. 2014 Jul 4:12():25. doi: 10.1186/1476-7120-12-25. Epub 2014 Jul 4 [PubMed PMID: 24993976]
Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheffield, England). 2017 Jun:13(2):100-111. doi: 10.1183/20734735.004717. Epub [PubMed PMID: 28620429]
Singh S, Kaur H, Singh S, Khawaja I. Basic Insights of Lung Ultrasonography in Critical Care Setting. Cureus. 2018 Dec 7:10(12):e3702. doi: 10.7759/cureus.3702. Epub 2018 Dec 7 [PubMed PMID: 30788191]
Raheja R, Brahmavar M, Joshi D, Raman D. Application of Lung Ultrasound in Critical Care Setting: A Review. Cureus. 2019 Jul 25:11(7):e5233. doi: 10.7759/cureus.5233. Epub 2019 Jul 25 [PubMed PMID: 31565634]
Pishbin E, Ahmadi K, Foogardi M, Salehi M, Seilanian Toosi F, Rahimi-Movaghar V. Comparison of ultrasonography and radiography in diagnosis of rib fractures. Chinese journal of traumatology = Zhonghua chuang shang za zhi. 2017 Aug:20(4):226-228. doi: 10.1016/j.cjtee.2016.04.010. Epub 2017 May 26 [PubMed PMID: 28687342]
Chichra A, Makaryus M, Chaudhri P, Narasimhan M. Ultrasound for the Pulmonary Consultant. Clinical medicine insights. Circulatory, respiratory and pulmonary medicine. 2016:10():1-9. doi: 10.4137/CCRPM.S33382. Epub 2016 Jun 29 [PubMed PMID: 27398039]
Parker JA, Coleman RE, Grady E, Royal HD, Siegel BA, Stabin MG, Sostman HD, Hilson AJ, Society of Nuclear Medicine. SNM practice guideline for lung scintigraphy 4.0. Journal of nuclear medicine technology. 2012 Mar:40(1):57-65. doi: 10.2967/jnmt.111.101386. Epub 2012 Jan 26 [PubMed PMID: 22282651]
Level 1 (high-level) evidenceThomas KS, Mann A, Williams J. Pulmonary (V/Q) Imaging. Journal of nuclear medicine technology. 2018 Jun:46(2):87-88 [PubMed PMID: 29871994]
Moore AJE, Wachsmann J, Chamarthy MR, Panjikaran L, Tanabe Y, Rajiah P. Imaging of acute pulmonary embolism: an update. Cardiovascular diagnosis and therapy. 2018 Jun:8(3):225-243. doi: 10.21037/cdt.2017.12.01. Epub [PubMed PMID: 30057872]
Glaser JE, Chamarthy M, Haramati LB, Esses D, Freeman LM. Successful and safe implementation of a trinary interpretation and reporting strategy for V/Q lung scintigraphy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011 Oct:52(10):1508-12. doi: 10.2967/jnumed.111.090753. Epub 2011 Jul 29 [PubMed PMID: 21803837]
Freeman LM, Krynyckyi B, Zuckier LS. Enhanced lung scan diagnosis of pulmonary embolism with the use of ancillary scintigraphic findings and clinical correlation. Seminars in nuclear medicine. 2001 Apr:31(2):143-57 [PubMed PMID: 11330785]
Chokkappan K, Kannivelu A, Srinivasan S, Babut SB. Review of diagnostic uses of shunt fraction quantification with technetium-99m macroaggregated albumin perfusion scan as illustrated by a case of Osler-Weber-Rendu syndrome. Annals of thoracic medicine. 2016 Apr-Jun:11(2):155-60. doi: 10.4103/1817-1737.180020. Epub [PubMed PMID: 27168866]
Level 3 (low-level) evidenceDoğan H, de Roos A, Geleijins J, Huisman MV, Kroft LJ. The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagnostic and interventional radiology (Ankara, Turkey). 2015 Jul-Aug:21(4):307-16. doi: 10.5152/dir.2015.14403. Epub [PubMed PMID: 26133321]
Chen S, Cheng R, Zhang G. [Comparison of value of Qanadli versus Mastora pulmonary embolism index in evaluating straddle-type pulmonary embolism]. Zhonghua yi xue za zhi. 2014 Dec 16:94(46):3629-32 [PubMed PMID: 25622952]
Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV Jr, Naidich DP, Sak DJ, Sostman HD, Tapson VF, Weg JG, Woodard PK, PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Annals of internal medicine. 2010 Apr 6:152(7):434-43, W142-3. doi: 10.7326/0003-4819-152-7-201004060-00008. Epub [PubMed PMID: 20368649]
Tsuchiya N, van Beek EJ, Ohno Y, Hatabu H, Kauczor HU, Swift A, Vogel-Claussen J, Biederer J, Wild J, Wielpütz MO, Schiebler ML. Magnetic resonance angiography for the primary diagnosis of pulmonary embolism: A review from the international workshop for pulmonary functional imaging. World journal of radiology. 2018 Jun 28:10(6):52-64. doi: 10.4329/wjr.v10.i6.52. Epub [PubMed PMID: 29988845]
Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015 Jun:147(6):1659-1670. doi: 10.1378/chest.14-1313. Epub [PubMed PMID: 26033127]
Zhou M, Leung A, Echegaray S, Gentles A, Shrager JB, Jensen KC, Berry GJ, Plevritis SK, Rubin DL, Napel S, Gevaert O. Non-Small Cell Lung Cancer Radiogenomics Map Identifies Relationships between Molecular and Imaging Phenotypes with Prognostic Implications. Radiology. 2018 Jan:286(1):307-315. doi: 10.1148/radiol.2017161845. Epub 2017 Jul 20 [PubMed PMID: 28727543]