Introduction
The Vagus nerve is the longest mixed cranial nerve associated structurally with the post olivary sulcus of the medulla oblongata. The literal translation of the vagus is 'wanderer,' which aptly represents its widespread interfacing of the cortex, brainstem, hypothalamus, and body. Its afferent and efferent pathways comprise about 80% and 20%, respectively. With a premise that venous hyperemia caused seizures, Dr. James Corning, a 19th-century neurologist from New York, devised instrumented carotid compression along with vagus nerve stimulation as a treatment intervention for seizures. His observations were not put to the test until the latter part of the 20th century. In the 1980s, various observational studies emerged in the cybernetic use of a vagus nerve stimulator (VNS) in refractory epilepsy.[1][2]
Currently, VNS is a Food and Drug Administration (FDA) approved treatment for various conditions like chronic epilepsy, refractory epilepsy, and depression. It is also being investigated in various other conditions like autoimmune and chronic inflammatory disorders.[3]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
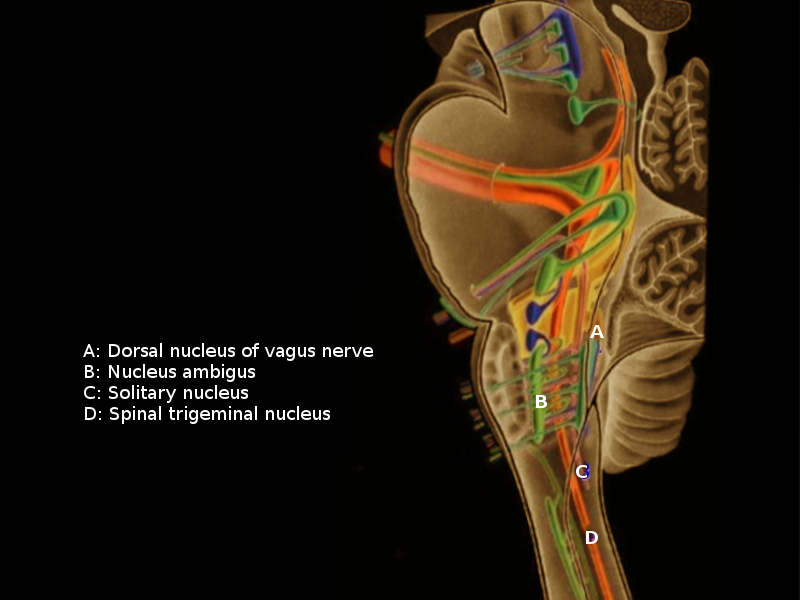
The Vagus nerve connects many visceral organs with the brainstem and the cortex given its widespread course and distribution compared to the rest of the cranial nerves through the autonomic nervous system interface. It originates in the medulla oblongata as eight to ten rootlets from four nuclei, namely:[4][5]
- Dorsal Motor Nucleus: This nucleus gives rise to the preganglionic parasympathetic visceromotor fibers.
- Nucleus Tractus Solitarius (NTS): This nucleus receives the viscerosensory input from the gastrointestinal and respiratory system as well as the afferent taste input via the chorda tympani nerve of the 7th cranial nerve. These sensory afferents constitute over 80% of the vagus nerve. The projections from NTS are extensive, involving different regions of the brain, brainstem (including locus coeruleus and raphe nucleus), and the hypothalamus.[6]
- Nucleus Ambiguous: This nucleus is associated with efferent outputs associated with the 9, 10, and 11 cranial nerves. It also contains the preganglionic parasympathetic neurons that innervate the postganglionic parasympathetic neurons to the heart.
- Spinal Nucleus of Trigeminal Nerve: This nucleus receives general somatic sensory input from the back of the ear and the external auditory meatus.
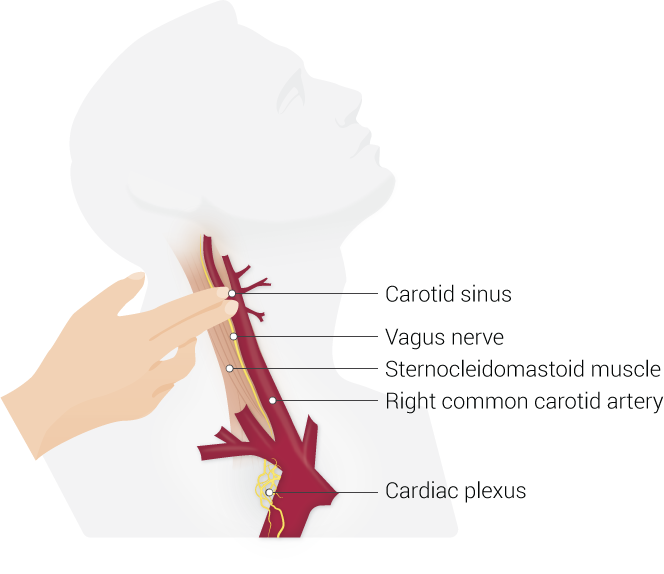
The vagus nerve, after originating from the medulla oblongata, exits the cranium through the jugular foramen and travels down the neck within the carotid sheath along with the common carotid artery and the internal jugular vein.
Indications
The premise of the vagal nerve stimulation is to activate various neurochemical coordinates arising from the NTS to different parts of the brain. The FDA approved indications are:
Epilepsy
An implantable vagus nerve stimulator was used in 1988 in a patient with pharmaco-resistant epilepsy for the first time.[7] Initially, in 1997, the US FDA approved VNS to treat pharmaco-refractory partial-onset seizures in patients above 12 years of age. Later in 2017, extended for use in children above four years of age. Fewer side effects meant broader clinical applications beyond the approved guidelines to additional conditions like Lennox Gestaut syndrome, Rett syndrome, and epilepsy in pregnant women due to the lack of detrimental and teratogenic side effects.[8][9][10] Studies have also shown that the risk of sudden unexpected death in epilepsy (SUDEP) is reduced with the long-term use of VNS.[11]Mechanisms of action: A precise mechanism of action is still inconclusive, but the following are generally agreed upon based on human and animal studies. In animal epilepsy models, VNS has caused abruption of an ongoing seizure and a decrease in the frequency of chronic seizures.[12][13] Zabara postulated that high synchronization of the cortical and thalamocortical loops are the basis of the complex partial seizures in animal models, VNS intervention breaks these synchronized networks and thus mitigates seizure activity.[14] NTS projections to the raphe nuclei and the locus coeruleus play an important role in the VNS neuromodulation therapy, purportedly by increasing the serotonergic and noradrenergic transmission, evidenced by the increased levels of serotonin, noradrenaline (NA) and its metabolites in the cerebrospinal fluid (CSF) of the patients undergoing VNS stimulation. Serotonin and NA have known anti-seizure effects.[15][16][17]
Depression
The use of VNS for treatment-resistant depression was approved in 2005.[18] This approval was preceded by several controlled and uncontrolled studies that observed improved standardized mood scores following treatment with VNS in patients with treatment-resistant depression.[19][20] It was also observed that the patients with clinical refractory depression who claimed improvement due to VNS use relapsed into clinical depression after the removal of a VNS device or repairs arising due to battery issues of the device.[21][22] A meta-analysis revealed a substantial difference in response rates between those treated as usual versus those treated as usual with adjunctive VNS therapy.[23]Mechanisms of action: Again, the exact role of VNS in the treatment of clinically refractory depression is inconclusive; however various explanations as to why the VNS works include; better NA synaptic transmission through the locus coeruleus and other pathways and VNS mediated changes to the anti-convulsant system in the brain, which improves depression.[18][24]
Other Investigational Clinical Applications
The Vagus nerve plays an important role in conveying information about peripheral proinflammatory cytokines in the body to the brain (NTS) as the vagal afferents are sensitive to the presence of interleukins and prostaglandins.[25][26] In turn, NTS relays this information to various levels like the hypothalamus, limbic lobe, and the pituitary leading to activation of the hypothalamo-pituitary adrenal (HPA) axis leading to the release of cortisol from the adrenal cortex. The anti-inflammatory role of the vagal efferents is mediated through the vagovagal reflex, where vagal afferents activate the vagal efferents. The vagal cholinergic output from the dorsal motor nucleus inhibits the release of cytokines like TNFα from the macrophages, and this is commonly termed as the cholinergic anti-inflammatory pathway.[27][28] These anti-inflammatory abilities of the vagus nerve make it a target for modulation by VNS to affect the inflammatory conditions of the gut like the inflammatory bowel disease (IBD), and also other non-gut inflammations like rheumatoid arthritis (RA), diabetes mellitus (DM), sepsis, cardiovascular diseases, Alzheimer disease, intractable hiccups, and chronic pain.[27][29][27]
Transcutaneous Auricular Vagus Nerve Stimulation (taVNS)
This is a non-invasive method of delivering the transcutaneous stimulation directly to the auricular branch of the vagus nerve and has been widely discussed in recent days.
There are two subsets for taVNS that are closed-loop:
1. Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS)[30]: This works on the principle that inhalation induces transient inhibition of vagus nerve activity. This has shown some promise in treating pain disorders and migraines.
2. Motor Activated Auricular Vagus Nerve Stimulation (MAAVNS)[31]: This pairs taVNS with motor activity and has been found to be a promising neurorehabilitation tool and in facilitating motor learning in neonates. This technique is also being studied in adult post-stroke rehabilitation trials.
Contraindications
Vagotomy: Since the right vagus nerve supplies the sinoatrial node, the VNS is usually implanted in the left vagus nerve to prevent any cardiac dysrhythmias.[32] So, VNS cannot be used in patients who had a bilateral or left cervical vagotomy.
Diathermy: Since diathermy treatment (therapeutic ultrasound, microwave or shortwave) could cause heating of the VNS system, well above temperatures that could cause tissue damage to the nerves and blood vessels, it is contraindicated in people who have VNS implants.[33] There is no contraindication to diagnostic ultrasound.
Equipment
Commonly used vagus nerve stimulator devices consist of an implantable, non-rechargeable battery-powered VNS therapy pulse generator and the VNS therapy lead. There is also an external programming system that is used to change the stimulation setting according to the requirement. They require new batteries in about six years. The VNS therapy lead is placed surgically around the left vagus nerve in the carotid sheath and connected to a subcutaneous programmable pacemaker device that is placed over the left chest wall. The branches of the right vagus nerve predominantly innervate the sinoatrial (SA) node and those from the left vagus nerve predominantly innervate the atrioventricular (AV) node. Insertion on the right side can cause bradycardia and other arrhythmias due to this, but this fact has recently been challenged.[34]
Electrical signals generated from the pulse generator are transmitted to the vagus nerve via the VNS therapy lead. The patients can deactivate (turn OFF) or give an additional burst of stimulation on demand by placing or swiping the magnet provided to them over the pulse generator, respectively. The previously programmed stimulation resumes after the magnet is removed. The device has three modes, manual, automatic, and chronic.[35][36][37]
Personnel
Vagus nerve stimulator device ideally should be implanted by a neurosurgeon, general surgeon, vascular surgeon, or an ear-nose-throat surgeon trained in this procedure.[38] It involves an interprofessional team to determine the eligibility of the patient for the procedure (screening) and a close long-term follow-up and education after the procedure for good outcomes. The settings of the device would require periodic adjustments as necessary to result in an optimal response for the indicated clinical indication.
Preparation
The procedure is done under general anesthesia in a supine position with the neck extended by placing a shoulder pillow.
Technique or Treatment
The VNS is placed by placing two incisions - one 2-3 cm below the left clavicle and the other skin crease incision on the left side at the level of the thyroid cartilage.
The battery is placed deep into the subcutaneous fat in the chest wall through the former incision.
Via the latter incision, the subplatysmal plane is dissected and sternomastoid muscle is retracted posteriorly and the carotid sheath is identified. Within this sheath, the vagus nerve is usually found deep and medial to the internal jugular vein and lateral to the common carotid artery. The nerve is dissected off the surrounding tissues and the leads of the stimulator are coiled around the nerve. The leads are connected to the stimulator via a silicone sheath passed through a subcutaneous tunnel connecting them both. Both the wounds are closed in layers.
The working of the VNS is confirmed by comparing the heart rate measured from the vagus nerve with the heart rate from the anesthesia monitor.
Complications
Randomized controlled trials observed the following early complications (in descending order):
- Voice alteration
- Hoarseness
- Cough
- Tingling
- Dyspnea
But generally, the patients show improved tolerance over a period of time. Intraoperative complications are rare and may include the following:
- Vocal cord paralysis
- Implant site infection
- Left facial nerve paralysis
- Horner syndrome
Chronic use of vagus nerve stimulation has not been evidenced to cause any significant or deleterious changes to cardio-respiratory functions.[39][40][41]
The VNS manufacturer's guidelines have to be strictly adhered to while performing magnetic resonance imaging scans of the cranium in patients having implanted VNS.[42]
The VNS battery usually has a lifetime of 3-5 years, after which it needs to be replaced.
Clinical Significance
The following uses for vagus nerve stimulation have been identified in the literature:
- Refractory epilepsy[43]
- Treatment-resistant depression[44]
- Facilitating neuro-plasticity[45]
- Cluster headaches and migraines[46]
- Adult stroke rehabilitation[47]
- Tinnitus[48]
- Alzheimer disease[49]
- Parkinson disease[50]
- Autistic Spectrum Disorders[51]
- Male infertility[52]
- As prophylaxis for the systemic inflammatory response syndrome and postoperative ileus[53]
- Inflammatory bowel diseases[54]
- Psoriatic arthritis and ankylosing spondylitis[55]
Enhancing Healthcare Team Outcomes
Vagus nerve stimulation is an effective antiseizure treatment, albeit without significant treatment-related side effects. Based on the evidence from randomized controlled trials, VNS therapy is an adjunctive treatment aimed towards a maximal reduction in seizure frequency with reduced use of antiepileptic medications.[56] The general trend in patients, both children, and adults, with chronic epilepsy treated with VNS therapy, has been increased quality of life. At the same time, this effect is most significant in those with the highest seizure frequency reduction. Besides the antiseizure effect, VNS therapy leads to improved mood symptoms.
According to the American Psychiatric Association (APA) adjuvant long-term VNS therapy is specified by FDA in treatment-refractory depression, either unipolar or bipolar, with a history of failure to respond to at least four antidepressant medications. Better efficacy of electroconvulsive therapy (ECT) for treatment-resistant depression should be discussed with the patient and should be considered before the use of the VNS.
The extensive interface of the vagus nerve, between mind, body, gut, and brain, opens a plethora of therapeutic possibilities from seizures and depression to immune modulation. As Dacher Keltner puts it: "The vagus nerve is one of the great mind-body nexuses in the human nervous system."
Overall, an interprofessional team effort involving multiple specialists (neurologists, epileptologists, neurosurgeons, psychiatrists) and medical staff personnel are essential to provide and enhance patients care to achieve good outcomes. Patient education is crucial since these devices require active involvement when there is a need to intervene and provide on-demand stimulation.
Nursing, Allied Health, and Interprofessional Team Interventions
The following parameters are set by the VNS nurse, in conjunction with the neurologist, after the insertion of the VNS by the surgeon[57]:
- Pulse width (microseconds) - length of time of a square pulse of current
- Current intensity (milliampere) - amplitude, or strength, of the electrical pulse. VNS is most often delivered as current-controlled, rather than voltage-controlled.
- Frequency (Hertz) - total number of cycles (the beginning of a pulse to the beginning of the next pulse) in a second.
- On-Off Time - "On-time" is the time period in which the stimulation is delivered above an intensity of 0 mA (active stimulation) and the “off-time” is the time period when no stimulation is delivered (0 mA) (rest period).
- Duration of stimulation - cumulative time of VNS treatment.
Media
(Click Image to Enlarge)
References
Lanska DJ. J.L. Corning and vagal nerve stimulation for seizures in the 1880s. Neurology. 2002 Feb 12:58(3):452-9 [PubMed PMID: 11839848]
Ohemeng KK, Parham K. Vagal Nerve Stimulation: Indications, Implantation, and Outcomes. Otolaryngologic clinics of North America. 2020 Feb:53(1):127-143. doi: 10.1016/j.otc.2019.09.008. Epub 2019 Nov 1 [PubMed PMID: 31685239]
Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. Journal of inflammation research. 2018:11():203-213. doi: 10.2147/JIR.S163248. Epub 2018 May 16 [PubMed PMID: 29844694]
Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: Evolving trends. Journal of natural science, biology, and medicine. 2013 Jan:4(1):8-13. doi: 10.4103/0976-9668.107254. Epub [PubMed PMID: 23633829]
Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Annals of the New York Academy of Sciences. 2001 Jun:940():237-46 [PubMed PMID: 11458681]
Level 3 (low-level) evidenceAbuAlrob MA, Tadi P. Neuroanatomy, Nucleus Solitarius. StatPearls. 2023 Jan:(): [PubMed PMID: 31751021]
Ben-Menachem E. Modern management of epilepsy: Vagus nerve stimulation. Bailliere's clinical neurology. 1996 Dec:5(4):841-8 [PubMed PMID: 9068884]
Level 3 (low-level) evidenceWilfong AA, Schultz RJ. Vagus nerve stimulation for treatment of epilepsy in Rett syndrome. Developmental medicine and child neurology. 2006 Aug:48(8):683-6 [PubMed PMID: 16836782]
Frost M, Gates J, Helmers SL, Wheless JW, Levisohn P, Tardo C, Conry JA. Vagus nerve stimulation in children with refractory seizures associated with Lennox-Gastaut syndrome. Epilepsia. 2001 Sep:42(9):1148-52 [PubMed PMID: 11580762]
Level 2 (mid-level) evidenceHouser MV, Hennessy MD, Howard BC. Vagal nerve stimulator use during pregnancy for treatment of refractory seizure disorder. Obstetrics and gynecology. 2010 Feb:115(2 Pt 2):417-419. doi: 10.1097/AOG.0b013e3181bd1a8b. Epub [PubMed PMID: 20093864]
Level 3 (low-level) evidenceAnnegers JF, Coan SP, Hauser WA, Leestma J. Epilepsy, vagal nerve stimulation by the NCP system, all-cause mortality, and sudden, unexpected, unexplained death. Epilepsia. 2000 May:41(5):549-53 [PubMed PMID: 10802760]
Level 2 (mid-level) evidenceWoodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1990:31 Suppl 2():S7-19 [PubMed PMID: 2226368]
Level 3 (low-level) evidenceLockard JS, Congdon WC, DuCharme LL. Feasibility and safety of vagal stimulation in monkey model. Epilepsia. 1990:31 Suppl 2():S20-6 [PubMed PMID: 2226362]
Level 3 (low-level) evidenceZabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992 Nov-Dec:33(6):1005-12 [PubMed PMID: 1464256]
Level 3 (low-level) evidencePérez-Carbonell L, Faulkner H, Higgins S, Koutroumanidis M, Leschziner G. Vagus nerve stimulation for drug-resistant epilepsy. Practical neurology. 2020 May:20(3):189-198. doi: 10.1136/practneurol-2019-002210. Epub 2019 Dec 31 [PubMed PMID: 31892545]
Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998 Jul:39(7):709-14 [PubMed PMID: 9670898]
Level 3 (low-level) evidenceDi Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Meglio M, Colicchio G, Barba C, Papacci F, Tonali PA. Effects of vagus nerve stimulation on cortical excitability in epileptic patients. Neurology. 2004 Jun 22:62(12):2310-2 [PubMed PMID: 15210904]
O'Reardon JP, Cristancho P, Peshek AD. Vagus Nerve Stimulation (VNS) and Treatment of Depression: To the Brainstem and Beyond. Psychiatry (Edgmont (Pa. : Township)). 2006 May:3(5):54-63 [PubMed PMID: 21103178]
Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR. A Pilot Study of Mood in Epilepsy Patients Treated with Vagus Nerve Stimulation. Epilepsy & behavior : E&B. 2000 Apr:1(2):93-99 [PubMed PMID: 12609137]
Level 3 (low-level) evidenceHandforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998 Jul:51(1):48-55 [PubMed PMID: 9674777]
Level 1 (high-level) evidenceMartinez JM, Zboyan HA. Vagus nerve stimulation therapy in a patient with treatment-resistant depression: a case report of long-term follow-up and battery end-of-service. CNS spectrums. 2006 Feb:11(2):143-7 [PubMed PMID: 16520692]
Level 3 (low-level) evidenceAshton AK. Depressive relapse after vagal nerve stimulator explantation. The American journal of psychiatry. 2010 Jun:167(6):719-20. doi: 10.1176/appi.ajp.2010.10010020. Epub [PubMed PMID: 20516167]
Level 3 (low-level) evidenceBerry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Medical devices (Auckland, N.Z.). 2013:6():17-35. doi: 10.2147/MDER.S41017. Epub 2013 Mar 1 [PubMed PMID: 23482508]
Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006 Jul:31(7):1345-55 [PubMed PMID: 16641939]
Level 3 (low-level) evidenceTracey KJ. The inflammatory reflex. Nature. 2002 Dec 19-26:420(6917):853-9 [PubMed PMID: 12490958]
Level 3 (low-level) evidencePavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain, behavior, and immunity. 2005 Nov:19(6):493-9 [PubMed PMID: 15922555]
Level 3 (low-level) evidenceBonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. The Journal of physiology. 2016 Oct 15:594(20):5781-5790. doi: 10.1113/JP271539. Epub 2016 May 1 [PubMed PMID: 27059884]
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nature reviews. Endocrinology. 2012 Dec:8(12):743-54. doi: 10.1038/nrendo.2012.189. Epub [PubMed PMID: 23169440]
Level 3 (low-level) evidenceTariq K, Das JM, Monaghan S, Miserocchi A, McEvoy A. A case report of Vagus nerve stimulation for intractable hiccups. International journal of surgery case reports. 2021 Jan:78():219-222. doi: 10.1016/j.ijscr.2020.12.023. Epub 2020 Dec 16 [PubMed PMID: 33360634]
Level 3 (low-level) evidenceGarcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, Napadow V. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain. 2017 Aug:158(8):1461-1472. doi: 10.1097/j.pain.0000000000000930. Epub [PubMed PMID: 28541256]
Cook DN, Thompson S, Stomberg-Firestein S, Bikson M, George MS, Jenkins DD, Badran BW. Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation. Brain stimulation. 2020 May-Jun:13(3):800-803. doi: 10.1016/j.brs.2020.02.028. Epub 2020 Feb 27 [PubMed PMID: 32289710]
Level 1 (high-level) evidenceSaper CB, Kibbe MR, Hurley KM, Spencer S, Holmes HR, Leahy KM, Needleman P. Brain natriuretic peptide-like immunoreactive innervation of the cardiovascular and cerebrovascular systems in the rat. Circulation research. 1990 Dec:67(6):1345-54 [PubMed PMID: 1978807]
Level 3 (low-level) evidenceHowland RH. Vagus Nerve Stimulation. Current behavioral neuroscience reports. 2014 Jun:1(2):64-73 [PubMed PMID: 24834378]
McGregor A, Wheless J, Baumgartner J, Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005 Jan:46(1):91-6 [PubMed PMID: 15660773]
Level 3 (low-level) evidenceCouch JD, Gilman AM, Doyle WK. Long-term Expectations of Vagus Nerve Stimulation: A Look at Battery Replacement and Revision Surgery. Neurosurgery. 2016 Jan:78(1):42-6. doi: 10.1227/NEU.0000000000000985. Epub [PubMed PMID: 26678088]
Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. 2017 Apr:58 Suppl 1():85-90. doi: 10.1111/epi.13678. Epub [PubMed PMID: 28386925]
Tronnier VM. Vagus Nerve Stimulation: Surgical Technique and Complications. Progress in neurological surgery. 2015:29():29-38. doi: 10.1159/000434653. Epub 2015 Sep 4 [PubMed PMID: 26393499]
Denski KM, Labiner DM. Should I offer vagus nerve stimulation as part of my neurology practice? Neurology. Clinical practice. 2014 Aug:4(4):313-318. doi: 10.1212/CPJ.0000000000000048. Epub [PubMed PMID: 29473565]
Veldsema-Currie RD, Labruyère WT, Langemeijer MW. Depletion of total acetylcholine by hemicholinium-3 in isolated rat diaphragm is less in the presence of dexamethasone. Brain research. 1984 Dec 24:324(2):305-12 [PubMed PMID: 6529621]
Level 3 (low-level) evidenceKim W, Clancy RR, Liu GT. Horner syndrome associated with implantation of a vagus nerve stimulator. American journal of ophthalmology. 2001 Mar:131(3):383-4 [PubMed PMID: 11239877]
Level 3 (low-level) evidence. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995 Feb:45(2):224-30 [PubMed PMID: 7854516]
Level 1 (high-level) evidenceFetzer S, Dibué M, Nagel AM, Trollmann R. A systematic review of magnetic resonance imaging in patients with an implanted vagus nerve stimulation system. Neuroradiology. 2021 Sep:63(9):1407-1417. doi: 10.1007/s00234-021-02705-y. Epub 2021 Apr 12 [PubMed PMID: 33846830]
Level 1 (high-level) evidenceJain P, Arya R. Vagus Nerve Stimulation and Seizure Outcomes in Pediatric Refractory Epilepsy: Systematic Review and Meta-Analysis. Neurology. 2021 Apr 13:():. pii: 10.1212/WNL.0000000000012030. doi: 10.1212/WNL.0000000000012030. Epub 2021 Apr 13 [PubMed PMID: 33849993]
Level 1 (high-level) evidenceMu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, Denslow S, Lomarev M, Moghadam P, Chae JH, George MS. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biological psychiatry. 2004 Apr 15:55(8):816-25 [PubMed PMID: 15050863]
Level 1 (high-level) evidenceBorland MS, Engineer CT, Vrana WA, Moreno NA, Engineer ND, Vanneste S, Sharma P, Pantalia MC, Lane MC, Rennaker RL, Kilgard MP. The Interval Between VNS-Tone Pairings Determines the Extent of Cortical Map Plasticity. Neuroscience. 2018 Jan 15:369():76-86. doi: 10.1016/j.neuroscience.2017.11.004. Epub 2017 Nov 10 [PubMed PMID: 29129793]
Nesbitt AD, Marin JC, Tompkins E, Ruttledge MH, Goadsby PJ. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015 Mar 24:84(12):1249-53. doi: 10.1212/WNL.0000000000001394. Epub 2015 Feb 20 [PubMed PMID: 25713002]
Level 2 (mid-level) evidenceShi C, Flanagan SR, Samadani U. Vagus nerve stimulation to augment recovery from severe traumatic brain injury impeding consciousness: a prospective pilot clinical trial. Neurological research. 2013 Apr:35(3):263-76. doi: 10.1179/1743132813Y.0000000167. Epub [PubMed PMID: 23485054]
Level 3 (low-level) evidenceYakunina N, Nam EC. Direct and Transcutaneous Vagus Nerve Stimulation for Treatment of Tinnitus: A Scoping Review. Frontiers in neuroscience. 2021:15():680590. doi: 10.3389/fnins.2021.680590. Epub 2021 May 28 [PubMed PMID: 34122002]
Level 2 (mid-level) evidenceSlater C, Wang Q. Alzheimer's disease: An evolving understanding of noradrenergic involvement and the promising future of electroceutical therapies. Clinical and translational medicine. 2021 Apr:11(4):e397. doi: 10.1002/ctm2.397. Epub [PubMed PMID: 33931975]
Level 3 (low-level) evidenceMondal B, Choudhury S, Banerjee R, Roy A, Chatterjee K, Basu P, Singh R, Halder S, Shubham S, Baker SN, Baker MR, Kumar H. Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson's disease in patients with freezing of gait. NPJ Parkinson's disease. 2021 May 27:7(1):46. doi: 10.1038/s41531-021-00190-x. Epub 2021 May 27 [PubMed PMID: 34045464]
van Hoorn A, Carpenter T, Oak K, Laugharne R, Ring H, Shankar R. Neuromodulation of autism spectrum disorders using vagal nerve stimulation. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2019 May:63():8-12. doi: 10.1016/j.jocn.2019.01.042. Epub 2019 Feb 4 [PubMed PMID: 30732986]
Derakhshan N, Yaghmaei S, Keshavarz P. Vagal nerve stimulation for the treatment of male factor infertility. Andrologia. 2021 Jul:53(6):e14043. doi: 10.1111/and.14043. Epub 2021 Apr 30 [PubMed PMID: 33929756]
van Beekum CJ, Willis MA, von Websky MW, Sommer NP, Kalff JC, Wehner S, Vilz TO. Electrical vagus nerve stimulation as a prophylaxis for SIRS and postoperative ileus. Autonomic neuroscience : basic & clinical. 2021 Nov:235():102857. doi: 10.1016/j.autneu.2021.102857. Epub 2021 Jul 29 [PubMed PMID: 34343825]
Bonaz B, Sinniger V, Pellissier S. Therapeutic Potential of Vagus Nerve Stimulation for Inflammatory Bowel Diseases. Frontiers in neuroscience. 2021:15():650971. doi: 10.3389/fnins.2021.650971. Epub 2021 Mar 22 [PubMed PMID: 33828455]
Brock C, Rasmussen SE, Drewes AM, Møller HJ, Brock B, Deleuran B, Farmer AD, Pfeiffer-Jensen M. Vagal Nerve Stimulation-Modulation of the Anti-Inflammatory Response and Clinical Outcome in Psoriatic Arthritis or Ankylosing Spondylitis. Mediators of inflammation. 2021:2021():9933532. doi: 10.1155/2021/9933532. Epub 2021 May 27 [PubMed PMID: 34135691]
Level 2 (mid-level) evidenceDasheiff RM, Sandberg T, Thompson J, Arrambide S, E03 and E05 Cooperative Study Group. Vagal nerve stimulation does not unkindle seizures. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2001 Jan:18(1):68-74 [PubMed PMID: 11290941]
Level 1 (high-level) evidenceThompson SL, O'Leary GH, Austelle CW, Gruber E, Kahn AT, Manett AJ, Short B, Badran BW. A Review of Parameter Settings for Invasive and Non-invasive Vagus Nerve Stimulation (VNS) Applied in Neurological and Psychiatric Disorders. Frontiers in neuroscience. 2021:15():709436. doi: 10.3389/fnins.2021.709436. Epub 2021 Jul 13 [PubMed PMID: 34326720]