Introduction
The limbic system is an aggregation of brain structures that are generally located lateral to the thalamus, underneath the cerebral cortex, and above the brainstem. In 1878, Paul Broca was the first to name this general region as the brain le grand lobe limbique. Later on, in 1949, the American physician and neuroscientist, Paul D. MacLean, called it the limbic lobe,[1] although now there is more current terminology used for the individual structures included in this region. Eventually, this region became understood to have links to emotional, memory, and motivational processes that connect to other parts of the brain.[2] Clinically, some specific disorders occur when parts of the limbic system suffer a lesion. While a full understanding of the limbic system is far from complete, advances in neurosciences have still given a better understanding of the role the individual components of the limbic system play, and some insight into their many connections.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
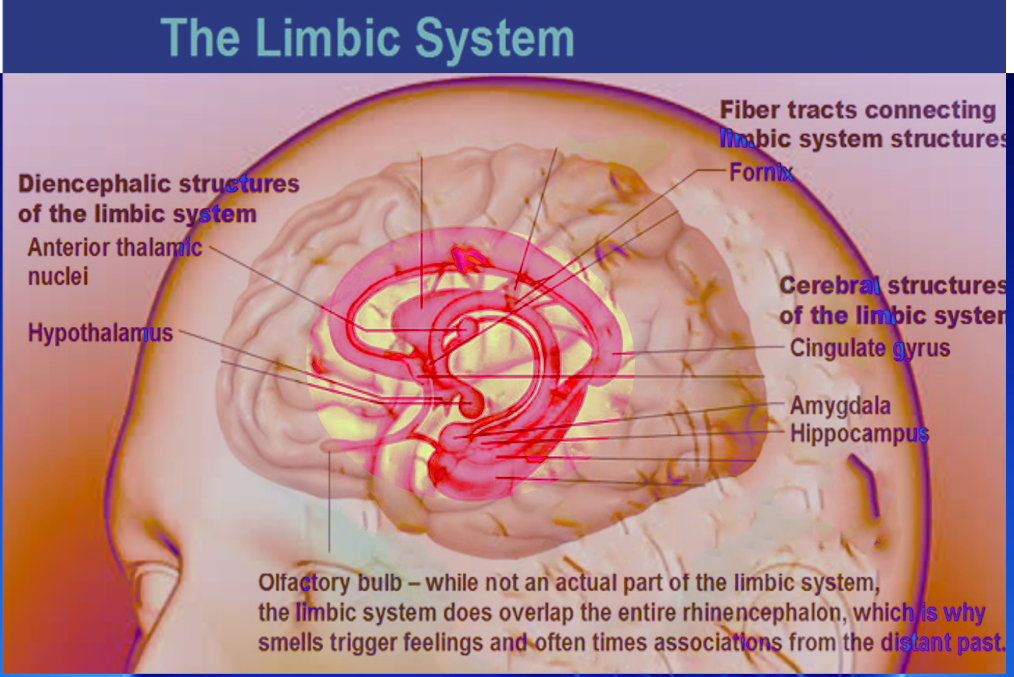
Because of advances in neuroscience, the structures included in the limbic system have undergone redefinition multiple times. However, the structures included in the limbic system are in the general region that borders the cerebral hemisphere and brainstem, lateral to the thalamus, underneath the cerebral cortex, but above the brainstem. The particular embryologic origins can separate the currently defined major structures of the limbic system. The mesencephalic components are from visual, auditory, and somatosensory inputs processed in the region. The diencephalic components are the hypothalamus, anterior thalamic nuclei, and habenular commissure. The telencephalic components contain the cortical and subcortical regions; which are the olfactory bulbs, hippocampus, parahippocampal gyrus, fornix, columns of the fornix, mammillary body, septum pellucidum, amygdala, cingulate gyrus, entorhinal cortex.[3]
While the limbic system was initially suggested to be the sole neurological system involved in regulating emotion, it is now considered only one part of the brain to regulate visceral, autonomic processes. In general, the limbic system assists in various processes relating to cognition; including spatial memory, learning, motivation, emotional processing, and social processing.[2]
Embryology
The limbic system forms from different components that rise from the mesencephalon, diencephalon, and telencephalon as described above.
Surgical Considerations
In 1953, a patient who was known as "H.M." had the anterior two-thirds of bilateral hippocampi resected in an attempt to cure his epilepsy. While he experienced a partial reduction of his epilepsy, for the remainder of his life, he was unable to form new memories.[4]
Clinical Significance
The hypothalamus plays many roles in maintaining homeostasis. However, its role in the limbic system receives less attention. Connections between the hypothalamus, nucleus accumbens, ventral tegmental area, hippocampus, and amygdala have been established. The neural interface between these structures is essential for behaviors such as food-seeking and escape and fear from predators. This interface has been described as the “limbic-motor interface,” it is a model for the initiation of actions by limbic forebrain structures and helps explain how the “emotive brain” and “cognitive brain” operate together to initiate a response.[5]
The olfactory bulbs are involved in the sense of smell. They transfer olfactory information to the amygdala, orbitofrontal cortex, and hippocampus for processing.[6] The amygdala then processes this information and uses it for associative learning. For example, by encoding odor cues associated with a positive or negative taste.[6][7]
The hippocampus is an allocortical structure that is important for the consolidation of information, including short-term, long-term, and spatial memory.[8] People with extensive bilateral hippocampal damage are likely to have anterograde amnesia, as demonstrated in the infamous case of “H.M.” Schizophrenic patients have been reported to have reductions in the size of their hippocampi.[9][10] The parahippocampal gyrus is the cortical region surrounding the hippocampus with roles in scene recognition, and memory encoding and retrieval.[11] Like the hippocampus, the parahippocampal gyrus has been observed to be asymmetrical in patients with schizophrenia.[12]
The fornix is the major output tract of the hippocampus. Its exact function is not clear, but lesions along the fornix have been shown to cause problems with recall memory.[13] The columns of the fornix end at the mammillary bodies. The mammillary body has limbic connections with the amygdala, hippocampus, and anterior thalamic nuclei. The mammillary bodies are important for episodic memory. Thiamine deficiency has been well described in causing damage to the mammillary bodies, most commonly through Wernicke-Korsakoff syndrome.[14]
The amygdala is a subcortical structure of the limbic system, located in the medial temporal lobe, whose role involves processing emotional responses- specifically fear, anxiety, and aggression. Additionally, the amygdala further processes memory and decision-making.[15] Fear conditioning processing takes place in the lateral nuclei of the amygdalae where memories form associations with the adverse stimuli through long-term potentiation.[16][17] Damage to the amygdalae has resulted in the impairment of fear conditioning.[18] Klüver-Bucy syndrome is another rare condition observed after bilateral lesions to the amygdalae occur. Symptoms include amnesia, docility, hyperphagia (both pica and overeating normal foods), hyperorality, hypersexuality, and visual agnosia.[19]
The cingulate gyrus is a cortical structure that lies immediately above the corpus callosum. Its major afferents are from the thalamus and neocortex. The cingulate gyrus, like most of the limbic system, is involved in emotion formation, learning, and memory.[20][21] It is responsible for linking behavior and motivational outcomes.[22] Some research has shown the cingulate gyri (specifically the anterior cingulate cortex) to have size differences in patients with mood disorders and schizophrenia.[23][24][25][26][27]
The entorhinal cortex is located in the medial temporal lobe and is the main gateway between the hippocampus and neocortex. The EC-hippocampus system is an essential part of the limbic system responsible for declarative memories, spatial memories, memory formation, and memory consolidation.[28][29] Clinically, in those who have Alzheimer's disease, magnetic resonance imaging has shown a loss of volume in the entorhinal cortex.[30][31]
Other Issues
With the advancement of the understanding of the complexity of higher cognitive processes, there are suggestions that the term limbic system is no longer relevant but that it functioned as a historical framework upon which to build our current understanding of neuroscience.[32][33] Others have suggested a revised limbic system model that includes three distinct networks. The first being the hippocampal-diencephalic and parahippocampal-retrosplenial network which has a role in memory and spatial orientation. The second being the temporo-amygdala-orbitofrontal network which associates emotion with cognition. And finally, the third being the default-mode network involved in autobiographical memory and introspection.[3]
In conclusion, many individual components comprise the limbic system, all of which play specific roles in the greater whole of the functionality of the limbic system. Emotion, memory, and social processing are essential functions when considering the whole of human health. Generally, clinical disorders involving bilateral lesions of individual parts of the limbic system are rare. However, in much more prevalent disorders, such as schizophrenia, asymmetry and cortical volume loss of limbic system components is common.
Media
References
Pessoa L, Hof PR. From Paul Broca's great limbic lobe to the limbic system. The Journal of comparative neurology. 2015 Dec 1:523(17):2495-500. doi: 10.1002/cne.23840. Epub 2015 Aug 10 [PubMed PMID: 26103943]
Level 2 (mid-level) evidenceHariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000 Jan 17:11(1):43-8 [PubMed PMID: 10683827]
Catani M, Dell'acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neuroscience and biobehavioral reviews. 2013 Sep:37(8):1724-37. doi: 10.1016/j.neubiorev.2013.07.001. Epub 2013 Jul 9 [PubMed PMID: 23850593]
SCOVILLE WB, MILNER B. Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry. 1957 Feb:20(1):11-21 [PubMed PMID: 13406589]
Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in neurobiology. 1980:14(2-3):69-97 [PubMed PMID: 6999537]
Level 3 (low-level) evidenceRoyet JP, Plailly J. Lateralization of olfactory processes. Chemical senses. 2004 Oct:29(8):731-45 [PubMed PMID: 15466819]
Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999 Mar 1:19(5):1876-84 [PubMed PMID: 10024371]
Level 3 (low-level) evidenceSquire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992 Apr:99(2):195-231 [PubMed PMID: 1594723]
Level 3 (low-level) evidenceHarrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004 Jun:174(1):151-62 [PubMed PMID: 15205886]
Level 3 (low-level) evidenceAntoniades M, Schoeler T, Radua J, Valli I, Allen P, Kempton MJ, McGuire P. Verbal learning and hippocampal dysfunction in schizophrenia: A meta-analysis. Neuroscience and biobehavioral reviews. 2018 Mar:86():166-175. doi: 10.1016/j.neubiorev.2017.12.001. Epub 2017 Dec 6 [PubMed PMID: 29223768]
Level 1 (high-level) evidenceEpstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998 Apr 9:392(6676):598-601 [PubMed PMID: 9560155]
McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: A postmortem study. The American journal of psychiatry. 2000 Jan:157(1):40-7 [PubMed PMID: 10618011]
Lövblad KO, Schaller K, Vargas MI. The fornix and limbic system. Seminars in ultrasound, CT, and MR. 2014 Oct:35(5):459-73. doi: 10.1053/j.sult.2014.06.005. Epub 2014 Jun 25 [PubMed PMID: 25217299]
Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010 Jul:48(8):2316-27. doi: 10.1016/j.neuropsychologia.2009.10.019. Epub 2009 Oct 30 [PubMed PMID: 19879886]
Level 3 (low-level) evidenceAmunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005 Dec:210(5-6):343-52 [PubMed PMID: 16208455]
Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends in neurosciences. 1999 Dec:22(12):561-7 [PubMed PMID: 10542437]
Level 3 (low-level) evidenceBlair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learning & memory (Cold Spring Harbor, N.Y.). 2001 Sep-Oct:8(5):229-42 [PubMed PMID: 11584069]
Level 3 (low-level) evidenceRessler K, Davis M. Genetics of childhood disorders: L. Learning and memory, part 3: fear conditioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2003 May:42(5):612-5 [PubMed PMID: 12707566]
Marlowe WB, Mancall EL, Thomas JJ. Complete Klüver-Bucy syndrome in man. Cortex; a journal devoted to the study of the nervous system and behavior. 1975 Mar:11(1):53-9 [PubMed PMID: 168031]
Level 3 (low-level) evidenceHadland KA, Rushworth MF, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003:41(8):919-31 [PubMed PMID: 12667528]
Level 3 (low-level) evidence. Cingulate binds learning. Trends in cognitive sciences. 1997 Apr:1(1):2. doi: 10.1016/S1364-6613(97)85002-4. Epub [PubMed PMID: 21223838]
Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Mar 3:30(9):3339-46. doi: 10.1523/JNEUROSCI.4874-09.2010. Epub [PubMed PMID: 20203193]
Level 3 (low-level) evidenceDrevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008 Aug:13(8):663-81 [PubMed PMID: 18704022]
Adams R, David AS. Patterns of anterior cingulate activation in schizophrenia: a selective review. Neuropsychiatric disease and treatment. 2007 Feb:3(1):87-101 [PubMed PMID: 19300540]
Fujiwara H, Hirao K, Namiki C, Yamada M, Shimizu M, Fukuyama H, Hayashi T, Murai T. Anterior cingulate pathology and social cognition in schizophrenia: a study of gray matter, white matter and sulcal morphometry. NeuroImage. 2007 Jul 15:36(4):1236-45 [PubMed PMID: 17524666]
Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophrenia research. 2004 Dec 1:71(2-3):249-62 [PubMed PMID: 15474896]
Costain G, Ho A, Crawley AP, Mikulis DJ, Brzustowicz LM, Chow EW, Bassett AS. Reduced gray matter in the anterior cingulate gyrus in familial schizophrenia: a preliminary report. Schizophrenia research. 2010 Sep:122(1-3):81-4. doi: 10.1016/j.schres.2010.06.014. Epub 2010 Jul 16 [PubMed PMID: 20638248]
Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science (New York, N.Y.). 2005 Jun 17:308(5729):1792-4 [PubMed PMID: 15961670]
Level 3 (low-level) evidenceJacobs J, Kahana MJ, Ekstrom AD, Mollison MV, Fried I. A sense of direction in human entorhinal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010 Apr 6:107(14):6487-92. doi: 10.1073/pnas.0911213107. Epub 2010 Mar 22 [PubMed PMID: 20308554]
Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nature neuroscience. 2014 Feb:17(2):304-11. doi: 10.1038/nn.3606. Epub 2013 Dec 22 [PubMed PMID: 24362760]
Level 3 (low-level) evidenceLópez ME, Bruña R, Aurtenetxe S, Pineda-Pardo JÁ, Marcos A, Arrazola J, Reinoso AI, Montejo P, Bajo R, Maestú F. Alpha-band hypersynchronization in progressive mild cognitive impairment: a magnetoencephalography study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014 Oct 29:34(44):14551-9. doi: 10.1523/JNEUROSCI.0964-14.2014. Epub [PubMed PMID: 25355209]
Blessing WW. Inadequate frameworks for understanding bodily homeostasis. Trends in neurosciences. 1997 Jun:20(6):235-9 [PubMed PMID: 9185301]
Level 3 (low-level) evidenceKötter R, Stephan KE. Useless or helpful? The "limbic system" concept. Reviews in the neurosciences. 1997 Apr-Jun:8(2):139-45 [PubMed PMID: 9344183]
Level 3 (low-level) evidence