Introduction
A pleural friction rub is an audible respiratory sound, often likened to the creaking of leather or the squeaking of a shoe on wet surfaces, that serves as a distinctive clinical sign indicative of pleural inflammation and is commonly associated with conditions such as pleurisy, pneumonia, a pulmonary embolism, and malignancy.[1] For clinicians, encountering this sound during auscultation can provide a valuable clue in diagnosing various underlying respiratory conditions.
Characterized by its localized, discontinuous, and grating nature, a pleural friction rub typically occurs during inspiration and expiration, resulting from the inflamed and roughened pleural surfaces rubbing against each other.[2] Understanding the significance of this auscultatory finding and its potential implications enables clinicians to diagnose and treat patients presenting with related respiratory complaints.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
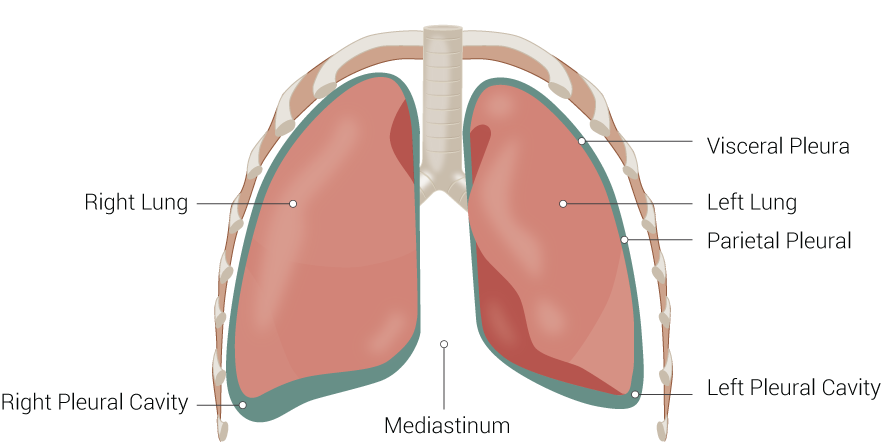
The pleurae comprise visceral and parietal tissues that overlay the lungs and line the thoracic cavity. A small amount of serous fluid typically coats the surfaces of these layers, allowing them to slide easily over one another and creating surface tension that pulls the parietal and visceral pleura together. This mechanism ensures that the lungs expand with the thorax during inspiration (see Image. Lung Anatomy). A pleural friction rub occurs when the usually smooth surfaces of the visceral and parietal layers roughen due to inflammation.
Any condition that causes pleurisy or a pleural effusion can produce a pleural friction rub. The following list contains possible underlying conditions associated with a pleural friction rub:
- Bacterial pneumonia
- Pulmonary embolism
- Viral infections
- Autoimmune conditions like systemic lupus erythematosus, systemic sclerosis, and rheumatoid arthritis
- Pulmonary infarction
- Tuberculosis
- Empyema
- Drugs that cause pleural disease
- End-stage renal disease
- Pancreatitis
- Malignancy [3]
Epidemiology
A friction rub is a common finding in patients with pleurisy. This clinical sign may also be detected in approximately 4% of patients with pneumonia and pulmonary embolism.[4][5] Human enteroviruses, such as poliovirus, group A and B coxsackieviruses, and echoviruses, cause a pleural friction rub in up to 25% of patients. Approximately 20% to 40% of patients who die of chronic renal insufficiency have fibrinous pleuritis, and 10% or less of patients with pancreatic pseudocysts develop pancreatic pulmonary effusions, which can also lead to a pleural friction rub.[6][7][8]
Pathophysiology
The pleural surfaces become thick and rough in conditions marked by inflammation or neoplastic growth, impeding easy motion and giving rise to a pleural friction rub. Prolonged inflammation causes pleural macrophage accumulation, triggering fibrocyte proliferation and producing the characteristic grating sound of a pleural rub as inflamed pleural surfaces slide past each other.[9]
Pleural effusions, whether transudative or exudative, can lead to pleural friction rubs with various underlying causes. Transudative effusions, often associated with conditions like heart failure and liver cirrhosis, contrast with exudative effusions, which stem from inflammatory or infectious conditions such as pneumonia, malignancy, connective tissue disorders, and pulmonary emboli. Complicated effusions, a subtype of exudative effusions, result from microorganisms directly invading the pleura, which activate the coagulation cascade, triggering fibrin deposition and loculation formation within the effusion. Without drainage, complicated effusions may progress to empyema, and marked fibroblast activity leads to pleural thickening and fibrosis.[10]
Malignancy can disrupt lymphatic drainage due to tumor invasion or lymph node involvement, leading to pleural effusion and subsequent friction rubs. The accumulation of tumor cells within the pleurae increases pleural fluid production, worsening the effusion.[11] Patients with chronic pancreatitis may have a disruption in the pancreatic duct, forming a fistula in the chest, followed by a pleural effusion. Uremic pleuritis results from chronic fibrinous inflammation caused by unknown pathogenetic mechanisms. The typical patient with a uremic pleural effusion undergoes hemodialysis or peritoneal dialysis for over 1 to 2 years.[12]
While the visceral pleura lacks somatic innervation and nociceptors, the sensation of pleuritic pain, characterized by sudden, intense, and sharp discomfort exacerbated by breathing, is relayed by somatic nerves innervating the parietal pleura. This pain may be referred to the neck or shoulder if the inflammation is near the diaphragm. Inflammatory mediators released into the pleural space trigger local pain receptors.
History and Physical
On auscultation, a pleural friction rub is a nonmusical, short sound described as creaking or grating and likened to walking on fresh snow. These sounds may vary in intensity, and clinicians may accentuate them by applying additional pressure to their stethoscope. While typically heard during inspiration and expiration, pleural friction rubs may be transient and occur only during a specific part of the respiratory cycle. Pleural friction rubs usually localize to a small area on the chest, do not change after coughing, and may be palpable, feeling like sandpaper or cracking eggshells.
Interestingly, pleural friction rubs may still be audible even in the presence of a large pleural effusion, which prevents direct contact between the pleural surfaces. Pleural effusion often presents with dullness to percussion, decreased tactile fremitus, and reduced or delayed chest expansion on the affected side. Clinicians typically find diminished or inaudible breath sounds and often appreciate egophony at the superior aspect of the effusion.
Patients may have chest pain, described as sudden and intense, sharp, stabbing, or burning pain when inhaling and exhaling. The accompanying presenting features depend on the underlying cause of the pleural friction rub. Patients with hemoptysis, weight loss, fatigue, night sweats, a history of smoking, change in bowel habits, breast lumps, or skin abnormalities may have an underlying malignancy. Individuals with a respiratory tract infection usually present with cough, sputum production, hemoptysis, fever, history of aspiration, or, possibly, immunosuppression.
Edema, orthopnea, paroxysmal nocturnal dyspnea, and jugular venous distension suggest heart failure. Jaundice and ascites suggest liver failure, while tachycardia, tachypnea, hypoxia, and hemoptysis suggest a pulmonary embolism. Historical features like diffuse skin thickening and hardening, arthritis, rash, photosensitivity, and oral ulcers may suggest an autoimmune disorder.
Evaluation
Because a pleural friction rub indicates an underlying process, all patients warrant an in-depth history and complete physical examination to guide further evaluation. Healthcare professionals should inquire about heart, liver, or kidney disease and conditions like systemic lupus erythematosus, hypothyroidism, amyloidosis, pancreatitis, lymphangioleiomyomatosis, and rheumatoid arthritis.
Imaging begins with chest radiography.[13] If a pleural effusion is present, an ultrasound can measure the size and evaluate its characteristics. Computed tomography with contrast is helpful when loculations are likely present, the effusion size is small, or more detailed information about the surrounding anatomy is necessary.
Diagnostic thoracentesis under ultrasound guidance is typically the next step in evaluating patients with a pleural effusion. Pleural fluid is tested for pH, total protein, lactate dehydrogenase (LDH), glucose, cholesterol, cell counts, and differential, along with serum protein and LDH levels, to determine whether the fluid is a transudate or exudate. The pH for transudates generally ranges from 7.40 to 7.55, while most exudates have pH values between 7.30 and 7.45. The Light criteria define an exudative effusion as:
- Pleural fluid-to-serum protein ratio greater than 0.5
- Pleural fluid-to-serum LDH ratio greater than 0.6
- Pleural fluid LDH greater than 0.67 or 2/3 of the upper limits of the laboratory's normal serum LDH [14]
The appearance of the fluid can help determine whether additional testing is necessary. For instance, green fluid should undergo testing for bilirubin levels, cytology, and rheumatoid factor. Black fluid should undergo fungal and bacterial cultures and testing for amylase, cytology, and hematocrit or pleurocrit. Milky fluid should be evaluated for lipid levels, while bloody fluid requires hematocrit and cytological analysis. Turbid fluid requires pH determination and microbiological assessment.
Malignancy
Clinicians perform a cell count and cytology on pleural fluid collected from patients with suspected malignancy. Leukemic involvement of the pleura is likely when basophils comprise more than 10% of nucleated cells in the pleural fluid. Eosinophils greater than 15% may also signify the presence of malignancy. Flow cytometry and immunohistochemical staining are essential for patients with suspected lymphoma or multiple myeloma. High pleural fluid protein concentrations of 7.0 to 8.0 g/dL may suggest Waldenström macroglobulinemia or multiple myeloma. See StatPearls' companion references, "Multiple Myeloma" and "Lymphoma," for a detailed discussion regarding evaluating patients with suspected multiple myeloma and lymphoma. Patients with suspected mesothelioma or solid pleural lesions or thickening may be evaluated with thoracoscopy or image-guided biopsy.
Infection
Empyema generally results from pneumonia. No specific laboratory tests establish the diagnosis of empyema.[15] However, the expected findings are leukocytosis with a left shift on a complete blood count with differential and an elevated C-reactive protein. Blood cultures may be helpful in the case of concurrent bacteremia. Pleural fluid should undergo pH testing, Gram staining, cell counts, and aerobic and anaerobic cultures.
Bacterial pneumonia is most often associated with a predominance of neutrophils, while lymphocyte predominance may indicate tuberculous or fungal etiologies. Polymorphonuclear cell counts above 50,000/µL from an exudative pleural effusion may suggest a complicated parapneumonic infection like empyema. Patients with counts above 10,000/µL from an exudative pleural effusion likely have bacterial pneumonia, acute pancreatitis, or lupus pleuritis. Individuals with counts below 5000/µL are likelier to have chronic exudates due to tuberculous pleurisy or malignancy.
Lymphocyte predominance may indicate a reactive process, a benign condition, or a neoplasm. A lymphocyte count of 85% to 90% of the nucleated cell count suggests tuberculous pleurisy or lymphoma. However, these findings may also indicate sarcoidosis, chronic rheumatoid pleurisy, yellow nail syndrome, chylothorax, or drug-induced pleural effusion. Malignant pleural effusions are generally associated with 50% to 70% lymphocytes.
Patients with pleural effusion and risk factors for tuberculous infection should undergo tuberculosis evaluation beginning with a chest radiograph and 3 sputum specimens obtained 8 hours apart for acid-fast bacillus (AFB), mycobacterial culture, and nucleic acid amplification testing. One sample should be an early morning sample.
Patients with poor immune status, including people with human immunodeficiency virus and CD4 counts below 100 cells/mm³, should also undergo blood and urine mycobacterial cultures. Clinicians obtain a pleural fluid specimen for AFB smears, mycobacterial cultures, and measurement of adenosine deaminase (ADA), an enzyme produced from lymphocytes and involved in purine metabolism.[16] AFB smears and mycobacterial cultures of pleural fluid are only positive in approximately 50% of cases, and the results can take several weeks. Pleural fluid ADA levels can establish a presumptive diagnosis in patients with lymphocyte-predominant effusions and who live in areas with a high tuberculosis incidence. The pleural fluid in patients with tuberculosis almost always has a total protein content above 4 g/dL.[17]
Autoimmunity
Rheumatoid arthritis, idiopathic juvenile arthritis, systemic lupus erythematosus (SLE), inflammatory bowel disease, and Sjögren syndrome are autoimmune conditions that can produce pleural friction rubs due to serositis or pleural effusion. Basic laboratory tests for suspected Sjögren syndrome are a complete blood count with differential, erythrocyte sedimentation rate (ESR), urinalysis, basic metabolic profile, and a spot urine-to-creatinine ratio. An ophthalmologist should evaluate the patient for dry eyes using Schirmer testing and tear break-up time. Additional assessments include anti-Sjögren-syndrome-related antigens A and B antibodies, antinuclear antibodies (ANA), and rheumatoid factor.
Besides meeting diagnostic criteria, clinicians evaluate patients with suspected SLE with ANA, anti-double-stranded deoxyribonucleic acid, antiphospholipid antibodies, immunoglobulins G (IgG) and M (IgM), anticardiolipin antibodies, IgG and IgM anti-β2-glycoprotein, C3 and C4 or CH50 complement levels, ESR or CRP levels, and a urine protein-to-creatinine ratio. Anti-Smith antibodies are highly specific for SLE. Pleural fluid ANA titers of 1:160 or higher are a good indicator of pleuritis in a patient with known SLE.[18]
Individuals with symptoms consistent with rheumatoid arthritis, such as symmetric pain, swelling, and morning stiffness of multiple joints, should undergo testing for rheumatoid factor and anticyclic citrullinated peptide antibodies. Pleural fluid may have elongated macrophages, unique multinucleated giant cells called "tadpole cells," and rheumatoid factor. Patients with suspected irritable bowel disease should undergo stool Clostridioides difficile toxin polymerase chain reaction, routine stool cultures, microscopy for ova and parasites, and Giardia stool antigen.[19] Fecal calprotectin and lactoferrin levels are elevated in irritable bowel disease and help distinguish this condition from irritable bowel syndrome. Endoscopy and biopsy follow.
Heart Failure
A pleural N-terminal pro-B-type natriuretic peptide (NT-proBNP) level exceeding 1500 pg/mL supports the presence of a transudative pleural effusion consistent with heart failure. However, this finding has limited utility since a serum NT-proBNP is equally reliable.
Renal Disease
Uremic pleuritis is a diagnosis of exclusion. Typical findings of uremia include elevated blood urea nitrogen and creatinine levels, metabolic acidosis, and altered mental status. However, kidney disease often coexists with other lung pathologies, including infectious and autoimmune conditions. Clinicians should carefully rule out other causes of pleuritis and consider uremia when no other potential cause is identified.
Pulmonary Embolism
Clinicians determine the pretest probability of a pulmonary embolism in hemodynamically stable patients using the Wells criteria.[20] Individuals with a low probability or a Wells score of less than 2 should have the pulmonary embolism rule-out criteria applied. Patients who meet all 8 criteria do not require any further testing, while the rest undergo a sensitive D-dimer. No further testing is necessary if the D-dimer is negative or less than 500 ng/mL. Patients with a positive D-dimer should undergo a computed tomography pulmonary angiogram (CTPA). Patients with an intermediate score of 2 to 6 undergo D-dimer testing with CTPA if positive. Some authors recommend proceeding directly with imaging if the Wells score is in the upper range of 4 to 6. Patients with a high probability or a Wells score greater than 6 proceed directly to CTPA.
Additional testing includes electrocardiography, chest radiography, and B-type natriuretic peptide and troponin levels to exclude other possible diagnoses. Hemodynamically unstable patients can undergo immediate CTPA if successfully resuscitated. Otherwise, a bedside echocardiogram and lower extremity Doppler are alternatives.
Medications
Pleural reactions from medications manifest as pleural effusions, pleural thickening, or pleuritic chest pain. Nitrofurantoin, dantrolene, methysergide, dasatinib, amiodarone, interleukin-2, procarbazine, methotrexate, clozapine, phenytoin, β-blockers, and ergot drugs can all cause pleuropulmonary reactions. When an apparent cause for pleural findings is not evident, in the setting of a pleural effusion with pleural fluid eosinophilia, clinicians should take a careful drug history, paying close attention to the temporal relationship between the start of therapy and the onset of symptoms.[21] A therapeutic trial of drug withdrawal before additional evaluation may be in order.
Gastrointestinal Causes
Patients with a history of chronic pancreatitis and a left-sided pleural effusion should undergo a pleural fluid pancreatic amylase level. A pancreaticopleural fistula should be ruled out in cases of large, rapidly recurring pleural effusion in the setting of chronic pancreatic disease.[22]
Treatment / Management
Addressing pleural friction rubs begins with identifying the underlying cause. Since these rubs often arise from various conditions, managing the underlying pathology should be the primary focus.
In pneumonia cases, appropriate antibiotic therapy is essential to resolve the infection and alleviate associated inflammation. Similarly, in malignancy-related pleural friction rubs, oncological treatment modalities such as chemotherapy, radiation therapy, and surgical excision may be necessary to target and reduce tumor burden, thereby alleviating pleural fluid accumulation and friction rubs.
Patients with pulmonary emboli require appropriate anticoagulation. Individuals with autoimmune disorders warrant a suitable disease-modifying therapy. Heart failure management requires treatment of the underlying cause and associated comorbid conditions. See StatPearls' companion references, "Acute Pulmonary Embolism," "Typical Bacterial Pneumonia," "Rheumatoid Arthritis," "Systemic Lupus Erythematosus," and "Active Tuberculosis," for additional information regarding further management of some of the underlying causes of pleural friction rubs.
Symptomatic relief may be provided by administering analgesics to manage pleuritic chest pain commonly associated with pleural friction rubs. Nonsteroidal anti-inflammatory drugs or opioids may be used, depending on the severity of pain and individual patient factors. Some patients may require corticosteroids due to underlying autoimmune conditions or inflammation, causing pleurisy. However, clinicians must monitor patients closely for adverse events associated with these medications, particularly in people with comorbidities such as renal impairment or gastrointestinal bleeding. Clinicians may perform interventional procedures like thoracentesis or pleurodesis to drain pleural fluid or prevent reaccumulation, especially in patients with large or recurrent effusions causing the friction rub.
Differential Diagnosis
Distinguishing a pleural friction rub from a pericardial rub is critical. A pericardial rub is highly specific for pericarditis, characterized by a grating sound as inflamed pericardial layers slide against each other. Unlike pleural friction rubs, pericardial rubs persist even while a patient is holding their breath. A pleural friction rub typically has 2 sounds, heard during inspiration and expiration. In comparison, pericardial rubs consist of 3 sounds heard during atrial systole, ventricular systole, and early ventricular diastole.[23]
Coarse crackles and rhonchi are 2 additional lung sounds that may resemble a pleural friction rub. Crackles are explosive, nonmusical sounds lasting for 25 ms or less and are usually heard during inspiration and, sometimes, during expiration. A proposed etiology of crackles is that small airways that collapse during expiration snap open during inspiration. Other authors suggest that crackles originate from the vibration in the walls of small airways. Coarse crackles are loud, low-pitched, and occur less frequently per breath, whereas fine crackles are soft, higher-pitched, and more numerous per breath.
Coarse crackles often resolve after coughing, but fine crackles may persist in some patients. Body position changes can alter the sound of fine crackles but not coarse crackles. Crackles are associated with pneumonia, chronic obstructive pulmonary disease, pulmonary edema, interstitial lung disease, and heart failure. Rhonchi are harsh, low-pitched rattling sounds heard during inspiration or expiration due to airway obstruction from secretions, edema, or inflammation.
Prognosis
The prognosis of a pleural friction rub depends on the underlying cause. Patients with a friction rub due to malignancy-related pleural effusion generally have a guarded prognosis and are likely terminal, with a 1-year mortality rate of 77%.[24] Study results have revealed a 20% to 90% incidence of pleuropulmonary disease in patients with systemic lupus erythematous, ranging from subclinical pleural effusions to diffuse alveolar hemorrhage, with a mortality rate of 68% to 75%.[25]
Patients with rheumatoid arthritis who develop interstitial lung disease have a 5-year mortality rate of 36% compared to 18% in patients without lung disease. The prognosis associated with empyema is good if treated early and aggressively. One study revealed that patients with uremic pleuritis have a high mortality of 60% at 3 years. The prognosis for a pulmonary embolism varies based on timing, embolus size, and treatment. The overall mortality is 30% if left untreated. In patients treated with anticoagulation, the mortality is between 2% and 11%.[26]
Complications
The complications associated with pleural friction rubs are linked to their underlying causes and the treatment regimens used, listed below.
- Corticosteroid use: Adrenal insufficiency, diabetes, cataracts, peptic ulcers, glaucoma, hypertension, mood changes, and decreased bone density
- Thoracentesis: Infection, intercostal nerve or artery injury, injury to the lung, diaphragm, heart, liver, or spleen, re-expansion pulmonary edema, pneumothorax, and death
- Pleurodesis: Systemic inflammation with hypotension and acute respiratory distress syndrome, empyema, decreased lung volume, and death
- Anticoagulant use: Intracranial hemorrhage, gastrointestinal bleeding, hemarthrosis, and hemothorax
- Immunosuppressant medications: Increased risk of skin cancer and infection, neutropenia, and lymphopenia
- Therapy with disease-modifying antirheumatic medications: Cirrhosis, hepatic fibrosis, neutropenia, megaloblastic anemia, thrombocytopenia, and pancytopenia
- Chemotherapy: Neuropathy, pancytopenia, infection, myocardial dysfunction, pulmonary fibrosis, secondary malignancy, and neurocognitive impairment
- Antibiotic use: Allergic reactions, cardiac conduction abnormalities with macrolides and fluoroquinolones
- Tendon rupture can occur with fluoroquinolones.
- Autoimmune diseases: Episcleritis, scleritis, osteopenia, osteoporosis, interstitial lung disease, pericarditis, myocarditis, heart failure, coronary artery disease, vasculitis, stroke, glomerulonephritis, Felty syndrome, anemia of chronic disease, lymphoma, monoclonal gammopathy, cryoglobulinemia, and colorectal cancer
- Pulmonary emboli: Death, shock, pulmonary hypertension, and stroke
- Empyema: Sepsis, lung abscess, fibrothorax, pneumothorax, and extension into the chest wall and soft tissue
Understanding these complications and their pathophysiological mechanisms enables clinicians to formulate proactive measures to ensure optimal care.
Deterrence and Patient Education
Clinicians are pivotal in educating patients about the significance of pleural friction rubs to enhance awareness and promote proactive management. Healthcare professionals should inform patients that pleural friction rubs are distinctive respiratory sounds indicating inflammation of the pleural surfaces. A pleural friction rub indicates an underlying condition, such as pneumonia, autoimmune disease, or pulmonary embolism, that requires further evaluation and management. Patients must understand that the root causes of this clinical sign may require long-term therapies and careful monitoring to prevent complications.
Understanding the significance of these sounds empowers patients to recognize potential respiratory issues early and seek prompt medical attention. Additionally, clinicians should discuss the importance of adhering to prescribed treatment regimens, maintaining regular follow-up appointments, and reporting new or worsening symptoms like dyspnea, hemoptysis, joint pain, and chest pain to allow clinicians to address and manage the underlying cause effectively. Moreover, lifestyle modifications, including smoking cessation and maintaining a healthy weight, can contribute to overall respiratory health and reduce the risk of complications associated with pleural friction rubs. By fostering patient engagement and providing comprehensive education, clinicians can empower individuals to actively manage their respiratory health and mitigate the impact of pleural friction rubs.
Enhancing Healthcare Team Outcomes
A pleural friction rub, characterized by its distinct grating sound reminiscent of creaking leather or squeaking shoes on wet surfaces, is a valuable diagnostic clue for pleural inflammation and associated underlying conditions. Roughened pleural surfaces moving against each other during breathing cause pleural friction rubs, which may arise from conditions like pneumonia, pulmonary embolism, malignancy, autoimmune disorders, or uremic pleuritis in patients undergoing long-term dialysis. Evaluation begins with a chest radiograph, followed by an ultrasound if a pleural effusion occurs. A thoracentesis helps differentiate the possible etiologies of pleural effusion. Further testing is guided based on concurrent symptoms.
Treatment involves addressing the underlying pathology, such as administering antibiotics for infections or initiating anticancer therapies for tumors, while providing symptomatic relief with analgesics to manage pleuritic chest pain. In some cases, interventional procedures like thoracentesis and pleurodesis may be necessary to drain pleural fluid and prevent reaccumulation, underscoring the need for comprehensive care and vigilant monitoring for potential complications.
An interprofessional approach involving clinicians, advanced clinicians, nurses, pharmacists, and other healthcare professionals is necessary to optimize patient-centered care, outcomes, patient safety, and team performance. Clinicians and advanced clinicians are crucial in accurately diagnosing and initiating treatment for underlying conditions contributing to pleural friction rubs. The clinical expertise of these healthcare professionals is essential in determining the appropriate diagnostic tests, interpreting results, and devising individualized treatment plans.
Nurses are instrumental in monitoring patients' respiratory status, administering medications, providing patient education on symptom management, and promptly recognizing and reporting any patient status changes. Pharmacists contribute by ensuring medication safety, reviewing drug interactions, and optimizing medication regimens to minimize adverse effects and enhance treatment efficacy.
Effective interprofessional communication is paramount, facilitated through precise documentation and open dialogue to discuss patient progress, address concerns, and adjust treatment plans collaboratively. By working cooperatively, healthcare team members can pool their diverse skills and knowledge to provide holistic care, address patient needs comprehensively, and improve outcomes for individuals with pleural friction rubs. This interprofessional approach fosters synergy within the healthcare team and enhances overall outcomes, reducing morbidity and mortality.
Media
(Click Image to Enlarge)
References
Sarkar M, Madabhavi I, Niranjan N, Dogra M. Auscultation of the respiratory system. Annals of thoracic medicine. 2015 Jul-Sep:10(3):158-68. doi: 10.4103/1817-1737.160831. Epub [PubMed PMID: 26229557]
Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. The New England journal of medicine. 2014 Feb 20:370(8):744-51. doi: 10.1056/NEJMra1302901. Epub [PubMed PMID: 24552321]
Reamy BV, Williams PM, Odom MR. Pleuritic Chest Pain: Sorting Through the Differential Diagnosis. American family physician. 2017 Sep 1:96(5):306-312 [PubMed PMID: 28925655]
Wang Y, Zhao H, Ou R, Zhu H, Gan L, Zeng Z, Yuan R, Yu H, Ye M. Epidemiological and clinical characteristics of severe hand-foot-and-mouth disease (HFMD) among children: a 6-year population-based study. BMC public health. 2020 May 27:20(1):801. doi: 10.1186/s12889-020-08961-6. Epub 2020 May 27 [PubMed PMID: 32460823]
Level 2 (mid-level) evidenceAlshalhoub M, Alhusain F, Alsulaiman F, Alturki A, Aldayel S, Alsalamah M. Clinical significance of elevated D-dimer in emergency department patients: a retrospective single-center analysis. International journal of emergency medicine. 2024 Apr 3:17(1):47. doi: 10.1186/s12245-024-00620-6. Epub 2024 Apr 3 [PubMed PMID: 38566042]
Level 2 (mid-level) evidenceShaik L, Thotamgari SR, Kowtha P, Ranjha S, Shah RN, Kaur P, Subramani R, Katta RR, Kalaiger AM, Singh R. A Spectrum of Pulmonary Complications Occurring in End-Stage Renal Disease Patients on Maintenance Hemodialysis. Cureus. 2021 Jun:13(6):e15426. doi: 10.7759/cureus.15426. Epub 2021 Jun 3 [PubMed PMID: 34249571]
Blatnik JA, Hardacre JM. Management of pancreatic fistulas. The Surgical clinics of North America. 2013 Jun:93(3):611-7. doi: 10.1016/j.suc.2013.02.011. Epub 2013 Mar 22 [PubMed PMID: 23632147]
Shaikh AT, Atiq I, Gul S, Gul O, Ali W. Recurrent Pleural Effusions Secondary to Pancreaticopleural Fistula: A Case Presentation. Cureus. 2023 Jul:15(7):e41625. doi: 10.7759/cureus.41625. Epub 2023 Jul 10 [PubMed PMID: 37575866]
Level 3 (low-level) evidenceForgacs P. The functional basis of pulmonary sounds. Chest. 1978 Mar:73(3):399-405 [PubMed PMID: 630938]
Ruiz E, Alemán C, Alegre J, Monasterio J, Segura RM, Armadans L, Vázquez A, Soriano T, Fernández de Sevilla T. Angiogenic factors and angiogenesis inhibitors in exudative pleural effusions. Lung. 2005 May-Jun:183(3):185-95 [PubMed PMID: 16078040]
Mierzejewski M, Korczynski P, Krenke R, Janssen JP. Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration. Respiratory research. 2019 Nov 7:20(1):247. doi: 10.1186/s12931-019-1204-x. Epub 2019 Nov 7 [PubMed PMID: 31699094]
Seo HM, Kim M, Kim H. Refractory exudative pleural effusion in patients with chronic kidney disease not receiving dialysis: A case report. Clinical case reports. 2019 Apr:7(4):675-679. doi: 10.1002/ccr3.2069. Epub 2019 Feb 19 [PubMed PMID: 30997062]
Level 3 (low-level) evidenceHeffner JE, Klein JS, Hampson C. Diagnostic utility and clinical application of imaging for pleural space infections. Chest. 2010 Feb:137(2):467-79. doi: 10.1378/chest.08-3002. Epub [PubMed PMID: 20133295]
Shen-Wagner J, Gamble C, MacGilvray P. Pleural Effusion: Diagnostic Approach in Adults. American family physician. 2023 Nov:108(5):464-475 [PubMed PMID: 37983698]
Kommedal Ø, Eagan TM, Fløtten Ø, Leegaard TM, Siljan W, Fardal H, Bø B, Grøvan F, Larssen KW, Kildahl-Andersen A, Hjetland R, Tilseth R, Hareide SKØ, Tellevik M, Dyrhovden R. Microbiological diagnosis of pleural infections: a comparative evaluation of a novel syndromic real-time PCR panel. Microbiology spectrum. 2024 Jun 4:12(6):e0351023. doi: 10.1128/spectrum.03510-23. Epub 2024 Apr 24 [PubMed PMID: 38656204]
Level 2 (mid-level) evidenceAggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PloS one. 2019:14(3):e0213728. doi: 10.1371/journal.pone.0213728. Epub 2019 Mar 26 [PubMed PMID: 30913213]
Level 1 (high-level) evidenceZhao T, Zhang J, Zhang X, Wang C. Clinical significance of pleural fluid lactate dehydrogenase/adenosine deaminase ratio in the diagnosis of tuberculous pleural effusion. BMC pulmonary medicine. 2024 May 15:24(1):241. doi: 10.1186/s12890-024-03055-0. Epub 2024 May 15 [PubMed PMID: 38750432]
Kaudewitz D, John L, Meis J, Frey N, Lorenz HM, Leuschner F, Blank N. Clinical and serological characterization of acute pleuropericarditis suggests an autoinflammatory pathogenesis and highlights risk factors for recurrent attacks. Clinical research in cardiology : official journal of the German Cardiac Society. 2024 Feb 15:():. doi: 10.1007/s00392-024-02390-w. Epub 2024 Feb 15 [PubMed PMID: 38358415]
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. The American journal of gastroenterology. 2019 Mar:114(3):384-413. doi: 10.14309/ajg.0000000000000152. Epub [PubMed PMID: 30840605]
Altabbaa G, Flemons W, Ocampo W, Babione JN, Kaufman J, Murphy S, Lamont N, Schaefer J, Boscan A, Stelfox HT, Conly J, Ghali WA. Deployment of a human-centred clinical decision support system for pulmonary embolism: evaluation of impact on quality of diagnostic decisions. BMJ open quality. 2024 Feb 12:13(1):. doi: 10.1136/bmjoq-2023-002574. Epub 2024 Feb 12 [PubMed PMID: 38350673]
Level 2 (mid-level) evidenceHuggins JT, Sahn SA. Drug-induced pleural disease. Clinics in chest medicine. 2004 Mar:25(1):141-53 [PubMed PMID: 15062606]
Sandhu M, Bernshteyn M, Banerjee S, Kuhn M. Rapidly Accumulating Pleural Effusion: A Sequela of Chronic Pancreatitis. Journal of investigative medicine high impact case reports. 2022 Jan-Dec:10():23247096221099269. doi: 10.1177/23247096221099269. Epub [PubMed PMID: 35593441]
Level 3 (low-level) evidencePadilla-Ortiz AL, Ibarra D. Lung and Heart Sounds Analysis: State-of-the-Art and Future Trends. Critical reviews in biomedical engineering. 2018:46(1):33-52. doi: 10.1615/CritRevBiomedEng.2018025112. Epub [PubMed PMID: 29717676]
DeBiasi EM, Pisani MA, Murphy TE, Araujo K, Kookoolis A, Argento AC, Puchalski J. Mortality among patients with pleural effusion undergoing thoracentesis. The European respiratory journal. 2015 Aug:46(2):495-502. doi: 10.1183/09031936.00217114. Epub 2015 Apr 2 [PubMed PMID: 25837039]
Shin JI, Lee KH, Park S, Yang JW, Kim HJ, Song K, Lee S, Na H, Jang YJ, Nam JY, Kim S, Lee C, Hong C, Kim C, Kim M, Choi U, Seo J, Jin H, Yi B, Jeong SJ, Sheok YO, Kim H, Lee S, Lee S, Jeong YS, Park SJ, Kim JH, Kronbichler A. Systemic Lupus Erythematosus and Lung Involvement: A Comprehensive Review. Journal of clinical medicine. 2022 Nov 13:11(22):. doi: 10.3390/jcm11226714. Epub 2022 Nov 13 [PubMed PMID: 36431192]
Donovan KM. Pulmonary Embolism Triage: When to Do What. Methodist DeBakey cardiovascular journal. 2024:20(3):65-67. doi: 10.14797/mdcvj.1394. Epub 2024 May 16 [PubMed PMID: 38765217]