Introduction
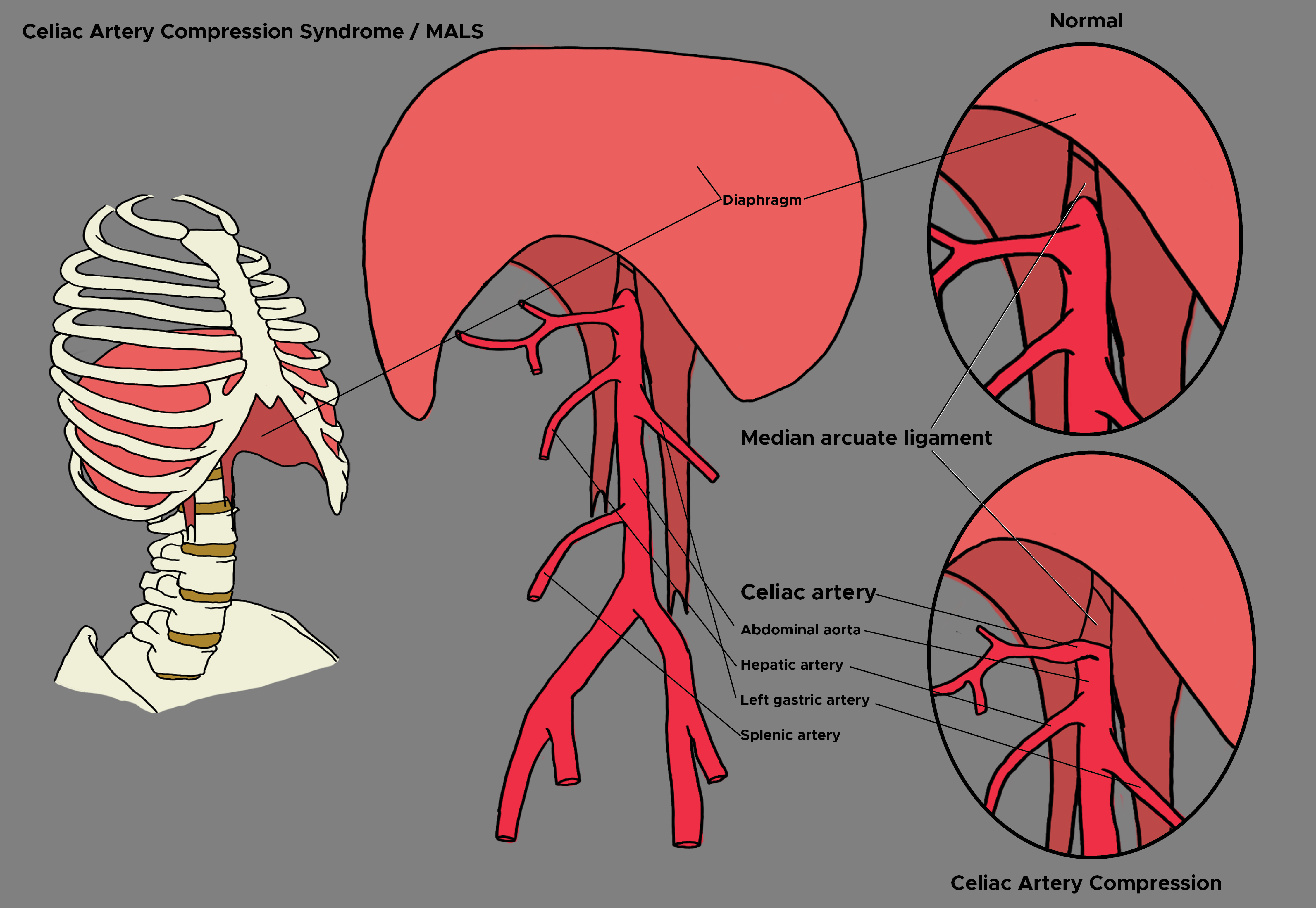
Celiac artery compression syndrome is also known as Dunbar syndrome or median arcuate ligament syndrome. It is a rare medical condition characterized by recurrent abdominal pain. The condition results from the compression of the celiac artery by a fibrous band of the diaphragm known as the median arcuate ligament. Lipshutz first reported the anatomical compression of the celiac artery in 1917. As a clinical entity, median arcuate ligament syndrome was first described by Harolja in 1963. Dunbar described the first clinical study on this entity in 1965.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of celiac artery compression syndrome is not fully understood. Uncertainty arises because the anatomic compression of the celiac artery by the median arcuate ligament can also be seen in asymptomatic patients. In other words, every patient with this anatomic compression does not develop symptoms. The median arcuate ligament is a fibrous band that connects the two medial borders of the diaphragmatic crura, usually near the level of the 12th thoracic or first lumbar vertebra. Compression of the celiac artery can occur in two anatomic situations: an abnormally cephalad origin of the celiac artery or an abnormally caudad insertion of the diaphragm. Congenital factors may influence the level of the insertion of the diaphragm or the origin of the celiac artery. This hypothesis is supported by a small series of studies in families and monozygotic twins. The compression of the celiac artery by the median arcuate ligament is believed to cause intermittent mesenteric ischemia. However, this explanation alone may not completely explain the condition as there is usually a rich collateral network of mesenteric vessels between the celiac artery and the superior mesenteric artery. Therefore, there may be a role for underlying celiac nerve plexus dysfunction as well when considering the etiology of this condition. Nerve dysfunction may lead to abnormal splanchnic vasoconstriction, leading to ischemia.[2]
Epidemiology
Celiac artery compression syndrome is a rare condition with a reported incidence of 2 per 100,000 population. It is commonly seen in young females between the ages of 30 to 50 years. It has a female to male ratio of 4:1. The condition has also been reported in children. The incidence of radiographic compression of the celiac axis has been reported to be between 10% to 24% in some populations; note that symptomatic celiac artery compression exists in a smaller percentage of the population.[3]
Pathophysiology
During expiration, there is compression of the celiac axis by the median arcuate ligament. During expiration, the diaphragm moves upwards, causing stretching of crura, which causes more compression of celiac axis [4]
Histopathology
Histopathologic findings in celiac artery compression syndrome develop over time. The celiac artery develops fibrotic changes in its wall due to the recurrent compression from the median arcuate ligament.
History and Physical
In history, it is important to ask about risk factors for atherosclerotic disease and to inquire about the use of non-steroidal anti-inflammatory medications to rule out competing etiologies. Often, these patients will present with a history of being on medications such as proton pump inhibitors that do not give any relief. Patients with celiac artery compression syndrome may complain of abdominal pain in the epigastric area, anorexia, and/or diarrhea. Typically, the onset of the pain is after food intake (post-prandial pain). The pain may be associated with nausea and emesis. The serial measurement of the patient’s weight may demonstrate a decline over time. On physical examination, patients may have mild tenderness to palpation in the epigastric area. Auscultation of the abdomen may reveal a bruit.
Evaluation
Celiac artery compression syndrome is a diagnosis of exclusion. Pertinent workup of this condition may include simple tests to rule out other etiologies causing diagnostic uncertainty. These can include colonoscopy, ultrasound of the liver, pancreas, and gallbladder, upper gastrointestinal endoscopy, complete blood count, hepatic function testing, serum amylase and lipase, C-reactive protein, and testing of certain antibodies such as anti-smooth muscle antibody (ASMA). Several diagnostic modalities can be employed to make a diagnosis of celiac artery compression syndrome. These can be categorized as non-invasive and invasive modalities.
The invasive modalities include conventional visceral angiography while the invasive modalities include Doppler ultrasound (US), magnetic resonance imaging (MRI), and computerized tomography angiography (CTA). On ultrasound, there is a demonstration of elevated celiac artery peak systolic velocities with deep expiration. More specifically, the following two criteria are supportive for the diagnosis of celiac artery compression syndrome on ultrasound: expiratory peak velocity of greater than 200 cm/s and deflection angle greater than 50 degrees.
Additionally, the following findings may also be encountered: the abnormal origin of the celiac artery, flow reversal in the hepatic artery and lowering of velocity in the celiac artery when the patient stands erect. Conventional visceral angiography shows partial to complete stenosis of the celiac artery secondary to extrinsic compression with possible post-stenotic dilation and retrograde filling of the celiac artery. During visceral angiography, intravascular ultrasound can be used to demonstrate ostial compression of the celiac artery with expiration. CTA shows compression of the celiac axis with focal stenosis and post-stenotic dilation. On these modalities, one can appreciate the difference in perfusion of the celiac artery during inspiration and expiration. Additional studies that may be done to diagnose celiac artery compression syndrome include gastric tonometry and percutaneous celiac ganglion block.[5]
Treatment / Management
As compression of the celiac artery is believed to be the primary pathology in celiac artery compression syndrome, therapy is directed at the release of this compression. There is no known medical treatment of celiac artery compression syndrome. As first-line therapy, the surgical division of median arcuate ligament can be performed in the following two ways: traditional open surgery or with the use of minimally invasive techniques. Traditional open surgery can involve either a retroperitoneal approach or a transperitoneal approach. The minimally invasive approach can involve laparoscopy or the use of a robot. The main advantage of minimally invasive techniques is a shorter hospital stay, improved pain control, use of smaller incisions, and avoidance of postoperative wound complications. The division of the ligament is often combined with neurolysis of the celiac nerve plexus and may contribute to improved symptom relief. In selected patients, additional procedures (also called co-surgical procedures) may be pursued. These include celiac artery bypass (such as an aorto celiac or aorto hepatic bypass), superior mesenteric artery transposition, splenic artery transposition, balloon angioplasty with or without stenting, and para-spinal ganglion nerve blocks. Angioplasty with or without stenting is typically reserved for refractory cases where the first-line surgical therapies may have proven unsuccessful in providing symptomatic relief.[6]
Differential Diagnosis
Celiac artery compression syndrome may mimic several other medical conditions posing a diagnostic challenge for the physician. It can be mistaken for other conditions that can cause post-prandial pain such as gallbladder disease including biliary dyskinesia and cholecystitis. Other intra-abdominal pathologies such as appendicitis, colorectal malignancy, hepatitis, gastroparesis and gastritis/peptic ulcer disease can also mimic celiac artery compression syndrome. Furthermore, chronic mesenteric ischemia secondary to atherosclerotic disease may have a similar presentation as celiac artery compression syndrome. Some of these conditions may co-exist simultaneously as well. Presence for abdominal bruit on examination may lead to consideration for other entities such as renal artery stenosis or arteriovenous malformations.
Prognosis
Following surgical intervention, about 60% to 70% of the patients report symptom relief. Symptom relief has been reported to be immediate in some instances. In others, it may take up to a few months for the resolution of the pain. The presence of postexertional pain has been shown to be correlated with symptomatic improvement after surgery while the presence of emesis and unprovoked pain preoperatively has been shown to be associated with suboptimal surgical outcomes postoperatively. Patients who have a good response to a diagnostic celiac plexus block preoperatively also seem to report better symptom relief following surgical intervention. The presence of atherosclerotic risk factors may predict poorer outcomes postoperatively.[7]
Pearls and Other Issues
Further studies are required to better define the underlying pathogenesis of celiac artery compression syndrome. The durability and long-term efficacy of endovascular options in the management of this condition need to be better evaluated in future studies. Additionally, a better understanding of the prognostic factors will help in improved patient selection for surgical intervention.
Enhancing Healthcare Team Outcomes
When the primary care provider, nurse practitioner, emergency department physician or internist encounter patients with postprandial pain and weight loss, celiac artery compression syndrome should be considered. The diagnosis and management of this syndrome are challenging the most patients require an exhaustive workup. Surgery is the only treatment but acceptable results are only seen in 75% of patients. Today, endovascular therapy has become an option and it does provide temporary relief from symptoms.[8][9][10][11]
Media
(Click Image to Enlarge)
References
Zambrano-Lara M,Gonzalez-Urquijo M,Lozano-Balderas G,Rodarte-Shade M,Fabiani MA, Median arcuate ligament syndrome as a rare cause of chronic abdominal pain. Revista de gastroenterologia de Mexico. 2020 Jul 14; [PubMed PMID: 32680594]
Matsuura H,Okita A,Suganami Y, Intermittent Severe Epigastric Pain and Abdominal Bruit Varying With Respiration. Gastroenterology. 2020 Mar; [PubMed PMID: 31560895]
Koç M,Artaş H,Serhatlıoğlu S, The investigation of incidence and multidetector computed tomography findings of median arcuate ligament syndrome Turkish journal of medical sciences. 2018 Dec 12; [PubMed PMID: 30541249]
Den B,Ignashov AM,Perleĭ VE,Gichkin AIu,Ustiuzhaninov AS, [The significance of respiratory and orthostatic tests in duplex scanning in diagnostics of celiac artery compression syndrome]. Vestnik khirurgii imeni I. I. Grekova. 2013; [PubMed PMID: 24000675]
Level 2 (mid-level) evidenceRömer C,Fischer T,Haase O,Möckel M,Hamm B,Lerchbaumer MH, Assessment of celiac artery compression using color-coded duplex sonography. Clinical hemorheology and microcirculation. 2020 Jul 16; [PubMed PMID: 32675404]
Coelho JCU,Hosni AVE,Claus CM,Aguilera YSH,Abot GP,Freitas ATC,Costa MARD, Treatment of median arcuate ligament syndrome: outcome of laparoscopic approach. Arquivos brasileiros de cirurgia digestiva : ABCD = Brazilian archives of digestive surgery. 2020; [PubMed PMID: 32428132]
Ho KKF,Walker P,Smithers BM,Foster W,Nathanson L,O'Rourke N,Shaw I,McGahan T, Outcome predictors in median arcuate ligament syndrome. Journal of vascular surgery. 2017 Jun; [PubMed PMID: 28189355]
Selvaraj BJ,Joshi M,Weber G,Yarmush J, Celiac plexus block as a diagnostic tool in suspected pediatric median arcuate ligament syndrome. Local and regional anesthesia. 2019; [PubMed PMID: 30881107]
Laxague F,Dreifuss N,Schlottmann F,Buxhoeveden R, Laparoscopic resolution of median arcuate ligament syndrome. Cirugia espanola. 2019 Mar 4; [PubMed PMID: 30846189]
Patel MV,Dalag L,Weiner A,Skelly C,Lorenz J, Inability of conventional imaging findings to predict response to laparoscopic release of the median arcuate ligament in patients with celiac artery compression. Journal of vascular surgery. 2019 Feb; [PubMed PMID: 30686339]
Berek P,Kopolovets I,Dzsinich,Sihotský V,Štefanič P,Frankovičová M, Celiac axis compression syndrome - diagnostic and surgical treatment. Rozhledy v chirurgii : mesicnik Ceskoslovenske chirurgicke spolecnosti. Summer 2018; [PubMed PMID: 30470123]