Introduction
The mammary gland is a highly evolved and specialized organ developing on each side of the anterior chest wall. This organ's primary function is to secrete milk. Though the gland is present in both sexes, it is well-developed in females but rudimentary in males.[1] The mammary gland is a vital accessory organ in the female reproductive system.
The mammary gland is classified as apocrine. Thus, the secretory cells' apical segment and a portion of their cytoplasm become part of the secretion. The mammary gland usually weighs between 500 and 1000 grams each. The organ is hemispherical in young adult females but becomes pendulous later in life. This article discusses the anatomy, function, and clinical importance of the mammary gland.[2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
The mammary gland is situated in the pectoral region in the superficial fascia. However, a segment called the "axillary tail of Spence" pierces the deep fascia and lies in the axilla up to the 3rd rib level.[3] The mammary gland extends vertically from the 2nd to the 6th rib. Horizontally, it spreads from the lateral sternal border to the mid-axillary line.
Deep to the mammary gland tissue is the retromammary space, a loose connective-tissue plane that gives free mobility to the gland. Below the retromammary space is the pectoral fascia, which covers the pectoralis muscle. The serratus anterior and external oblique are other muscles that lie deep in the mammary gland.
The mammary gland is divided into 3 parts: skin, parenchyma, and stroma (see Image. Breast Sagittal View).
Skin
The skin consists of the nipple and areola. The nipple is a conical eminence situated in the 4th intercostal space (ICS). Piercing the nipple are 15 to 20 lactiferous ducts.[4] The nipple contains richly innervated circular and longitudinal smooth muscle fibers, which make it erect upon stimulation. The nipple usually has no sweat glands, fat, or hairs.
The areola is the dark pinkish-brown area around the nipple. This area is rich in modified sebaceous glands (tubercles of Montgomery) during pregnancy and lactation. These modified glands produce oily secretions that prevent nipple and areolar cracking. Notably, the areola is devoid of fat and hair.
Parenchyma
Glandular tissue is comprised of branching ducts and terminal secretory lobules. One lactiferous duct drains 15 to 20 lobes. These ducts enlarge to form the lactiferous sinus before they open separately into the nipple. Milk collects in the lactiferous sinuses and is released in response to the baby's suckling. The lactiferous ducts are arranged radially in the nipple. Hence, incisions in this area must be oriented radially to avoid cutting through multiple lactiferous ducts.
Stroma
The stroma is the supporting framework of the breast parenchyma. The stroma has fibrous and fatty areas.
Fibrous stroma gives rise to septa called "suspensory ligaments of Cooper," which separate the lobes and suspend the mammary gland from the pectoral fascia.[2] In patients with breast cancer, contraction of these ligaments causes breast rigidity and puckering of the overlying skin.[5] In breast cancer, the Peau d'orange sign arises from lymphatic obstruction and subsequent cutaneous edema and fibrosis.[6]
The bulk of the mammary gland is filled with variable amounts of stromal fat.
Mammary Gland Quadrants
The mammary gland is best examined by dividing it into 4 quadrants, with vertical and horizontal imaginary lines passing through the nipple (see Image. Mammary Gland Quadrants).[7] The regions are designated as the upper outer (UOQ), upper inner (UIQ), lower inner (LIQ), and lower outer (LOQ) quadrants.[8][9]
Function
The primary purpose of the mammary gland is to secrete milk for infant breastfeeding. This organ also plays an essential role in female sexuality.
Breast development is minimal and comparable in both sexes until females reach puberty. Estrogen, progesterone, and growth hormone spurt during the pubertal phase and induce greater development in females than males. The smooth contour of female breasts is due to increased adipose tissue.
The female breasts further increase in size in early pregnancy, with estrogen and progesterone inducing parenchymal growth and ductal branching. Secretory alveoli and the surrounding connective tissue start developing at duct terminals.[10] In the later stages of pregnancy, these alveoli are filled with milk under prolactin's influence. When lactation stops, the secretory alveoli shrink, decrease in number, and disappear. However, the mammary gland never returns to prepubertal form. After menopause, the breasts regress in size due to diminishing circulating estrogen levels.[11]
Embryology
Genetic and hormonal factors influence ectodermal cells to form the human breasts starting in the 4th week of embryonic life. Ectodermal thickenings, known as mammary ridges (milk ridge or milk line), emerge on the chest around the 4th intercostal space, giving rise to rudimentary mammary buds by the 5th week of gestation. These primary mammary buds extend downward, developing into secondary buds and intricate mammary lobules over the next 7 weeks. The breast stroma, fat, ligaments, nerves, arteries, veins, and lymphatics undergo development throughout the gestational period.
Beyond the 12th week, the secondary buds continue their growth, elongating and branching into 15 to 20 solid cords that differentiate into the lactiferous breast ducts and their branches. The ducts later canalize under the influence of maternal sex hormones, connecting the developing nipple to the expanding mammary lobules.
The fetal nipple is inverted but everts at birth due to the proliferation of its modified sebaceous glands (future Montgomery glands) and muscle tissue. Areolar pigmentation also increases at birth.[9]
Blood Supply and Lymphatics
Arterial Supply
The following arteries supply the mammary glands:
- The internal thoracic artery's perforating branches from the 2nd to the 6th ICS provide circulation to the medial gland regions.
- The lateral thoracic artery supplies the superolateral breast parenchyma.
- The axillary artery's superior thoracic, thoracoacromial, subscapular, and thoracodorsal branches supply a portion of the superior breast parenchyma.
- The musculophrenic artery originates from the internal thoracic artery and supplies inferior breast segments.
- The branches of the anterior and posterior intercostal arteries penetrate the chest wall muscles and supply the deep central breast parenchymal tissues.
- Lateral intercostal arteries travel alongside the pectoralis and serratus anterior muscles and send perforating branches into the deep breast parenchymal tissues.[12]
Venous Drainage
Breast veins are divided into superficial and deep veins. Superficial veins commonly drain the central and peripheral breast areas. The central veins form a venous plexus known as the "circulus venosus of Haller." Blood flows from this venous network into the internal thoracic vein medially, lateral thoracic veins laterally, and the superficial neck veins superiorly. The deep breast veins drain into the internal thoracic, axillary, and posterior intercostal veins. Valves are typically absent in breast veins, and intramammary venous anastomoses are frequently observed.
Lymphatic Drainage
Principal Lymph Nodes Draining the Breasts
Lymph from the breasts drains primarily toward the axillary and internal mammary groups, though a small amount also empties to nearby lymph nodes. These lymph nodes are clinically important, as they are common sites for breast cancer metastasis.[13][14]
- Axillary nodes: The axillary lymph nodes are located in 5 regions in the axillary fat pad. The axillary nodes include the following:
- Pectoral (Anterior or External): This lymph node group lies on the inferior pectoralis minor border along the lateral thoracic vessels and drains the outer quadrants of the breast.
- Subscapular (Posterior or Scapular): This group lies on the axilla's posterior wall along the lower subscapularis margin. The subscapular nodes drain the LOQ.
- Humeral (Lateral): This lymph node group lies over the axilla's lateral wall beside the humerus and drains minimal lymph from the breast.
- Central: This group lies in the axillary base and receives lymph from the anterior, posterior, and lateral lymph node groups.
- Apical (Subclavicular): This lymph node group lies deep in the axilla's apex and receives lymph from all the above lymph nodes. The apical lymph nodes also directly drain the UIQ.
- Interpectoral (Rotter nodes): This group lies between the pectoralis major and minor—the muscles posterior to the breast. These nodes drain the breasts directly. Tumor spread to the Rotter nodes may cause breast cancer recurrence, as these nodes may be missed during surgery.[44]
- Internal mammary (parasternal) nodes: These lymph nodes are situated lateral to the sternum's lateral border, surrounding the internal mammary artery. Some cross to the contralateral side and drain to the contralateral group of parasternal nodes. These nodes drain the inner breast quadrants. This route is often where cancer from one breast metastasizes to the other and becomes bilateral.
The axillary lymph node group is the main breast drainage site, collecting 75% to 80% of lymph from the breasts. The pectoral group drains most of the lymph going to the axillary nodes. The remaining 20% to 25% of breast lymph drains to the internal mammary nodes.[15]
Other Lymph Nodes Draining the Breasts
A small amount of breast lymph drains into the following nodes:
- Supraclavicular nodes
- Cephalic (deltopectoral) nodes
- Posterior intercostal nodes
- Subdiaphragmatic and subperitoneal lymph plexuses
The subdiaphragmatic and subperitoneal lymph plexuses are the routes where breast cancer metastasizes toward the abdomen.
Nerves
The literature has reported vast differences in breast tissue innervation, especially in the areola and nipple. However, most agree that the anterior and lateral cutaneous branches innervate the mammary gland from the 2nd to 6th intercostal and supraclavicular nerves. These nerves form a plexus deep into the areola in the subdermal tissue and supply the nipple and areola.[16][17] The nipple and areola are highly sensitive to touch, and these sensations are carried mainly by the T4 spinal nerve dermatome.
The breast's nerve supply, particularly in the nipple and areolar areas, is the subject of recent studies. Innervation in these regions must be preserved during breast surgical reconstruction.[18][19][20]
Muscles
The mammary gland is a modified sweat gland comprised of parenchymal and stromal tissues covered by skin. As previously mentioned, the nipple area becomes erect when stimulated due to its smooth muscle fibers. Besides these muscles, chest wall skeletal muscles are situated deep to the breasts.
The pectoralis muscle is the predominant skeletal muscle posterior to the mammary gland. The pectoral fascia covers this muscle. The pectoralis minor lies posterolateral to the pectoralis major. Other skeletal muscles deep into the breasts include the serratus anterior and external oblique.
The skeletal muscles in the mammary region are surgically important. Successful breast reconstruction and implantation require an intact pectoralis muscle.[21][22] Meanwhile, the muscle flap most frequently used for breast reconstruction is the latissimus dorsi.[23][24]
Physiologic Variants
The mammary gland shows physiological variations in size, shape, contour, density, spacing, and volume. These variations depend on factors like age, height, weight, genetic composition, race, nourishment, and environment. Breast asymmetry is observed in 25% of females, with the size and shape differing in the same person.
Nipple inversion is also quite frequently seen in females. This condition can be physiological and correct spontaneously over time or during pregnancy. However, nipple inversion may also be an early indicator of underlying pathology.[25]
Surgical Considerations
Surgery, ranging from lumpectomy to radical mastectomy, is the treatment of choice for breast lesions (See Image. Total Mastectomy). As mentioned earlier, breast malignancies can metastasize through the lymphatics (see Image. Cancer Metastasis Sites). Knowledge of the mammary glands' lymphatic drainage helps surgeons identify the affected lymph nodes before dissection.[26]
Recurrence is common, making adjuvant radiotherapy necessary after breast cancer surgery. Patients with a higher recurrence risk may also receive adjuvant chemotherapy, which may prolong their disease-free overall survival period. Hormonal therapy is helpful when breast tumors are estrogen- and progesterone-receptor-positive. Tamoxifen has been proven effective in estrogen-positive breast cancer patients.
The global rise in breast carcinoma cases has resulted in an increase in surgical mastectomy and demand for surgical breast reconstruction, breast implantation, and mammoplasty.[27][28] This trend has resulted in increased interest in understanding breast anatomy and developing new surgical breast treatments.[29][30][31]
Clinical Significance
Breast lesions may be benign or malignant. Most breast lesions are benign, but they have gained little attention because they are usually non-fatal.[32] Fibrocystic changes are the most common benign lesions, constituting almost 40% of all cases, even during the premenopausal years.
Fibrocystic breast changes occur in the duct and lobular epithelia and are classified into proliferative and nonproliferative. Examples of nonproliferative lesions are cystic and fibrotic changes. Epithelial hyperplasia and sclerosing adenosis are examples of proliferative breast changes.
Fibroadenomas are benign stromal tumors constituting 7% of all breast lesions. These outgrowths are more common in young adult women than menopausal women. Fibroadenomas are hormonally responsive, developing during pregnancy and late menstrual cycle stages and regressing after menopause. The phyllodes tumor and intraductal papilloma are other common benign neoplasms.[33]
Breast cancer has generated significant concern worldwide, as it is the most common malignancy after lung cancer and a leading cause of cancer-related deaths among women.[34][35] Breast carcinoma may also affect males, though the risk is lower.[36] The UOQ is the site most commonly affected by breast carcinoma, seen in almost 60% of the cases.[37]
Breast cancer risk factors include age older than 40 years, nulliparity, late first pregnancy, oral contraceptive use, obesity, high-fat diet, positive family history, genetic factors, alcohol consumption, cigarette smoking, and ionizing radiation exposure. Breast cancer is more common in Western countries. Afro-American females have a higher breast cancer risk than Asians and Africans.[38][39]
Malignant lesions are classified into invasive and noninvasive types. Noninvasive types include ductal carcinoma in situ (DCIS), also known as Paget disease of the nipple, and lobular carcinoma in situ (LCIS). Invasive or infiltrative carcinomas have many types, including invasive ductal carcinoma, invasive lobular carcinoma, medullary carcinoma, colloid carcinoma, and tubular carcinoma. Invasive ductal carcinoma accounts for 70% of breast malignancies. A rare mammary gland neoplasm is myoepithelioma, which is diagnosed mainly by immunohistochemical and histological features.[40]
Cancer cells may spread to the spinal cord via the communication between the valveless deep breast veins and the Batson plexus in the vertebral area. Vertebral metastasis can cause spinal fractures that can injure the cord[41]
The prognosis of breast carcinoma in females depends on tumor size, lymph node involvement, tumor grading, the presence of metastasis, and structural features (see Image. Metastasis Sites).[35] Screening helps improve breast cancer prognosis as it increases the likelihood of early detection.
Breast self-examinations increase the likelihood of detecting breast cancer early.[45] Imaging studies used for breast cancer screening include mammography and infrared breast thermography (see Image. Mammogram).[42] In patients younger than 40 years, mammography's sensitivity in detecting breast tumors is low due to high breast tissue density. Ultrasound is the recommended modality in these patients. In individuals who previously had breast implants, magnetic resonance imaging (MRI) is the recommended screening method for breast cancer.[46] A biopsy confirms the diagnosis.[43][44]
Other Issues
Common Congenital Anomalies
Common congenital anomalies include the following:[45][46][45]
- Ectopic breast: The mammary gland develops away from its usual site. Ectopic breasts may appear on another part of the chest or outside the thorax.[47]
- Polymastia (supernumerary breast): The growth of more than one breast on each side.[48]
- Polythelia: Multiple nipples, usually 2 or 3, grow on a single breast. Often, one of them is functional, while the others are rudimentary. Polymastia and polythelia combined occur in 0.2% to 5.6% of women.[49]
- Micromastia: The mammary gland tissue fails to develop fully and remains small.[50]
- Macromastia: The mammary gland grows much more than the usual size and hypertrophies.[51]
- Gynecomastia: Breast enlargement in males, though the glands remain non-functional. About 80% of patients with Klinefelter syndrome have gynecomastia.
- Retracted or inverted nipple: Incomplete nipple development, typically linked to fibrous nipple tethering within a hypoplastic ductal system. [52]
Of these mammary gland conditions, the most common is the ectopic breast.
Other Breast Disorders
Other conditions involving the breasts include the following:
Congenital Hypoplastic Breast Anomalies
Three conditions fall under this category: athelia, amazia, and amastia. Athelia is the absence of the nipple-areolar complex. Amazia is the absence of breast glandular tissue. Amastia is the absence of both the nipple and glandular tissue. These conditions can manifest unilaterally or bilaterally. Congenital breast hypoplasia is typically closely linked to a congenital syndrome or deformity, most commonly ectodermal dysplasia.[53]
Acquired Hypoplastic Breast Anomalies
Acquired hypoplastic breast anomalies include hypomastia and hypoplasia. These conditions can stem from hormonal irregularities, leading to insufficient breast development during puberty. Secondary breast underdevelopment may also arise from iatrogenic causes. Surgical procedures, like chest tube thoracostomy during the early stages of breast development, can cause scarring that impedes normal postpubertal growth.[54]
Poland Syndrome
This congenital condition presents with the absence or underdevelopment of the pectoralis major and associated abnormalities of the pectoralis minor, sternum, ribs, and upper limbs. The breast on the ipsilateral side may also be underdeveloped, though this anomaly may not be clinically evident until after the onset of thelarche.[55][56]
Endocrine Abnormalities
Endocrine disorders affecting breast tissue development include Turner, Noonan, and Van Maldergem syndromes. These conditions are commonly associated with a lack of postpubertal breast development.[57][58][59]
Stenotic Breast (tuberous malformation)
A stenotic breast has an irregular shape and growth pattern, resembling a tuberous plant's root. This condition leads to areolar enlargement and intermammary cleft widening.[60]
Mammary Gland Tuberculosis
Tuberculous mammary gland infection is rare in developed countries, with an incidence of 0.1% of all breast lesions. This condition is more common in developing countries.[61]
Media
(Click Image to Enlarge)
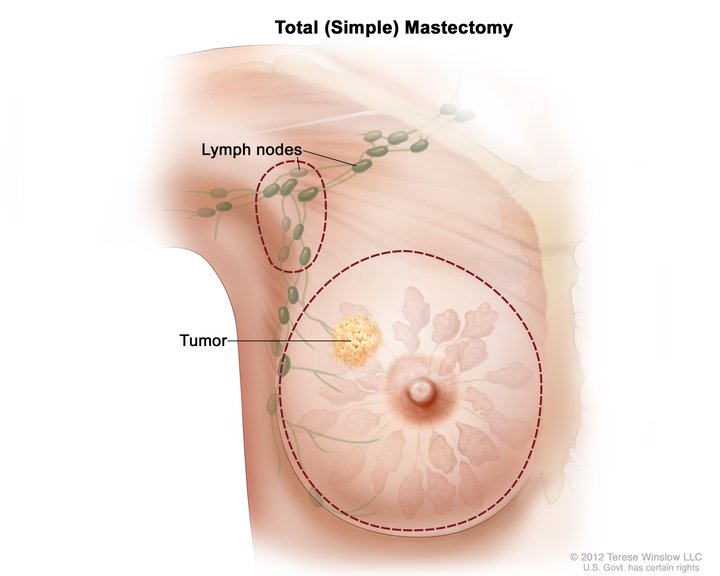
Total Simple Mastectomy. This illustration shows a scheme for removing the breast and lymph nodes in a total (simple) mastectomy. The dotted line shows where the breast incision is made. The procedure may also include axillary lymph node dissection.
National Cancer Institute, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
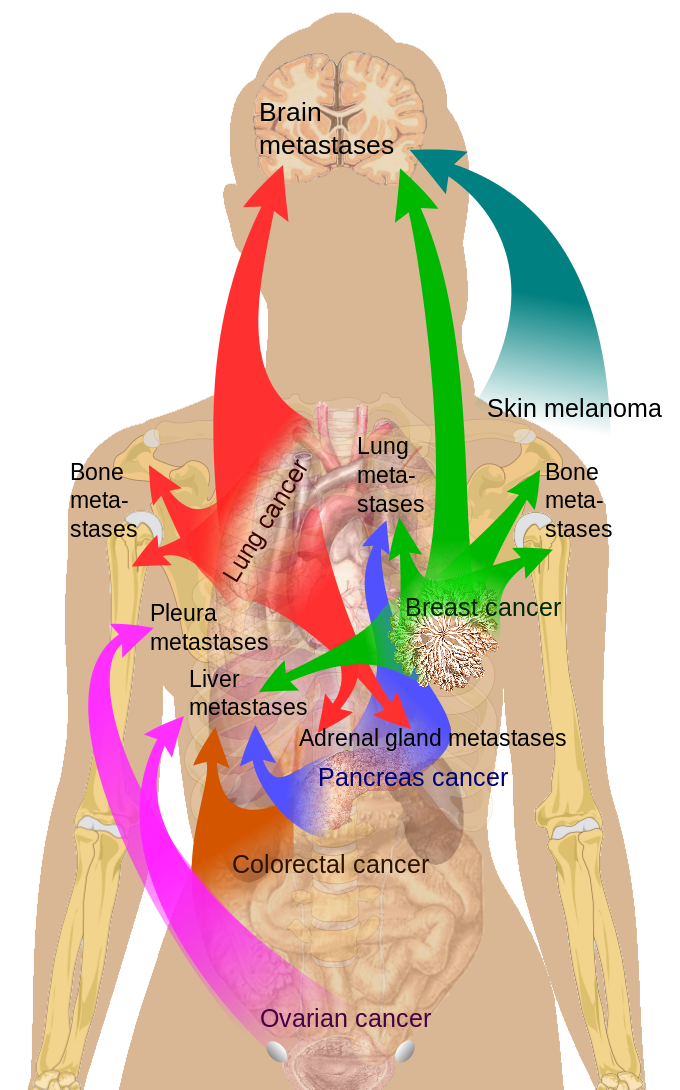
Common Sites of Breast Cancer Metastasis.
Medical Gallery of Mikael Häggström, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
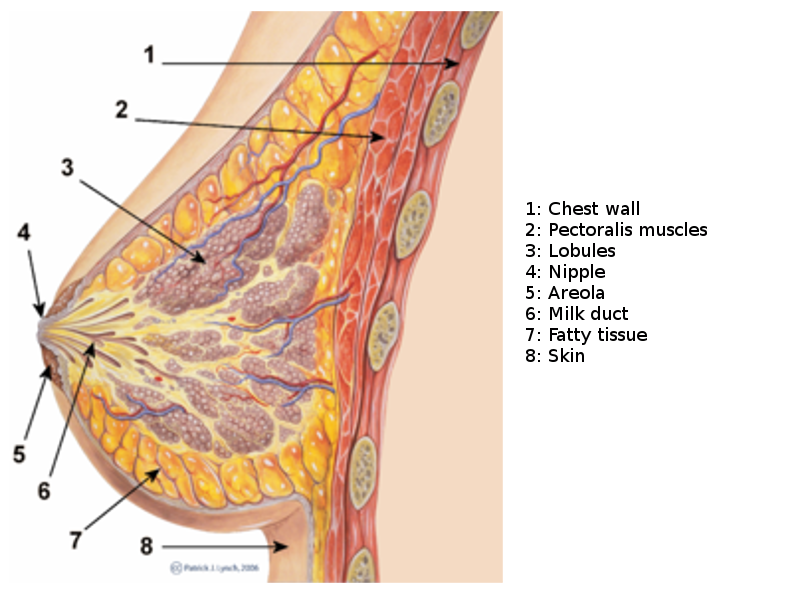
Breast Sagittal View. This illustration shows the chest wall, pectoralis, lobules, nipple, areola, milk duct, fatty tissue, and skin.
PJ Lynch and Morgoth666, Public Domain, via Wikimedia Commons.
References
Davies BP, Crew RC, Cochrane ALK, Davies K, Figueiredo Baptista A, Jeckel S, McCrone IS, Niu Y, Strugnell BW, Waine K, Fowden AL, Bryant CE, Wills JW, Giussani DA, Hughes K. An ovine model for investigation of the microenvironment of the male mammary gland. Journal of anatomy. 2024 Sep:245(3):405-419. doi: 10.1111/joa.14055. Epub 2024 May 12 [PubMed PMID: 38735860]
Hall MI, Suarez-Venot A, Lindvall T, Plochocki JH, Grossman A, Rodriguez-Sosa JR, Voegele GM, Valdez DR, Georgi JA. A reinterpretation of human breast anatomy includes all the layers of the anterior body wall. Anatomical record (Hoboken, N.J. : 2007). 2024 Nov:307(11):3564-3573. doi: 10.1002/ar.25456. Epub 2024 Apr 29 [PubMed PMID: 38682340]
Lee KE, Kang J, Kang WY, Woo OH. A Case Report on Ductal Carcinoma in situ Arising from Axillary Accessory Breast Tissue. Journal of the Korean Society of Radiology. 2025 Jan:86(1):154-159. doi: 10.3348/jksr.2024.0029. Epub 2025 Jan 21 [PubMed PMID: 39958511]
Level 3 (low-level) evidenceAbdelwahab RM, Aghazadeh Mohandesi N, Sturgis CD, Alavi A. Squamous Metaplasia of Lactiferous Ducts (Zuska's Disease) of the Breast: Clinical and Histopathologic Manifestations. Dermatology (Basel, Switzerland). 2024 Nov 14:():1-6. doi: 10.1159/000542622. Epub 2024 Nov 14 [PubMed PMID: 39541956]
Rancati A, Angrigiani C, Lamas G, Rancati A, Berrino V, Barbosa K, Dorr J, Irigo M. Current status of prepectoral breast reconstruction in Argentina. Gland surgery. 2024 Aug 31:13(8):1552-1560. doi: 10.21037/gs-23-291. Epub 2024 Aug 28 [PubMed PMID: 39282046]
Chen J, Zhong Z, Sun Y, Yip J, Yick KL. Dynamic simulation of breast behaviour during different activities based on finite element modelling of multiple components of breast. Scientific reports. 2025 Jan 29:15(1):3659. doi: 10.1038/s41598-024-83598-8. Epub 2025 Jan 29 [PubMed PMID: 39880851]
Soares Domingues Polita B, Lapinš J, Ģīlis A, Grucki M, Irmejs A, Gardovskis J, Maksimenko J. Evaluation of Breast Skin/Nipple-Areolar Complex Sensation and Quality of Life after Nipple-Sparing Mastectomy Followed by Reconstruction. Medicina (Kaunas, Lithuania). 2024 Oct 9:60(10):. doi: 10.3390/medicina60101655. Epub 2024 Oct 9 [PubMed PMID: 39459441]
Level 2 (mid-level) evidenceMcGhee DE, Steele JR. Breast Biomechanics: What Do We Really Know? Physiology (Bethesda, Md.). 2020 Mar 1:35(2):144-156. doi: 10.1152/physiol.00024.2019. Epub [PubMed PMID: 32027563]
Jesinger RA. Breast anatomy for the interventionalist. Techniques in vascular and interventional radiology. 2014 Mar:17(1):3-9. doi: 10.1053/j.tvir.2013.12.002. Epub [PubMed PMID: 24636325]
Ohta T. Characterization of breast changes in the early gestational period on automated breast ultrasound. Journal of medical ultrasonics (2001). 2024 Jan:51(1):103-108. doi: 10.1007/s10396-023-01370-3. Epub 2023 Oct 24 [PubMed PMID: 37875639]
Katulski K, Katulski A, Nykowska A, Beutler K, Kozielek K, Antczak S, Katulska K. Physiological changes in the mammary glands during a female's life. Polish journal of radiology. 2024:89():e386-e390. doi: 10.5114/pjr/189566. Epub 2024 Aug 9 [PubMed PMID: 39257924]
Jayalakshmi J, Thomas TR, N S S, N S S, Rajani CV, K M L, S M, Varghese R, T V A, N A, T D B, Darvin P, Chandrasekhar L. Gross anatomy of vascular supply and drainage of mammary fat pads in mice models. Anatomia, histologia, embryologia. 2024 May:53(3):e13045. doi: 10.1111/ahe.13045. Epub [PubMed PMID: 38735038]
Pandya S, Moore RG. Breast development and anatomy. Clinical obstetrics and gynecology. 2011 Mar:54(1):91-5. doi: 10.1097/GRF.0b013e318207ffe9. Epub [PubMed PMID: 21278507]
Casoli V, Vacher C. [Embryology and anatomy of the thorax and breast]. Annales de chirurgie plastique et esthetique. 2022 Nov:67(5-6):278-290. doi: 10.1016/j.anplas.2022.07.013. Epub 2022 Aug 13 [PubMed PMID: 35970650]
Xing N, Liu D, Chen L, Wang G, Tian Y, Yang C, Leng Y, Jiang X, Li C, Xie R, Nie Z, Zhang T. Quantitative analysis of pressure levels in manual lymphatic drainage across stages of breast cancer-related lymphedema: implications for optimized treatment protocols. Breast cancer research and treatment. 2025 Feb:210(1):95-103. doi: 10.1007/s10549-024-07540-2. Epub 2024 Nov 4 [PubMed PMID: 39489842]
Sarhadi NS, Shaw Dunn J, Lee FD, Soutar DS. An anatomical study of the nerve supply of the breast, including the nipple and areola. British journal of plastic surgery. 1996 Apr:49(3):156-64 [PubMed PMID: 8785595]
Sarhadi NS, Shaw-Dunn J, Soutar DS. Nerve supply of the breast with special reference to the nipple and areola: Sir Astley Cooper revisited. Clinical anatomy (New York, N.Y.). 1997:10(4):283-8 [PubMed PMID: 9213048]
Schulz S, Zeiderman MR, Gunn JS, Riccio CA, Chowdhry S, Brooks R, Choo JH, Wilhelmi BJ. Safe Plastic Surgery of the Breast II: Saving Nipple Sensation. Eplasty. 2017:17():e33 [PubMed PMID: 29213346]
Stromps JP, Bozkurt A, Grieb G, Kim BS, Wiezik M, Pallua N. Spontaneous Reinnervation of Deep Inferior Epigastric Perforator Flaps after Delayed Breast Reconstruction. Journal of reconstructive microsurgery. 2016 Mar:32(3):169-77. doi: 10.1055/s-0035-1564062. Epub 2015 Sep 15 [PubMed PMID: 26372687]
Kostidou E, Schmelz M, Hasemaki N, Kokotis P. Objective Methods for Breast Sensibility Testing. Plastic and reconstructive surgery. 2019 Feb:143(2):398-404. doi: 10.1097/PRS.0000000000005200. Epub [PubMed PMID: 30688881]
Chen G, Zhang Y, Xue J, Zhu X, Liu C, Sun L, Gu X, Zhang H, Liu C. Surgical Outcomes of Implant-based Breast Reconstruction Using TiLoop Bra Mesh Combined With Pectoralis Major Disconnection. Annals of plastic surgery. 2019 Oct:83(4):396-400. doi: 10.1097/SAP.0000000000001867. Epub [PubMed PMID: 31524731]
Leonardis JM, Lyons DA, Giladi AM, Momoh AO, Lipps DB. Functional integrity of the shoulder joint and pectoralis major following subpectoral implant breast reconstruction. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2019 Jul:37(7):1610-1619. doi: 10.1002/jor.24257. Epub 2019 Apr 17 [PubMed PMID: 30816589]
Liu C, Luan J, Ouyang Y, Zhuang Y, Xu B, Chen L, Li S, Fu S, Xin M. Breast Reconstruction in Poland Syndrome Patients with Latissimus Dorsi Myo Flap and Implant: An Efficient Endoscopic Approach Using Single Transverse Axillary Incision. Aesthetic plastic surgery. 2019 Oct:43(5):1186-1194. doi: 10.1007/s00266-019-01346-0. Epub 2019 Mar 14 [PubMed PMID: 30877446]
Delay E, Florzac AS, Frobert P. [Breast reconstruction with the autologous latissimus dorsi flap]. Annales de chirurgie plastique et esthetique. 2018 Nov:63(5-6):422-436. doi: 10.1016/j.anplas.2018.07.004. Epub 2018 Aug 28 [PubMed PMID: 30170856]
Scutt D, Lancaster GA, Manning JT. Breast asymmetry and predisposition to breast cancer. Breast cancer research : BCR. 2006:8(2):R14 [PubMed PMID: 16563179]
Level 2 (mid-level) evidenceAl-Gaithy ZK, Yaghmoor BE, Koumu MI, Alshehri KA, Saqah AA, Alshehri HZ. Trends of mastectomy and breast-conserving surgery and related factors in female breast cancer patients treated at King Abdulaziz University Hospital, Jeddah, Saudi Arabia, 2009-2017: A retrospective cohort study. Annals of medicine and surgery (2012). 2019 May:41():47-52. doi: 10.1016/j.amsu.2019.03.012. Epub 2019 Apr 2 [PubMed PMID: 31245000]
Level 2 (mid-level) evidenceKarabeg R, Jakirlic M, Karabeg A, Crnogorac D, Aslani I. The New Method of Pocket Forming for Breast Implant Placement in Augmentation Mammaplasty: Dual Plane Subfascial. Medical archives (Sarajevo, Bosnia and Herzegovina). 2019 Jun:73(3):178-182. doi: 10.5455/medarh.2019.73.178-182. Epub [PubMed PMID: 31404122]
Nuzzi LC, Firriolo JM, Pike CM, DiVasta AD, Labow BI. Complications and Quality of Life following Reduction Mammaplasty in Adolescents and Young Women. Plastic and reconstructive surgery. 2019 Sep:144(3):572-581. doi: 10.1097/PRS.0000000000005907. Epub [PubMed PMID: 31461005]
Level 2 (mid-level) evidenceSchrauder MG, Brunel-Geuder L, Häberle L, Wunderle M, Hoyer J, Csorba R, Reis A, Schulz-Wendtland R, Beckmann MW, Lux MP. Cost effectiveness of bilateral risk-reducing mastectomy and salpingo-oophorectomy. European journal of medical research. 2019 Sep 14:24(1):32. doi: 10.1186/s40001-019-0391-8. Epub 2019 Sep 14 [PubMed PMID: 31521205]
Pallara T, Cagli B, Fortunato L, Altomare V, Loreti A, Grasso A, Manna E, Persichetti P. Direct-To-Implant and 2-Stage Breast Reconstruction After Nipple Sparing Mastectomy: Results of a Retrospective Comparison. Annals of plastic surgery. 2019 Oct:83(4):392-395. doi: 10.1097/SAP.0000000000001893. Epub [PubMed PMID: 31524730]
Level 2 (mid-level) evidenceGuo R, Li L, Su Y, Xiu B, Zhang Q, Wang J, Chi W, Yang B, Zhang Y, Cao A, Shao Z, Wu J. Current practice and barriers of mesh-assisted implant-based breast reconstruction in China: A nationwide cross-sectional survey of 110 hospitals. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2020 Jan:46(1):65-70. doi: 10.1016/j.ejso.2019.09.001. Epub 2019 Sep 5 [PubMed PMID: 31519428]
Level 2 (mid-level) evidenceGuray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. The oncologist. 2006 May:11(5):435-49 [PubMed PMID: 16720843]
Yao Y, Zhao Y, Guo X, Xu X, Fu B, Cui H, Xue J, Tian J, Lu K, Zhang L. Deep Learning for Distinguishing Mucinous Breast Carcinoma From Fibroadenoma on Ultrasound. Clinical breast cancer. 2025 Jan:25(1):75-84. doi: 10.1016/j.clbc.2024.09.001. Epub 2024 Sep 4 [PubMed PMID: 39317636]
Voicu-Măceşeanu A, Nitu M, Olteanu M, Bică D. [Epidemiology of lung cancer]. Pneumologia (Bucharest, Romania). 2007 Apr-Jun:56(2):78-84 [PubMed PMID: 18019752]
Ahmad A. Breast Cancer Statistics: Recent Trends. Advances in experimental medicine and biology. 2019:1152():1-7. doi: 10.1007/978-3-030-20301-6_1. Epub [PubMed PMID: 31456176]
Level 3 (low-level) evidenceTraoré B, Koulibaly M, Diallo A, Bah M. Molecular profile of breast cancers in Guinean oncological settings. The Pan African medical journal. 2019:33():22. doi: 10.11604/pamj.2019.33.22.18189. Epub 2019 May 14 [PubMed PMID: 31312338]
Dhadlie S, Whitfield J, Hendahewa R. Synchronous bilateral breast cancer: A case report of heterogeneous estrogen receptor status. International journal of surgery case reports. 2018:53():102-106. doi: 10.1016/j.ijscr.2018.10.016. Epub 2018 Oct 24 [PubMed PMID: 30391732]
Level 3 (low-level) evidenceWright N, Akinyemiju T, Subhedar P, Rida P, Aneja R. Targeting risk factors for reducing the racially disparate burden in breast cancer. Frontiers in bioscience (Scholar edition). 2019 Mar 1:11(1):136-160 [PubMed PMID: 30844741]
Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, Alo RA, Payton M, Tchounwou PB. Health and Racial Disparity in Breast Cancer. Advances in experimental medicine and biology. 2019:1152():31-49. doi: 10.1007/978-3-030-20301-6_3. Epub [PubMed PMID: 31456178]
Level 3 (low-level) evidenceHabu T, Soh J, Toji T, Shien K, Niman E, Namba K, Sato H, Yamamoto H, Sugimoto S, Yamane M, Toyooka S. Myoepithelioma occurring in the posterior mediastinum harboring EWSR1 rearrangement: a case report. Japanese journal of clinical oncology. 2018 Sep 1:48(9):851-854. doi: 10.1093/jjco/hyy100. Epub [PubMed PMID: 30053180]
Level 3 (low-level) evidenceJu DG, Yurter A, Gokaslan ZL, Sciubba DM. Diagnosis and surgical management of breast cancer metastatic to the spine. World journal of clinical oncology. 2014 Aug 10:5(3):263-71. doi: 10.5306/wjco.v5.i3.263. Epub [PubMed PMID: 25114843]
Singh D, Singh AK. Role of image thermography in early breast cancer detection- Past, present and future. Computer methods and programs in biomedicine. 2020 Jan:183():105074. doi: 10.1016/j.cmpb.2019.105074. Epub 2019 Sep 7 [PubMed PMID: 31525547]
Biggs KW. Screening for Breast Cancer in Average-Risk Women. Annals of internal medicine. 2019 Sep 17:171(6):449-450. doi: 10.7326/L19-0470. Epub [PubMed PMID: 31525745]
Lee MV, Bennett DL, Appleton CM. Screening for Breast Cancer in Average-Risk Women. Annals of internal medicine. 2019 Sep 17:171(6):451. doi: 10.7326/L19-0471. Epub [PubMed PMID: 31525746]
Kulkarni D, Dixon JM. Congenital abnormalities of the breast. Women's health (London, England). 2012 Jan:8(1):75-86; quiz 87-8. doi: 10.2217/whe.11.84. Epub [PubMed PMID: 22171777]
Wysokinska EM, Keeney G. Breast cancer occurring in the chest wall: rare presentation of ectopic milk line breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Apr 1:32(10):e35-6. doi: 10.1200/JCO.2012.47.8958. Epub 2014 Jan 21 [PubMed PMID: 24449239]
Level 3 (low-level) evidenceDe la Torre M, Lorca-García C, de Tomás E, Berenguer B. Axillary ectopic breast tissue in the adolescent. Pediatric surgery international. 2022 Oct:38(10):1445-1451. doi: 10.1007/s00383-022-05184-1. Epub 2022 Jul 19 [PubMed PMID: 35852592]
Andaloussi MS, Mahdaoui S, Kawtari S, Bouffetal H, Samouh N. A rare case of unilateral axillary supernumerary breast. Radiology case reports. 2021 Sep:16(9):2804-2807. doi: 10.1016/j.radcr.2021.06.074. Epub 2021 Jul 23 [PubMed PMID: 34367399]
Level 3 (low-level) evidenceSanogo SA, Sylla C, Diawara B, Adane A, Bocoum A, Fané S, Tegueté I, Traoré Y, Dembélé BT, Togo AP, Mounkoro N. [Polythelia: About A Case And Litterature Review]. Le Mali medical. 2024:39(2):66-67 [PubMed PMID: 39584624]
Level 3 (low-level) evidenceLi H, Mu D. Analysis of Factors Affecting the Choice of Implant-based or Autologous Fat Grafting Breast Augmentation: A Retrospective Study. The Journal of craniofacial surgery. 2024 Jul-Aug 01:35(5):e447-e450. doi: 10.1097/SCS.0000000000010172. Epub 2024 May 6 [PubMed PMID: 38709046]
Level 2 (mid-level) evidenceAref Y, Bono CM, Najafian A. Back pain in patients with macromastia: what a spine surgeon should know? The spine journal : official journal of the North American Spine Society. 2025 Mar:25(3):403-410. doi: 10.1016/j.spinee.2024.10.009. Epub 2024 Nov 4 [PubMed PMID: 39505016]
Qi F, Zhang F, Zhang Y, Torres-Guzman R, Chaker SC, Lineaweaver W, Liu J. Surgical Management of Postoperative Nipple Necrosis After Inverted Nipple Correction: Experiences From a Series of 25 Cases. Annals of plastic surgery. 2024 Aug 1:93(2S Suppl 1):S43-S46. doi: 10.1097/SAP.0000000000003966. Epub 2024 May 22 [PubMed PMID: 38775260]
Level 2 (mid-level) evidenceIshida LH, Alves HR, Munhoz AM, Kaimoto C, Ishida LC, Saito FL, Gemperlli R, Ferreira MC. Athelia: case report and review of the literature. British journal of plastic surgery. 2005 Sep:58(6):833-7 [PubMed PMID: 15950955]
Level 3 (low-level) evidenceSadove AM, van Aalst JA. Congenital and acquired pediatric breast anomalies: a review of 20 years' experience. Plastic and reconstructive surgery. 2005 Apr:115(4):1039-50 [PubMed PMID: 15793443]
Level 2 (mid-level) evidenceRomanini MV, Calevo MG, Puliti A, Vaccari C, Valle M, Senes F, Torre M. Poland syndrome: A proposed classification system and perspectives on diagnosis and treatment. Seminars in pediatric surgery. 2018 Jun:27(3):189-199. doi: 10.1053/j.sempedsurg.2018.05.007. Epub 2018 Jun 8 [PubMed PMID: 30078491]
Level 3 (low-level) evidenceReisenbichler E, Hanley KZ. Developmental disorders and malformations of the breast. Seminars in diagnostic pathology. 2019 Jan:36(1):11-15. doi: 10.1053/j.semdp.2018.11.007. Epub 2018 Nov 17 [PubMed PMID: 30503250]
Richard-Eaglin A. Male and Female Hypogonadism. The Nursing clinics of North America. 2018 Sep:53(3):395-405. doi: 10.1016/j.cnur.2018.04.006. Epub [PubMed PMID: 30100005]
Sharaf B, Sabbagh MD, Roh SG. Breast reconstruction in a patient with Noonan syndrome. BMJ case reports. 2017 Nov 1:2017():. pii: bcr-2017-222325. doi: 10.1136/bcr-2017-222325. Epub 2017 Nov 1 [PubMed PMID: 29092974]
Level 3 (low-level) evidenceSotos J, Miller K, Corsmeier D, Tokar N, Kelly B, Nadella V, Zhong H, Wetzel A, Adler B, Yu CY, White P. A patient with van Maldergem syndrome with endocrine abnormalities, hypogonadotropic hypogonadism, and breast aplasia/hypoplasia. International journal of pediatric endocrinology. 2017:2017():12. doi: 10.1186/s13633-017-0052-z. Epub 2017 Oct 13 [PubMed PMID: 29046692]
Mandrekas AD, Zambacos GJ. Aesthetic reconstruction of the tuberous breast deformity: a 10-year experience. Aesthetic surgery journal. 2010 Sep:30(5):680-92. doi: 10.1177/1090820X10383397. Epub [PubMed PMID: 20884897]
Galego MA, Lage G, Shekhovtsova M, Duarte R. Tuberculosis of the breast: an uncommon presentation of an old disease. BMJ case reports. 2019 Feb 22:12(2):. doi: 10.1136/bcr-2018-227014. Epub 2019 Feb 22 [PubMed PMID: 30798273]
Level 3 (low-level) evidence