Introduction
This article will review the embryological development of the testicles. However, to understand the embryology of the testicles, it is essential to have a basic understanding of the normal anatomy of the testicles.
The male gonads, otherwise known as the testicles, are sex glands that have both an exocrine secretory function in the production of sperm and an endocrinological function as part of the hypothalamic-pituitary-gonadal axis in men through the production of androgens. The normal anatomy of the testicles is that of an oval shape located in the scrotum, further separated by the scrotal septum. The length of the testis is between 3 cm to 5 cm, whereas the width is between 2 cm to 3 cm.
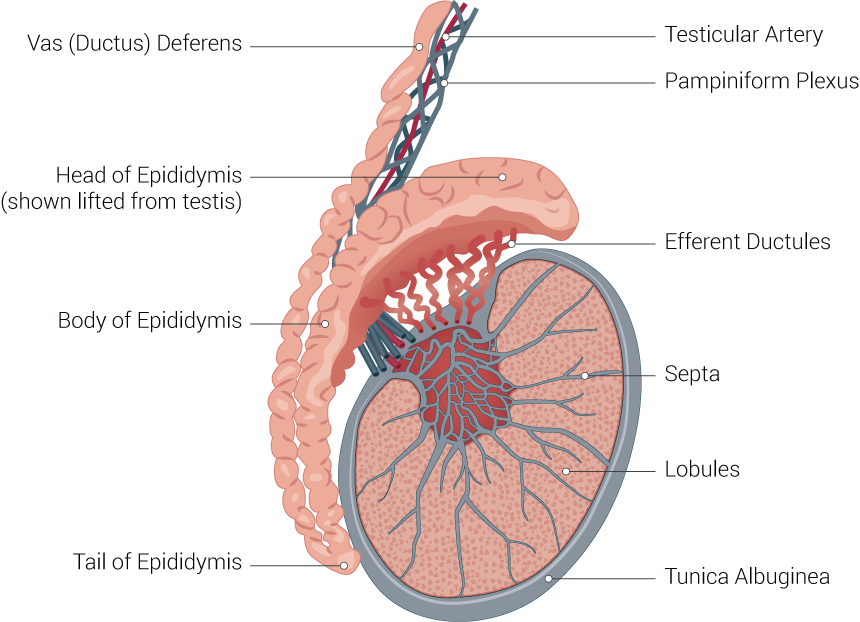
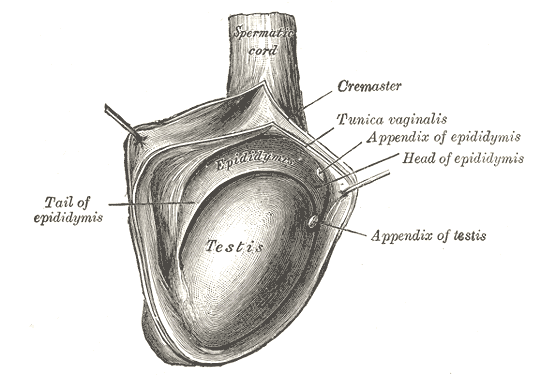
The consistency of normal testicles on palpation is smooth and soft. The testes are suspended superiorly by the spermatic cord and inferior to the scrotum by the scrotal ligament. During embryological development, the scrotal ligament is also known as the gubernaculum.[1][2]
The tunica vaginalis is a double-layered structure that covers all of the testes apart from the posterior and superior borders, which represent the attachment of the epididymis and spermatic cord. The posterior lateral testis has a small space between the body of the epididymis and the testis. This small space is known as the sinus of the epididymis. The tunica albuginea is found deep in the tunica vaginalis. It is a thick fibrous sheath that covers the testes.
The descent of the testicles is a complex stepwise process that involves an interaction between many anatomical structures, environmental influences, regulatory hormones, and inherited genetic factors.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
Development and Sex Determination of Gonads
The beginning of testicular development is with the formation of the genital ridge. The origin of the genital ridge is from the intermediate mesoderm. The intermediate mesoderm forms paired structures that reside beside the dorsal mesentery, specifically in the coelomic cavity.
The proliferation of coelomic epithelial cells found on the ventromedial surface of the mesonephroi initiates the formation of the genital ridge. The mesonephros contains the mesonephric duct (Wolffian duct). This structure is a primordial urogenital tissue that contributes to the formation of the epididymis, seminal vesicles, and vas deferens after male sex determination.[3][4]
The paramesonephric duct (Mullerian duct) is also found in the mesonephros and runs in parallel to the mesonephric duct. This structure is essentially the female equivalent of the mesonephric duct (Wolffian duct) and contributes to the formation of the uterus, fallopian tube, and vagina following female sex determination. The mesonephros and genital ridge form a structure known as the urogenital ridge (UGR).[5]
A dense layer of pseudostratified epithelial cells forms following the proliferation of coelomic epithelial cells, which are present on the ventromedial side of the mesonephros. Basement membrane fragmentation facilitates the migration of epithelial cells dorsally towards the mesonephros.[6] These cells undergo a transition from epithelial type cells to mesenchymal type cells (EMT) and begin to invade the area between coelomic epithelium and mesonephros.
Primordial germ cells (PGC) originate from an area around the yolk sac. PGCs begin to colonize the gonads by approximately five weeks of gestation. By the sixth week of gestation, the PGCs will have invaded the genital ridges. Primordial germ cells divide to reach approximately 3000 cells and undergo further specialization with the activation of gametogenic genes and inactivation of pluripotency genes; this is a process known as licensing.[7]
Following this process of the activation of gametogenic genes and inactivation of pluripotent genes, the primordial germ cells are known as gametogenesis-competent cells, which will either differentiate into male or female specialized cells in response to cues from the cells of the mesonephric tissue.
In genetically male embryos, the primordial germ cells have an XY chromosome complex. In males, there is a sex-specific gene that initiates the determination of gender by activating the production of regulatory sex-determining proteins. This testis-determining factor (TDF) is also known as the sex-determining region of the Y chromosome (SRY). It is the gene resting on the Y chromosome, specifically on its short arm (Yp11).[8] The SRY gene is the only gene on the Y chromosome, which is necessary for testis determination.[9][10] The simple absence of this gene will lead to the development of a female embryo.
The SRY gene encodes for a transcription factor that activates the testis-specific enhancer (TESCO) of a related autosomal gene known as SOX9 (SRY-Box Transcription Factor 9). The SOX9 gene is essential for the initiation of Sertoli cell differentiation from supporting cell precursors. These supporting cells would otherwise differentiate into follicle cells (specifically granuloma cells).[11] Besides SRY, there are other factors such as steroidogenic factor 1 (which is encoded by the gene NR5A1), which contribute to Sertoli cell differentiation.[12] SRY expresses exclusively in the supporting cells in the genital ridges, which differentiate into Sertoli cells.[11]
SRY upregulates levels of SOX9. Once the cellular/tissue levels of SRY and SOX9 are high enough, the SOX9 protein begins to maintain its transcription at high levels in Sertoli cells. Once there are enough SOX9 positive cells in the gonad, it begins to undergo morphological changes, which will lead to the formation of the testes.[13] This process includes the differentiation of interstitial cell lineages (peritubular myoid cells and Leydig cells), the mitotic arrest of germ cells, epithelialization of the Sertoli cells, and the formation of the testicular cords.[14]
By approximately the fifth to the sixth week, the gender of the embryo is determined.
Testicular Descent
There is a biphasic model for the explanation of testicular descent. This process divides into two morphologically and hormonally distinct stages.[15] The first stage of testicular descent (transabdominal) occurs by the eighth gestational week.
Transabdominal Stage
The transabdominal phase of testicular descent consists of the movement of the testes from their starting position on the posterior abdominal wall adjacent to the kidney down to the internal inguinal ring. This phase is dependent on a non-androgenic hormone and starts by the eighth gestational week. Between the eighth and fifteenth gestational weeks, the gubernaculum enlarges causally, leading to the formation of the gubernacular bulb, whereas between the 10th to 15th gestational weeks, the cranial suspensory ligament (CSL) regresses. Regression of the CSL is a necessary step for testicular descent; however, regression of the CSL alone is not sufficient to initiate gonadal descent.[16]
The gubernaculum anchors the testes to the future inguinal region by traction. The gubernaculum shortens as it progressively incorporates into the gubernacular bulb.[17] The enlargement of the gubernaculum during TTD is the "gubernacular swelling reaction" (GSR). Cellular proliferation and extracellular matrix deposition lead to enlargement of the caudal gubernaculum.[18][19] The growth of the gubernaculum is non-androgen dependant. Leydig insulin-like hormone (Insl3) is a "relaxin-like factor" and is a member of the relaxin family of proteins. It contributes to the TTD stage of testicular descent via the activation of the leucine-rich repeat-containing G-protein coupled receptor 8 (LGR8).[20]
Inguinoscrotal Stage
The second stage of testicular descent (inguinoscrotal) begins by the 26th gestational week. This stage is androgen-dependent and consists of the passage of the testes from the internal inguinal ring down into the scrotum.[21] The inguinoscrotal stage of testicular descent (ISTD) has specific pre-requisite steps, includes the formation of the processus vaginalis, dilation of the inguinal canal by the gubernacular bulb, and abdominal pressure to push the testes through the inguinal canal. The processus vaginalis is a herniation of the abdominal peritoneum. It develops as the gubernaculum elongates caudally. When the processus vaginalis enters the caudal abdominal wall, the development of the inguinal canal starts. After testicular descent has finished, the gubernaculum regresses, leaving behind a remnant testo-scrotal attachment. The contraction of the gubernaculum, along with intra-abdominal pressure, facilitates the movement of the testes through the inguinal canal into the scrotum.
The scrotum is effectively a continuation of skin and subcutaneous tissue lining the anterior abdominal wall. As the testes descend through the inguinal canal, it takes layers of the abdominal wall with it. The layers of the abdominal wall involved in testicular descent continue as analogous layers in the scrotum. The processus vaginalis obliterates through a process of programmed smooth muscle cell death in response to a decrease in androgen levels in the third trimester.[22] From superficial to deep, the Scarpa's fascia of the abdominal wall contributes to the dartos fascia and smooth muscle of the scrotum. The fascia of the external oblique muscle contributes to the external spermatic fascia. The internal oblique and transversus abdominis muscles and aponeurosis contribute to the cremasteric fascia and muscle. The transversalis fascia contributes to the internal spermatic fascia. The peritoneum of the abdominal wall contributes to the tunica vaginalis.[23]
In the majority of fetuses, the testes will have descended into the scrotum by the 33rd gestational week.[24] Whereas by the 25th + 4 gestational week, only 7.7% of testes will have descended into the scrotum.[25] By the 27th gestational week, only 12.5% of the testes will have descended into the scrotum.[25] Hence by birth, the testes will be in the scrotum, and it is advisable for the pediatricians to routinely check the scrotum of the newborn to confirm the descent.
Cellular
Sertoli Cells
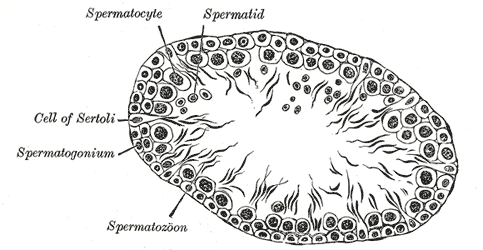
Sertoli cells are epithelial supportive cells found in the seminiferous tubules. They support the germ cells by providing the required nutrients to complete spermatogenesis.[26] Sertoli cells also undertake a range of other roles, such as contributing to the formation of the blood-testis barrier, phagocytosis of apoptotic spermatocytes, and the secretion of endocrinological signals.[26]
Also known as sustentacular cells, they are the only somatic cells in the entire seminiferous epithelium.[27] They are pyramidal-shaped, with an oval nucleus towards the base. The cells rest on a basement membrane. A large number of germ cells under different stages of development, especially the spermatids, are seen to be embedded near its apex. The spermogonia and spermatocytes invaginate near the base. The base of the Sertoli cells points towards the peritubular myoid cells (PMCs) surrounding the seminiferous tubule, and the apex points towards the lumen of the seminiferous tubule.[26] Inter-Sertolian junctions at the basal lamina of the seminiferous tubules contribute to the formation of the blood-testis barrier. Sertoli cells transport new primary spermatocytes through an active process towards the adluminal compartment of the seminiferous tubules.[28]
Sertoli cells have an epithelial nature. Sertoli cells are the target of hormones such as FSH and testosterone. This activity leads to the regulation of spermatogenesis through regulatory cross-talk between Sertoli cells and germ cells.[29]
Functions of The Sertoli Cells
- Not only provide support to the developing germ cells but also house them, provide with required nutrition, and protect them.[30]
- Secrete lipids, carbohydrates, vitamins, amino acids, and metal ions required for the proper development of the developing germ cells.[31]
- Regulate cholesterol metabolism during spermatogenesis, which plays a vital role in sperm production and male fertility.[31]
- Secrete hormone inhibin B, which helps in suppressing the secretion of FSH, thus bettering the function of Sertoli cells, spermatogenic status, and sperm number.[32][33]
- Act as macrophages by engulfing the apoptotic bodies, bacteria, all the dead, and worn-out cells.[34]
- Secrete an androgen-binding protein (ABP) [31]
- Convey the mature germ cells to the lumen to be transported further.
Leydig Cells
The primary source of steroid hormones in a normal male is the Leydig cell. The normal function of the Leydig cell through the production of steroid hormones ensures normal reproductive function and fertility in males.[35] Besides the production of steroid hormones, the Leydig cells have many other essential functions, such as the production of POMC derivatives, calcium-binding proteins, and growth factors.[36]
They are generally large polyhedral, pale cells with an eccentric nucleus lying in the connective tissue present between the seminiferous tubules. The cytoplasm contains rod-shaped Reinke's crystalloid bodies and vacuoles, which are yellowish in color containing various enzymes.[37] Mature adult Leydig cells originate from undifferentiated mesenchymal-like stem cells, which are present in the interstitial part of the early testis.[38]
Molecular Level
As mentioned earlier, there are specific genetic and molecular messengers that are essential for normal testicular descent.
SRY
In 1990 Sinclair et al. identified a gene on the Y chromosome, which they named the sex-determining region of the Y chromosome.[9] This gene is within a 35-kb sex-determining region on the Y chromosome. This gene encodes a 204-amino acid protein which has a molecular mass of 24kD. A DNA binding HMG motif is in the middle of this protein.[39] It is a member of the high mobility group (HMG)-box of DNA binding proteins.
The SRY gene becomes transactivated by the product of the WT1, which binds to the SRY promoter region.[40] SRY binds to a testis-specific enhancer of SOX9 core (TESCO) in mice along with steroidogenic factor 1 (SF1).[12] SF1 and SRY work to upregulate levels of SOX9. When the production of SRY reduces SOX9, and SF1 maintains its expression.
SOX9
The SOX9 (SRY-Box Transcription Factor 9) gene encodes a 509 amino acid long transcription factor, which is necessary for sexual development. It is found on the long arm of chromosome 17 and is a member of HMG transcription factors having a molecular mass of 56kD.[41][42]The SRY gene initiates a sequence of gene interactions along with SOX9, which is necessary for testicular development.[12]
SRY begins a feed-forward loop between Glia-activating factor (FGF9) and SOX9; this causes the upregulation of FGF9 and inhibits WNT4. WNT4 promotes female sex development, whereas FGF9 stimulates male sex development. This upregulation of FGF9 and SOX9 and downregulation of WNT4 stimulate testis development.[43] In conjunction with steroidogenic factor 1, SOX9 can stimulate the production of anti-mullerian hormone (AMH) to inhibit female sexual differentiation.[44]
SF1
Steroidogenic factor 1 (SF1), also known as NR5A1, is a transcription factor that consists of 461 amino acids.[45] SF1 is present in primary steroidogenic tissues, including the adrenal cortex, ovarian theca cells, and Leydig cells of the testis. Along with SOX9, SF1 regulates the production of AMH.[46][44]
AMH
Anti-Mullerian hormone (AMH), also known as Mullerian inhibiting substance (MIS) is a 560 amino acid long protein.[47] The gene for AMH is on chromosome 19.[48] AMH functions to prevent the development of the Mullerian ducts into female structures.[49] The suppression of the development of Mullerian structures takes place within the first eight weeks of development. AMH is also produced in motor neurons and is suspected to be responsible for subtle behavioral differences between males and females.[50][51]
INSL3
Insulin-like peptide 3 (INSL3), otherwise known as Leydig cell-specific relaxin-like factor, is a hormone encoded by the INSL3 gene found on chromosome 19.[52] It has a similar structure to insulin and relaxin and belongs to the “insulin-like hormone superfamily,” which includes other hormones such as insulin, relaxin, and insulin-like growth factors (I and II).[53] INSL3 is essential for the development of the gubernaculum in male embryos and is a crucial factor in testicular descent.[54]
Function
The Normal Function of the Testes
There are two main functions of the testes. These are the endocrine production of androgens and exocrine production of spermatozoa.
The endocrine function of the testis is under neuroendocrine control. The production of gonadotrophin-releasing hormone (GnRH) by the anterior hypothalamus stimulates the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the anterior pituitary.[55] LH and FSH bind to receptors found on Sertoli and Leydig cells. Luteinizing hormone stimulates Leydig cells to produce testosterone, whereas FSH stimulates Sertoli cells to produce androgen binding proteins (ABPs) along with the regulation of spermatogenesis.[56]
Endocrine Function
GnRH is released in repeated pulses in response to excitatory neurotransmitters such as glutamate and kisspeptin.[57][58] GnRH binds to a G-protein seven-transmembrane receptor (GPCR) known as the gonadotrophin-releasing hormone receptor (GnRHR) on gonadotrophic cells of the anterior pituitary. FSH becomes stimulated when GnRH pulses are a low frequency, whereas stimulation of LH occurs when GnRH pulses are at a high frequency.[59][60]
Luteinizing hormone stimulates the production of androgens in Leydig cells by binding to a GPCR.[61] During testicular development, blood testosterone levels peak three times. The first time is between 12 to 14 weeks of gestation when Leydig cell development occurs.[62] The second time is two months post-partum, which leads to the proliferation of Leydig cells. However, there is subsequent atrophy of Leydig cells leading to the interstitium of the testis becoming inhabited by inactive precursor cells. Mature adult Leydig cells develop during puberty.[63] The third peak of testosterone is during the onset of puberty.
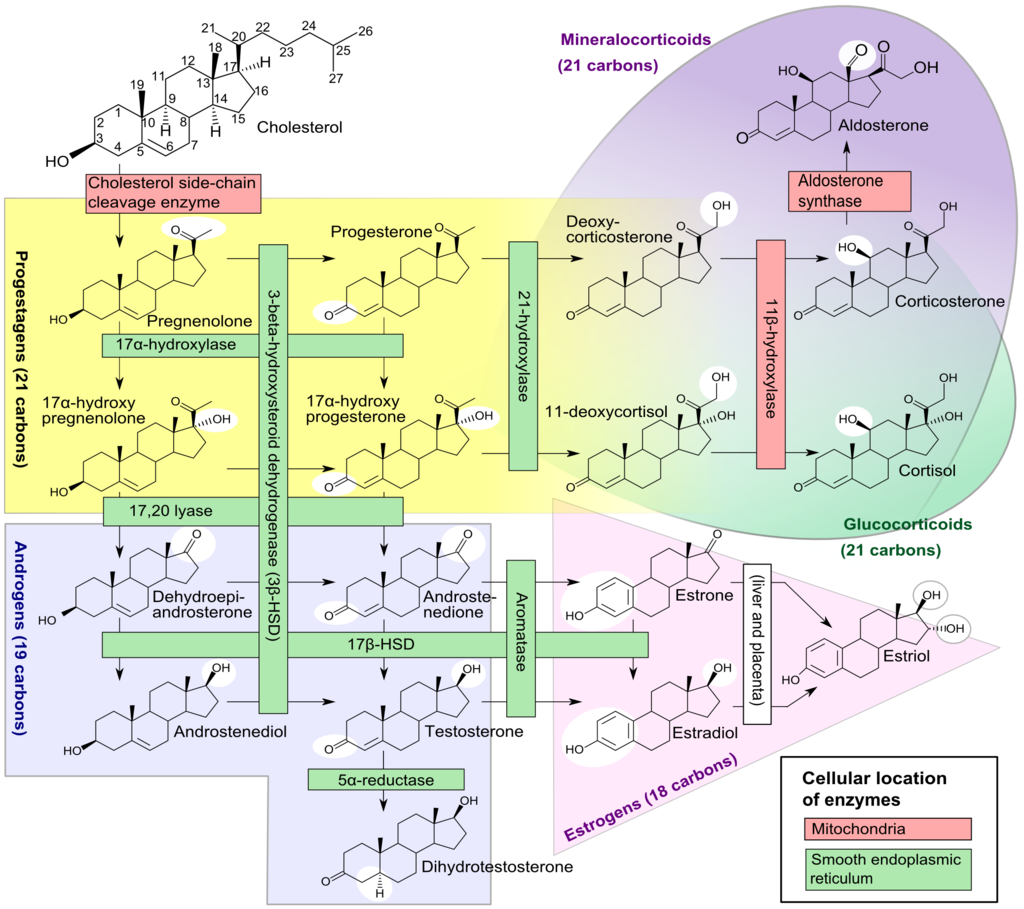
Leydig cell androgen production is dependent on the activity of four enzymes. These are cytochrome P450 17a-hydroxylase (P450 17a), P450 side-chain cleavage enzyme (P450scc), 17b-hydroxysteroid dehydrogenase (17b HSD) and 3b-hydroxysteroid dehydrogenase (3b-HSD).[64] Leydig cells convert cholesterol into pregnenolone, and pregnenolone then converts into progesterone. Progesterone then metabolizes into testosterone. A mutation in the genes that code for the enzymes P450 17a, P450scc, or 3b-HSD can cause congenital adrenal hyperplasia, whereas a mutation in the gene for 17b-HSD causes 17b-HSD deficiency. These can all present with impaired virilization.
Exocrine Function
Spermatozoa are produced from spermatogonia through a stepwise process. The spermatogonia are at the basement membrane of the seminiferous tubules. The spermatogonia can be either stem cells (type A spermatogonia) or differentiated spermatogonia (Type B spermatogonia). Type A spermatogonia either produce more type A spermatogonia or type B spermatogonia.[65][66][67] Type B spermatogonia divide and produce primary spermatocytes. The primary spermatocytes then enter the prophase of meiosis 1. During this period, they move away from the basement membrane towards the lumen of the seminiferous tubule.
After completing the prophase of meiosis 1, the spermatocytes rapidly complete meiosis 1. This process results in the formation of haploid secondary spermatocytes. The secondary spermatocytes complete meiosis 2, leading to the production of four haploid spermatids from one primary spermatocyte.[55] Spermatids undergo spermiogenesis, the process during which the spermatids become spermatozoa and acquire the characteristic features of spermatozoa, such as the formation of a tail and loss of cytoplasm.[55] Sertoli cells, also known as ‘nursemaid cells,’ provide nutrients, growth factors, and organization to the developing spermatids.[68]
Functional Significance of Testicular Descent
According to the ‘temperature-dependent hypothesis,’ testicular descent is necessary because a low-temperature environment is needed to produce viable sperm. Normal spermatogenesis and storage in the epididymis require temperatures below that of the core body temperature.[69][70][71]
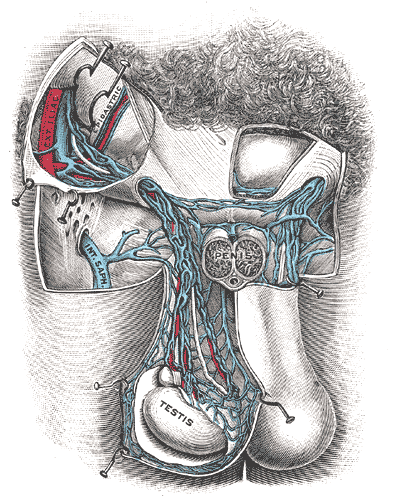
A testicular temperature of 2 to 7 degrees C below core body temperature is a requirement for effective spermatogenesis.[72][69] The testes have anatomical features that optimize temperature regulation. Thin scrotal skin with a large number of sweat glands, the pampiniform plexus which is a venous plexus surrounding the testes, the cremaster muscle which will contract or relax in response to temperature, the tunica dartos, and a lack of adipose tissue also have an essential role in regulating the temperature.[69]
Testing
Imaging
In the context of undescended testis, imaging methods can serve to determine the anatomical location of undescended testes; this allows for better pre-surgical planning and reduces the extent of exploration and anesthesia time.[73] Magnetic resonance imagining (MRI) and ultrasound (US) are useful in diagnosing and locating undescended testis. These techniques are preferable to others as they are non-invasive and do not use ionizing radiation. The diagnostic accuracy of MRI and US are similar to other methods.[74][75][74]
Karyotyping
Chromosomal changes are detectable through the use of a technique known as karyotyping, which allows the identification of the chromosomes so that in situations where there may be an extra or missing chromosome, it is detectable through karyotyping.[76] Karyotyping is a recommended investigation in all patients who present with disorders of sexual differentiation such as cryptorchidism and proximal hypospadias.[77]
Pathophysiology
Testicular Developmental Disorders
Testicular Dysgenesis
Testicular dysgenesis syndrome (TDS) is a constellation of poor-quality semen, testicular germ cell cancer, cryptorchidism, and hypospadias. It is a consequence of disruption to embryonal programming and gonad development.[78] The belief is that testicular dysgenesis syndrome is the result of environmental disrupting chemicals (EDCs), intrauterine growth disorders, genetic and lifestyle factors.
Reduced production of androgen receptors, disrupted hormone levels along with exposure to endocrine disrupters are associated with the development of TDS.[79] The thought is that this occurs due to Leydig-cell dysfunction and reduced INSL3 secretion from Leydig cells, which consequently leads to the disorders associated with TDS.[80] Furthermore, a disruption in Sertoli cell development contributes to the development of TDS.[81]
As mentioned before, researchers believe that EDCs contribute to the development of TDS through the interference with the production, transport, metabolism, binding, release, action, and elimination of hormones associated with developmental processes.[82] This result can be through anti-androgenic effects, estrogenic effects of steroidogenic enzyme inhibitory effects.[83][84]
There are historical examples of maternal exposure to chemicals contributing the testicular developmental disorders; the synthetic estrogenic drug known as diethylstilboestrol (DES) was historically used for the prevention of the complications of pregnancy but was shown to increase the risk of testicular malformations.[85] Another environmental toxin that appears to contribute to testicular malformations is bisphenol A (BPA). This chemical has weak estrogenic effects and is used in the production of polycarbonates and epoxy resins.[86] Phthalate esters are used in vinyl plastics to improve their flexibility. However, it seems that they have an endocrine-disrupting effect.[79]
Cryptorchidism
It is a state wherein there is an absence of the testes in the scrotum may be unilaterally or bilaterally. The etiology of cryptorchidism can divide into anatomical, genetic, or hormonal causes.[87][88] Anatomical causes of cryptorchidism include conditions that cause an anatomical abnormality of the inguinal canal, vas deferens, or testis themselves.[89] Genetic causes of cryptorchidism include 5α-reductase gene mutations, androgen receptor mutations, mutations in INSL3 genes.[90][91][90][92] Hormonal causes of cryptorchidism include a deficiency or insensitivity to AMH, INSL3, androgen, or GNRH/LH.[87][93][94] Cryptorchidism is problematic as it can lead to an increased likelihood of infertility and an increased likelihood of developing testicular cancer.[95][93]
As the main functional significance of the descent of the testes according to the ‘temperature-dependent hypothesis’ is the maintenance of a low-temperature environment to produce viable sperm, failure of testicular descent will lead to an increase in the testicular temperature. An increase in testicular temperature causes a reduction in effective spermatogenesis. Furthermore, damage to Sertoli cells may occur due to the production of reactive oxygen species.[69][96]
Cryptorchidism appears to demonstrate associations with an increased risk of testicular intraepithelial neoplasm (TIN), which may lead to testicular carcinoma in situ (CIS).[97] It is thought that the high temperatures associated with cryptorchidism lead to a failure of the development of neonatal gonocytes to differentiate into primary spermatogonia, which allows some gonocytes to persist as a potential source of malignancy.[95][93]
Anorchia
By definition, anorchia is the absence of testes in a person who has a 46 XY karyotype and an external male phenotype. It is also known as vanishing testis syndrome or embryonic testicular regression.[98][99][100] The exact etiology of congenital anorchia is not well known. However, there appears to be a genetic component due to the familial inheritance associated with anorchia.[98] However, in previous studies, mutations in genes such as SRY, INSL3/INSL3 receptor do not have an association with anorchia.[101] But mutations in genes such as NR5A1, which codes for SF1, correlate with anorchia.[102]
Because patients with anorchia have a normal male phenotype, scientists suspect that the testes were functionally normal during some point of embryological development.[103] Other suspected etiologies are testicular torsion, vascular occlusion, and trauma during the descent of the testes.[104]
Monorchism
Monorchism is, by definition, the state of only having one testis in the scrotum. Monorchism may result from trauma, testicular torsion, or surgical removal because of testicular malignancy.[105] Congenital monorchism is when a child is born with only one testis in the scrotum; this can be due to cryptorchidism or vascular pathology, which causes unilateral regression of the testis (anorchia/vanishing testis syndrome) such as spermatic cord torsion or thrombosis.[106][107] Monorchism may also occur in disorders of sexual development (DSD).[108] However, endocrine abnormalities are unlikely to be the sole cause of unilateral congenital monorchism. Men with congenital monorchism do not have a significant difference in hormonal excretory function compared to men with two testes.[109]
Polyorchidism
Polyorchidism refers to a congenital anomaly in which more than two testes are present in the scrotum.[110] Even though this is a very rare condition; 40 % of polyorchidism is associated with undescended testes.[111] The most common type of polyorchidism is triorchidism, which refers to the presence of three testicles.[112] It appears that polyorchidism occurs due to abnormal separation of the genital ridge during the development of the testes in the embryo.[113]
Leung described a way of classifying polyorchidism based on embryological differentiation.[114]
- Type I: An extra testis without an epididymis or vas which attaches to the ipsilateral testis.
- Type II: The extra testis drains through the epididymis of the ipsilateral testis, and they share a common vas deferens.
- Type III: The extra testis has its own epididymis separate from the ipsilateral testis, both of those structures draining into a common vas deferens.
- Type IV: The extra testis has a separate epididymis and vas deferens from the ipsilateral testis.
Testicular Torsion
Testicular torsion is a state in which the spermatic cord twisted upon itself, leading to a restriction of blood supply and venous drainage of the ipsilateral testis. If emergency treatment is not delivered quickly, it may lead to necrosis of the testis.
Researchers believe that the majority of postnatal testicular torsion is associated with the "bell clapper anomaly."[115] Hypermobility of the testicle is thought to be inherited through an X-linked dominant or Y-linked mechanism.[116][117][118] The bell clapper anomaly refers to the posterior attachment of the parietal lamina of the tunica vaginalis (TVPL). In a normal testis, the TVPL is attached to the posterolateral aspect of the testis, whereas in patients with the bell clapper anomaly, the TVPL attachment encircles and connects proximally to the spermatic cord, which leads to free-hanging testis in the tunica vaginalis.[119]
Testicular torsion is extravaginal or intravaginal.[120] Extravaginal testicular torsion occurs more frequently in neonates, it is due to the testis, epididymis, and tunica vaginalis twisting along with the spermatic cord.[121] In contrast, intravaginal testicular torsion occurs secondary to the bell clapper deformity.[120]
Swyer Syndrome
Swyer syndrome, also known as pure gonadal dysgenesis, occurs when there is abnormal sexual differentiation during embryological development, leading to insufficient intrauterine virilization with undifferentiated gonads with associated female phenotype.[122] The external genitalia is non-ambiguous and well-formed; however, the gonads are dysgenic and improperly formed.[123] Patients with Swyer syndrome have an XY 46 karyotype.
Swyer syndrome occurs due to reduced production of testosterone and AMH leading to a failure of gonadal differentiation.[122] Particular mutations associated with Swyer syndrome include mutations in NR5A1 (which encodes for SF1), WNT4, and the SRY gene, which mutates in 10 to 15% of patients with Swyer syndrome.[124][123]
Klinefelters Syndrome
Klinefelters syndrome is a common congenital chromosomal disorder with an incidence of 0.1% to 0.2% of newborns.[125] It is a common cause of primary hypogonadism.[126] The majority of patients (80%) have the 47 XXY karyotype, whereas 20% of patients with Klinefelter have supernumerary X chromosomes (49 XXXXY or 48 XXXY), mosaicism of 47 XXY/46XY, or more than one Y chromosome (XXYY).[126][127][128]
Androgen Insensitivity Syndrome
A child can have a disorder of sexual development (DSD) without a chromosomal abnormality such as Klinefelters syndrome. The most commonly seen 46 XY DSD is androgen insensitivity syndrome (AIS).[129] The severity of AIS can range from complete androgen insensitivity syndrome (CAIS), in which female genitalia is present, to mild androgen insensitivity syndrome (MAIS) in which normal external male genitalia is present with or without gynecomastia.[130][131]
AIS occurs due to molecular defects in the gene that codes for the androgen receptor (AR).[132] The gene for the AR is on the X chromosome at position Xq11-12.[133] Mutations in the gene that provides information for the AR known as NR3C4 cause AIS.[134] The AR is a nuclear receptor that binds to androgens such as testosterone and dihydrotestosterone (DHT).
As patients with AIS have a Y chromosome, they will produce male gonads. However, as the fetal cells do not respond to androgens, there will be abnormal development of the reproductive system. This situation may manifest as undescended testes. Furthermore, as androgens play a part in the inhibition of female characteristics through SOX9 and SF1, a loss of response to androgens may lead to a female phenotype.[135]
5-ARD Deficiency
The enzyme 5-alpha reductase 2 (5alpha-RD2) converts testosterone into DHT.[136] As mentioned earlier, androgens are essential during the development of male genitalia. A failure of the conversion of testosterone into DHT will lead to abnormal development of the genitalia.
5-alpha reductase deficiency is a 46 XY DSD. It presents with a female phenotype or improperly virilized external genitalia along with testes. The gene for 5 alpha-RD2, also known as SRD5A2, is found on the second chromosome on position 2p23, it is an autosomal recessive condition.[137][136]
Congenital Adrenal Hyperplasia
The most common inborn error of adrenal function is congenital adrenal hyperplasia (CAH) secondary to a deficiency of the steroid 21-hydroxylase enzyme.[138] This condition occurs due to deletions or mutations in the gene CYP21A2, which codes for the steroid 21-hydroxylase enzyme found on chromosome 6p21.[139][140] This mutation leads to an impairment of the production of cortisol and aldosterone alongside excessive adrenal androgen production. However, this form of congenital adrenal hyperplasia does not cause anatomical disorders in males.
In infant females, the most common form of congenital adrenal hyperplasia (21-hydroxylase deficiency) may present with a salt-wasting disease or virilization of the external genitalia. In both males and females, it can present with precocious puberty.[140]
Other less common forms of congenital adrenal hyperplasia include the 3b-hydroxysteroid dehydrogenase (3b-HSD) deficiency which occurs due to mutations in the HSD3B2 gene, the 17a-hydroxylase (P450 17a) deficiency which occurs due to mutations in the CYP17A1 gene, and the P450 side-chain cleavage enzyme (P450scc) deficiency which occurs due to mutations in the CYP11A1 gene.[140]
A deficiency in the 3b-HSD enzyme causes a failure of the conversion of 17-a hydroxypregnenolone to 17a hydroxyprogesterone, pregnenolone to progesterone, and DHEA to androstenedione, resulting in the accumulation of pregnenolone, DHEA, and 17-a hydroxypregnenolone and a reduction in the production of sex steroids. It may also cause salt-wasting disease.[141] Reduced production of androgenic steroid hormones may present as pseudohermaphroditism associated with cryptorchidism.[141]
A deficiency in the P450 17a enzyme leads to a failure of the conversion of pregnenolone to 17a-hydroxypregnenolone, progesterone to 17a- hydroxyprogesterone, and reduced conversion of 17-hydroxypregnenolone into DHEA. This process leads to the accumulation of pregnenolone, progesterone, and reduced production of glucocorticoids and sex steroids, leading to incomplete virilization in males associated with ambiguous external genitalia and cryptorchidism.[142][143]
A deficiency in the P450scc enzyme leads to a failure of the conversion of cholesterol into pregnenolone; this is the first step in the synthesis of all steroid hormones. As a result, there is a severe deficiency in steroid hormones.[144] Regardless of the chromosomal gender, children with a mutation in P450scc are born phenotypically female.[145] However, due to a failure of the production of androgens, the testes fail to descend.[146]
Other Related Conditions
Congenital Inguinal Hernia
The lifetime risk of inguinal hernias is significantly higher in men (27%) than in women (3%).[147] As such, one of the main risk factors for a primary inguinal hernia is the male gender.[148] Other risk factors for primary inguinal hernias include patency of the processus vaginalis. Obliteration of the processus vaginalis occurs typically by the third trimester through programmed cell death of smooth muscle cells.[149] Non-obliteration of this process vaginalis results in abnormal protruding of abdominal contents through the inguinal canal into the scrotum.
Clinical Significance
Testicular Developmental Disorders
Cryptorchidism
Congenital undescended testes (cryptorchidism) is a very common congenital urogenital malformation with a prevalence among normal birth weight boys varying between 1.8 to 8.4%.[150]
By definition, cryptorchidism is a condition where either one or both of the testes do not descend into the scrotum.[151] The undescended testes can be found in the usual route of descent or an abnormal ectopic position, leading to a way of classifying undescended testes according to their position:
- Cryptorchid – This refers to a testis that is not in the scrotum and cannot be manipulated so that they enter the scrotum.
- Ectopic – This refers to a testis that has not followed the regular developmental route of testicular descent. This condition can be femoral, pubopenile, crossed scrotal, or perineal.[152]
- Gliding – This refers to a testis that can be manipulated to enter into the upper scrotum, but with the removal of manipulation, they retract.[153]
- Retractile – This refers to the testis, which can be manipulated to enter into the scrotum; they do not retract when tension is released.
- Acquired – This refers to the testis, which initially had normal descent that ascends spontaneously.[154]
The diagnosis of cryptorchidism is by manual palpation; this is an essential part of the newborn physical examination and should be diagnosed during infancy.[155] Further investigation of undescended testicles includes various imaging techniques such as ultrasound, CT, and MRI.[73][156]
Anorchia
Anorchia occurs in 1/20,000 males and is also associated with 1/177 of those with cryptorchidism.[157][158] The majority of patients with anorchia have a normal phenotype, but some may present with abnormalities of the external genitalia.[159] The characteristic phenotype in a patient with anorchia is the absence of gonads despite having an XY karyotype. Most cases are associated with small tubular rudimentary Mullerian/Wolffian remnants. The phenotypical presentation can range from phenotypic females with a small, well-formed uterus to a phenotypical male with no testes.[160]
It is crucial to distinguish anorchia from cryptorchidism by examination of the abdomen and inguinal canal. Furthermore, as congenital adrenal hyperplasia can present similarly, plasma 17a-hydroxylase levels should be determined to rule out CAH. The diagnosis of anorchia can be made through undetectable plasma levels of AMH and inhibin B associated with raised plasma levels of FSH.[98] Testosterone therapy can be given to ensure the patient reaches a preferable height during puberty.
Monorchism
Clinically, it is not always possible to determine the exact etiology of monorchism as a child presenting with monorchism may have cryptorchidism, testicular agenesis, or VTS. Further investigation, such as laparoscopy or imaging techniques, may be performed. However, the accuracy of imaging techniques and the invasive nature of diagnostic laparoscopy means are not optimal methods for determining the cause of monorchism.[161] Alternative methods to determine the cause of monorchism is the volume of the contralateral testis in adults. However, in younger patients, this is not useful in predicting the cause of monorchism.[162][161] Fixation of the contralateral testicle is considered in children with monorchism to reduce the risk of torsion later in life to avoid a total loss of reproductive and endocrine function.[105]
Polyorchidism
The majority of patients with polyorchidism are asymptomatic.[163] It is usually a coincidental finding on the investigation of other symptoms such as hernia (30%), torsion (15%), mal-descent (40%), malignancy (6%), and hydrocele (9%).[164][165][166] In the majority of cases, the supernumerary testis is found on the left side [167]. The supernumerary testis may be found in the inguinal region (23%), scrotum (66%), or the abdomen (9%).[164][165]
Testicular Torsion
The prevalence of testicular torsion in males below the age of 25 years is between 2.9 to 3.8 patients per 100,000 people.[168][169][170] The classical clinical presentation of testicular torsion is acute severe scrotal pain, which occurs at rest, associated with some type of trauma. The severe pain can cause the patient to become nauseous or to vomit.[171] Testicular torsion tends to be associated with an absent cremasteric reflex.[171] Testicular torsion has a significant impact on the patient's future fertility and can be associated with psychological trauma.[172] Surgical intervention in testicular torsion has to be prompt. There is a 90 to 100% salvage rate if operation occurs within 6 hours of the onset of testicular torsion. The salvage rate reduces with time.[173]
Testicular torsion is diagnosable using a color doppler ultrasound; this is the gold standard for the evaluation of the acute scrotum.[174] Testicular torsion management involves emergency surgical exploration. Following this, the testis is untwisted and wrapped in a warm soaked gauze. The testis is then observed to see if there is an improvement in the color of the testis. To reduce the likelihood of the contralateral testis undergoing torsion, it is fixed along with the testis, which underwent torsion.[171]
Swyer Syndrome
Swyer syndrome has an incidence of 1 in 80,0000.[122] Swyer syndrome patients present with an above average height, underdeveloped breasts with normal axillary/pubic hair. The external genitalia is distinctly female; however, the uterus is small. The underdeveloped gonads are dysgenetic strips that do not have any endocrinological or reproductive function.[122] Patients with Swyer syndrome have an increased incidence of gonadal tumors such as gonadoblastomas or dysgerminomas.[122] One-third of patients with Swyer syndrome may suffer from dysgerminomas.[122]
Klinefelters Syndrome
In Klinefelter's syndrome, the primordial germ cells fail to develop appropriately such that by puberty, no germ cells remain, resulting in insufficient spermatogenesis.[175] Klinefelters syndrome is also associated with characteristic phenotypical features such as [125]:
- Small firm testes
- Above-average height
- Erectile issues
- Sparse male-pattern body hair
- Bilateral gynecomastia
- Infertility
5-ARD Deficiency
The phenotypical presentation of 5-ARD deficiency does not tend to match the genotypical presentation such that patients with the same genotype may present with different phenotypes.[176] However, phenotypical presentations include ambiguous genitalia with unfused labioscrotal folds resembling the labia majora or a phallus with a clitoris-like appearance.[177] Nevertheless, the internal genitalia such as the vas deferens, epididymis will be present. Undescended testes are also associated with 5-ARD deficiency.[177]
Congenital Adrenal Hyperplasia
A deficiency in 3B-HSD accounts for between 1% and 10% of CAH cases.[178][179] A deficiency in the P450 17a enzyme accounts for approximately 1% of CAH cases.[180] Deficiencies in the P50scc enzyme are incredibly rare and tend to present with a significant clinical variation.[146]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Tsili AC, Sofikitis N, Stiliara E, Argyropoulou MI. MRI of testicular malignancies. Abdominal radiology (New York). 2019 Mar:44(3):1070-1082. doi: 10.1007/s00261-018-1816-5. Epub [PubMed PMID: 30386879]
Mäkelä JA, Koskenniemi JJ, Virtanen HE, Toppari J. Testis Development. Endocrine reviews. 2019 Aug 1:40(4):857-905. doi: 10.1210/er.2018-00140. Epub [PubMed PMID: 30590466]
Yang Y, Workman S, Wilson M. The molecular pathways underlying early gonadal development. Journal of molecular endocrinology. 2018 Jul 24:():. pii: JME-17-0314. doi: 10.1530/JME-17-0314. Epub 2018 Jul 24 [PubMed PMID: 30042122]
Avellar MCW, Ribeiro CM, Dias-da-Silva MR, Silva EJR. In search of new paradigms for epididymal health and disease: innate immunity, inflammatory mediators, and steroid hormones. Andrology. 2019 Sep:7(5):690-702. doi: 10.1111/andr.12654. Epub 2019 Jun 17 [PubMed PMID: 31207127]
Acién P. Embryological observations on the female genital tract. Human reproduction (Oxford, England). 1992 Apr:7(4):437-45 [PubMed PMID: 1522183]
Gropp A, Ohno S. The presence of a common embryonic blastema for ovarian and testicular parenchymal (follicular, interstitial and tubular) cells in cattle Bos taurus. Zeitschrift fur Zellforschung und mikroskopische Anatomie (Vienna, Austria : 1948). 1966:74(4):505-28 [PubMed PMID: 5988471]
Level 3 (low-level) evidenceGill ME, Hu YC, Lin Y, Page DC. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proceedings of the National Academy of Sciences of the United States of America. 2011 May 3:108(18):7443-8. doi: 10.1073/pnas.1104501108. Epub 2011 Apr 19 [PubMed PMID: 21504946]
Level 3 (low-level) evidenceHoare BS, Khan YS. Anatomy, Abdomen and Pelvis: Female Internal Genitals. StatPearls. 2025 Jan:(): [PubMed PMID: 32119488]
Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19:346(6281):240-4 [PubMed PMID: 1695712]
Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19:346(6281):245-50 [PubMed PMID: 2374589]
Level 3 (low-level) evidenceAlbrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Developmental biology. 2001 Dec 1:240(1):92-107 [PubMed PMID: 11784049]
Level 3 (low-level) evidenceSekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008 Jun 12:453(7197):930-4. doi: 10.1038/nature06944. Epub 2008 May 4 [PubMed PMID: 18454134]
Level 3 (low-level) evidenceWarr N, Greenfield A. The molecular and cellular basis of gonadal sex reversal in mice and humans. Wiley interdisciplinary reviews. Developmental biology. 2012 Jul-Aug:1(4):559-77. doi: 10.1002/wdev.42. Epub 2012 Feb 28 [PubMed PMID: 23801533]
Level 3 (low-level) evidenceLarney C, Bailey TL, Koopman P. Switching on sex: transcriptional regulation of the testis-determining gene Sry. Development (Cambridge, England). 2014 Jun:141(11):2195-205. doi: 10.1242/dev.107052. Epub [PubMed PMID: 24866114]
Level 3 (low-level) evidenceHutson JM. A biphasic model for the hormonal control of testicular descent. Lancet (London, England). 1985 Aug 24:2(8452):419-21 [PubMed PMID: 2863447]
Level 3 (low-level) evidenceEmmen JM, McLuskey A, Grootegoed JA, Brinkmann AO. Androgen action during male sex differentiation includes suppression of cranial suspensory ligament development. Human reproduction (Oxford, England). 1998 May:13(5):1272-80 [PubMed PMID: 9647559]
Level 3 (low-level) evidenceWensing CJ. Testicular descent in some domestic mammals. 3. Search for the factors that regulate the gubernacular reaction. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. Series C. Biological and medical sciences. 1973:76(2):196-202 [PubMed PMID: 4267621]
Level 3 (low-level) evidenceHeyns CF, Human HJ, De Klerk DP. Hyperplasia and hypertrophy of the gubernaculum during testicular descent in the fetus. The Journal of urology. 1986 May:135(5):1043-7 [PubMed PMID: 2421019]
Level 3 (low-level) evidenceHeyns CF, Human HJ, Werely CJ, De Klerk DP. The glycosaminoglycans of the gubernaculum during testicular descent in the fetus. The Journal of urology. 1990 Mar:143(3):612-7 [PubMed PMID: 2106043]
Level 3 (low-level) evidenceKumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. The Journal of biological chemistry. 2002 Aug 30:277(35):31283-6 [PubMed PMID: 12114498]
Level 3 (low-level) evidenceHeyns CF. The gubernaculum during testicular descent in the human fetus. Journal of anatomy. 1987 Aug:153():93-112 [PubMed PMID: 2892824]
Tanyel FC. Obliteration of processus vaginalis: aberrations in the regulatory mechanism result in an inguinal hernia, hydrocele or undescended testis. The Turkish journal of pediatrics. 2004:46 Suppl():18-27 [PubMed PMID: 15499794]
Patel AP. Anatomy and physiology of chronic scrotal pain. Translational andrology and urology. 2017 May:6(Suppl 1):S51-S56. doi: 10.21037/tau.2017.05.32. Epub [PubMed PMID: 28725619]
Malas MA, Sulak O, Oztürk A. The growth of the testes during the fetal period. BJU international. 1999 Oct:84(6):689-92 [PubMed PMID: 10510117]
Nemec SF, Nemec U, Weber M, Kasprian G, Brugger PC, Krestan CR, Rotmensch S, Rimoin DL, Graham JM Jr, Prayer D. Male sexual development in utero: testicular descent on prenatal magnetic resonance imaging. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011 Dec:38(6):688-94. doi: 10.1002/uog.8964. Epub 2011 Feb 17 [PubMed PMID: 21337441]
Level 2 (mid-level) evidenceBarrionuevo F, Burgos M, Jiménez R. Origin and function of embryonic Sertoli cells. Biomolecular concepts. 2011 Dec 1:2(6):537-47. doi: 10.1515/BMC.2011.044. Epub [PubMed PMID: 25962053]
Chojnacka K, Zarzycka M, Mruk DD. Biology of the Sertoli Cell in the Fetal, Pubertal, and Adult Mammalian Testis. Results and problems in cell differentiation. 2016:58():225-51. doi: 10.1007/978-3-319-31973-5_9. Epub [PubMed PMID: 27300181]
Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiological reviews. 2002 Oct:82(4):825-74 [PubMed PMID: 12270945]
Level 3 (low-level) evidenceSilva FR, Leite LD, Wassermann GF. Rapid signal transduction in Sertoli cells. European journal of endocrinology. 2002 Sep:147(3):425-33 [PubMed PMID: 12213681]
Level 3 (low-level) evidenceNi FD, Hao SL, Yang WX. Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell death & disease. 2019 Jul 17:10(8):541. doi: 10.1038/s41419-019-1782-z. Epub 2019 Jul 17 [PubMed PMID: 31316051]
Shi JF, Li YK, Ren K, Xie YJ, Yin WD, Mo ZC. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis (Review). Molecular medicine reports. 2018 Jan:17(1):705-713. doi: 10.3892/mmr.2017.8000. Epub 2017 Nov 7 [PubMed PMID: 29115523]
Makanji Y, Zhu J, Mishra R, Holmquist C, Wong WP, Schwartz NB, Mayo KE, Woodruff TK. Inhibin at 90: from discovery to clinical application, a historical review. Endocrine reviews. 2014 Oct:35(5):747-94. doi: 10.1210/er.2014-1003. Epub 2014 Jul 22 [PubMed PMID: 25051334]
O'Connor AE, De Kretser DM. Inhibins in normal male physiology. Seminars in reproductive medicine. 2004 Aug:22(3):177-85 [PubMed PMID: 15319820]
Level 3 (low-level) evidenceShiratsuchi A, Osada Y, Nakanishi Y. Differences in the mode of phagocytosis of bacteria between macrophages and testicular Sertoli cells. Drug discoveries & therapeutics. 2013 Apr:7(2):73-7 [PubMed PMID: 23715505]
Level 3 (low-level) evidenceSvechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, Söder O. Origin, development and regulation of human Leydig cells. Hormone research in paediatrics. 2010:73(2):93-101. doi: 10.1159/000277141. Epub 2010 Feb 9 [PubMed PMID: 20190545]
Davidoff MS, Schulze W, Middendorff R, Holstein AF. The Leydig cell of the human testis--a new member of the diffuse neuroendocrine system. Cell and tissue research. 1993 Mar:271(3):429-39 [PubMed PMID: 8472301]
Mahran AM, Elgamal DA, Ghafeer HH, Abdel-Maksoud SA, Farrag AA. Histological alterations in Leydig cells and macrophages in azoospermic men. Andrologia. 2017 Oct:49(8):. doi: 10.1111/and.12714. Epub 2016 Oct 6 [PubMed PMID: 27709649]
Chen H, Wang Y, Ge R, Zirkin BR. Leydig cell stem cells: Identification, proliferation and differentiation. Molecular and cellular endocrinology. 2017 Apr 15:445():65-73. doi: 10.1016/j.mce.2016.10.010. Epub 2016 Oct 12 [PubMed PMID: 27743991]
Su H, Lau YF. Identification of the transcriptional unit, structural organization, and promoter sequence of the human sex-determining region Y (SRY) gene, using a reverse genetic approach. American journal of human genetics. 1993 Jan:52(1):24-38 [PubMed PMID: 8434602]
Level 3 (low-level) evidenceHossain A, Saunders GF. The human sex-determining gene SRY is a direct target of WT1. The Journal of biological chemistry. 2001 May 18:276(20):16817-23 [PubMed PMID: 11278460]
Massardier J, Roth P, Michel-Calemard L, Rudigoz RC, Bouvier R, Dijoud F, Arnould P, Combourieu D, Gaucherand P. Campomelic dysplasia: echographic suspicion in the first trimester of pregnancy and final diagnosis of two cases. Fetal diagnosis and therapy. 2008:24(4):452-7. doi: 10.1159/000176299. Epub 2008 Nov 26 [PubMed PMID: 19033726]
Level 3 (low-level) evidenceWagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994 Dec 16:79(6):1111-20 [PubMed PMID: 8001137]
Level 3 (low-level) evidenceKim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS biology. 2006 Jun:4(6):e187 [PubMed PMID: 16700629]
Level 3 (low-level) evidenceDe Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Molecular and cellular biology. 1998 Nov:18(11):6653-65 [PubMed PMID: 9774680]
Level 3 (low-level) evidenceOba K, Yanase T, Nomura M, Morohashi K, Takayanagi R, Nawata H. Structural characterization of human Ad4bp (SF-1) gene. Biochemical and biophysical research communications. 1996 Sep 4:226(1):261-7 [PubMed PMID: 8806624]
Level 3 (low-level) evidenceShen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994 Jun 3:77(5):651-61 [PubMed PMID: 8205615]
Level 3 (low-level) evidenceCate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6:45(5):685-98 [PubMed PMID: 3754790]
Level 3 (low-level) evidenceCohen-Haguenauer O, Picard JY, Mattéi MG, Serero S, Nguyen VC, de Tand MF, Guerrier D, Hors-Cayla MC, Josso N, Frézal J. Mapping of the gene for anti-müllerian hormone to the short arm of human chromosome 19. Cytogenetics and cell genetics. 1987:44(1):2-6 [PubMed PMID: 3028714]
Level 3 (low-level) evidenceBehringer RR. The in vivo roles of müllerian-inhibiting substance. Current topics in developmental biology. 1994:29():171-87 [PubMed PMID: 7828438]
Level 3 (low-level) evidenceWang PY, Koishi K, McGeachie AB, Kimber M, Maclaughlin DT, Donahoe PK, McLennan IS. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2005 Nov 8:102(45):16421-5 [PubMed PMID: 16260730]
Level 3 (low-level) evidenceWang PY, Protheroe A, Clarkson AN, Imhoff F, Koishi K, McLennan IS. Müllerian inhibiting substance contributes to sex-linked biases in the brain and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2009 Apr 28:106(17):7203-8. doi: 10.1073/pnas.0902253106. Epub 2009 Apr 9 [PubMed PMID: 19359476]
Level 3 (low-level) evidenceBurkhardt E, Adham IM, Brosig B, Gastmann A, Mattei MG, Engel W. Structural organization of the porcine and human genes coding for a Leydig cell-specific insulin-like peptide (LEY I-L) and chromosomal localization of the human gene (INSL3). Genomics. 1994 Mar 1:20(1):13-9 [PubMed PMID: 8020942]
Level 3 (low-level) evidenceAdham IM, Burkhardt E, Benahmed M, Engel W. Cloning of a cDNA for a novel insulin-like peptide of the testicular Leydig cells. The Journal of biological chemistry. 1993 Dec 15:268(35):26668-72 [PubMed PMID: 8253799]
Level 3 (low-level) evidenceKlonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Developmental biology. 2004 Jun 1:270(1):1-18 [PubMed PMID: 15136137]
Level 3 (low-level) evidenceShalet SM. Normal testicular function and spermatogenesis. Pediatric blood & cancer. 2009 Aug:53(2):285-8. doi: 10.1002/pbc.22000. Epub [PubMed PMID: 19343782]
Ulloa-Aguirre A, Reiter E, Crépieux P. FSH Receptor Signaling: Complexity of Interactions and Signal Diversity. Endocrinology. 2018 Aug 1:159(8):3020-3035. doi: 10.1210/en.2018-00452. Epub [PubMed PMID: 29982321]
Urbański HF, Kohama SG, Garyfallou VT. Mechanisms mediating the response of GnRH neurones to excitatory amino acids. Reviews of reproduction. 1996 Sep:1(3):173-81 [PubMed PMID: 9414455]
Level 3 (low-level) evidenceKauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends in neurosciences. 2007 Oct:30(10):504-11 [PubMed PMID: 17904653]
Level 3 (low-level) evidenceWildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981 Aug:109(2):376-85 [PubMed PMID: 6788538]
Level 3 (low-level) evidenceSavoy-Moore RT, Swartz KH. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Advances in experimental medicine and biology. 1987:219():641-5 [PubMed PMID: 3124522]
Level 3 (low-level) evidenceDufau ML. The luteinizing hormone receptor. Annual review of physiology. 1998:60():461-96 [PubMed PMID: 9558473]
Level 3 (low-level) evidenceMüller J, Skakkebaek NE. The prenatal and postnatal development of the testis. Bailliere's clinical endocrinology and metabolism. 1992 Apr:6(2):251-71 [PubMed PMID: 1616445]
Prince FP. Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. The Anatomical record. 1990 Dec:228(4):405-17 [PubMed PMID: 2178325]
Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation. Biology of reproduction. 2018 Jul 1:99(1):101-111. doi: 10.1093/biolre/ioy059. Epub [PubMed PMID: 29566165]
Clermont Y. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops). The American journal of anatomy. 1969 Sep:126(1):57-71 [PubMed PMID: 4982042]
Level 3 (low-level) evidenceClermont Y, Antar M. Duration of the cycle of the seminiferous epithelium and the spermatogonial renewal in the monkey Macaca arctoides. The American journal of anatomy. 1973 Feb:136(2):153-65 [PubMed PMID: 4684877]
Level 3 (low-level) evidenceSchulze C. Morphological characteristics of the spermatogonial stem cells in man. Cell and tissue research. 1979 May 18:198(2):191-9 [PubMed PMID: 466665]
Cortes D, Müller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. International journal of andrology. 1987 Aug:10(4):589-96 [PubMed PMID: 3654012]
Setchell BP. The Parkes Lecture. Heat and the testis. Journal of reproduction and fertility. 1998 Nov:114(2):179-94 [PubMed PMID: 10070346]
Level 3 (low-level) evidenceEHRENBERG L, VON EHRENSTEIN G, HEDGRAN A. Gonad temperature and spontaneous mutation-rate in man. Nature. 1957 Dec 21:180(4599):1433-4 [PubMed PMID: 13493561]
Speit G. Effects of temperature on sister chromatid exchanges. Human genetics. 1980:55(3):333-6 [PubMed PMID: 7203467]
Level 3 (low-level) evidenceRitzén EM. Undescended testes: a consensus on management. European journal of endocrinology. 2008 Dec:159 Suppl 1():S87-90. doi: 10.1530/EJE-08-0181. Epub 2008 Aug 26 [PubMed PMID: 18728121]
Level 3 (low-level) evidenceKanemoto K, Hayashi Y, Kojima Y, Maruyama T, Ito M, Kohri K. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of non-palpable testis. International journal of urology : official journal of the Japanese Urological Association. 2005 Jul:12(7):668-72 [PubMed PMID: 16045560]
Sarihan H, Sari A, Abeş M, Dinç H. Nonpalpable undescending testis. Value of magnetic resonance imaging. Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 1998 Dec:50(4):233-6 [PubMed PMID: 9973808]
Maghnie M, Vanzulli A, Paesano P, Bragheri R, Palladini G, Preti P, Del Maschio A, Severi F. The accuracy of magnetic resonance imaging and ultrasonography compared with surgical findings in the localization of the undescended testis. Archives of pediatrics & adolescent medicine. 1994 Jul:148(7):699-703 [PubMed PMID: 7912611]
Schreck RR, Distèche C. Karyotyping. Current protocols in human genetics. 2001 May:Appendix 4():Appendix 4A. doi: 10.1002/0471142905.hga04as18. Epub [PubMed PMID: 18428228]
Cox MJ, Coplen DE, Austin PF. The incidence of disorders of sexual differentiation and chromosomal abnormalities of cryptorchidism and hypospadias stratified by meatal location. The Journal of urology. 2008 Dec:180(6):2649-52; discussion 2652. doi: 10.1016/j.juro.2008.08.058. Epub 2008 Oct 31 [PubMed PMID: 18951572]
Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human reproduction (Oxford, England). 2001 May:16(5):972-8 [PubMed PMID: 11331648]
Level 3 (low-level) evidenceXing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder? Life sciences. 2018 Feb 1:194():120-129. doi: 10.1016/j.lfs.2017.11.039. Epub 2017 Nov 26 [PubMed PMID: 29183799]
van den Driesche S, Kolovos P, Platts S, Drake AJ, Sharpe RM. Inter-relationship between testicular dysgenesis and Leydig cell function in the masculinization programming window in the rat. PloS one. 2012:7(1):e30111. doi: 10.1371/journal.pone.0030111. Epub 2012 Jan 11 [PubMed PMID: 22253897]
Level 3 (low-level) evidenceChen GR, Dong L, Ge RS, Hardy MP. [Relationship between phthalates and testicular dysgenesis syndrome]. Zhonghua nan ke xue = National journal of andrology. 2007 Mar:13(3):195-200 [PubMed PMID: 17393778]
He M, Yang C, Geng R, Zhao X, Hong L, Piao X, Chen T, Quinto M, Li D. Monitoring of phthalates in foodstuffs using gas purge microsyringe extraction coupled with GC-MS. Analytica chimica acta. 2015 Jun 16:879():63-8. doi: 10.1016/j.aca.2015.02.066. Epub 2015 Feb 27 [PubMed PMID: 26002478]
Cevasco A, Urbatzka R, Bottero S, Massari A, Pedemonte F, Kloas W, Mandich A. Endocrine disrupting chemicals (EDC) with (anti)estrogenic and (anti)androgenic modes of action affecting reproductive biology of Xenopus laevis: II. Effects on gonad histomorphology. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2008 Mar:147(2):241-51 [PubMed PMID: 18032117]
Level 3 (low-level) evidenceMills LJ, Gutjahr-Gobell RE, Zaroogian GE, Horowitz DB, Laws SC. Modulation of aromatase activity as a mode of action for endocrine disrupting chemicals in a marine fish. Aquatic toxicology (Amsterdam, Netherlands). 2014 Feb:147():140-50. doi: 10.1016/j.aquatox.2013.12.023. Epub 2013 Dec 31 [PubMed PMID: 24418745]
Level 3 (low-level) evidenceTroisi R, Hyer M, Hatch EE, Titus-Ernstoff L, Palmer JR, Strohsnitter WC, Herbst AL, Adam E, Hoover RN. Medical conditions among adult offspring prenatally exposed to diethylstilbestrol. Epidemiology (Cambridge, Mass.). 2013 May:24(3):430-8. doi: 10.1097/EDE.0b013e318289bdf7. Epub [PubMed PMID: 23474687]
Cabaton NJ, Canlet C, Wadia PR, Tremblay-Franco M, Gautier R, Molina J, Sonnenschein C, Cravedi JP, Rubin BS, Soto AM, Zalko D. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environmental health perspectives. 2013 May:121(5):586-93. doi: 10.1289/ehp.1205588. Epub 2013 Feb 21 [PubMed PMID: 23425943]
Level 3 (low-level) evidenceVirtanen HE, Bjerknes R, Cortes D, Jørgensen N, Rajpert-De Meyts E, Thorsson AV, Thorup J, Main KM. Cryptorchidism: classification, prevalence and long-term consequences. Acta paediatrica (Oslo, Norway : 1992). 2007 May:96(5):611-6 [PubMed PMID: 17462053]
Level 3 (low-level) evidenceBerkowitz GS, Lapinski RH, Dolgin SE, Gazella JG, Bodian CA, Holzman IR. Prevalence and natural history of cryptorchidism. Pediatrics. 1993 Jul:92(1):44-9 [PubMed PMID: 8100060]
Niedzielski JK, Oszukowska E, Słowikowska-Hilczer J. Undescended testis - current trends and guidelines: a review of the literature. Archives of medical science : AMS. 2016 Jun 1:12(3):667-77. doi: 10.5114/aoms.2016.59940. Epub 2016 May 18 [PubMed PMID: 27279862]
Suzuki Y, Sasagawa I, Itoh K, Ashida J, Ogata T. 5Alpha-reductase type 2 genes in Japanese males do not appear to be associated with cryptorchidism. Fertility and sterility. 2002 Aug:78(2):330-4 [PubMed PMID: 12137870]
Level 1 (high-level) evidenceSilva-Ramos M, Oliveira JM, Cabeda JM, Reis A, Soares J, Pimenta A. The CAG repeat within the androgen receptor gene and its relationship to cryptorchidism. International braz j urol : official journal of the Brazilian Society of Urology. 2006 May-Jun:32(3):330-4; discussion 335 [PubMed PMID: 16813680]
Level 2 (mid-level) evidenceZimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Molecular endocrinology (Baltimore, Md.). 1999 May:13(5):681-91 [PubMed PMID: 10319319]
Level 3 (low-level) evidenceNef S, Parada LF. Hormones in male sexual development. Genes & development. 2000 Dec 15:14(24):3075-86 [PubMed PMID: 11124800]
Level 3 (low-level) evidenceHutson JM, Hasthorpe S. Testicular descent and cryptorchidism: the state of the art in 2004. Journal of pediatric surgery. 2005 Feb:40(2):297-302 [PubMed PMID: 15750918]
Level 3 (low-level) evidenceHutson JM, Li R, Southwell BR, Petersen BL, Thorup J, Cortes D. Germ cell development in the postnatal testis: the key to prevent malignancy in cryptorchidism? Frontiers in endocrinology. 2012:3():176. doi: 10.3389/fendo.2012.00176. Epub 2013 Jan 3 [PubMed PMID: 23316184]
Ivell R, Hartung S. The molecular basis of cryptorchidism. Molecular human reproduction. 2003 Apr:9(4):175-81 [PubMed PMID: 12651898]
Level 3 (low-level) evidenceWood HM, Elder JS. Cryptorchidism and testicular cancer: separating fact from fiction. The Journal of urology. 2009 Feb:181(2):452-61. doi: 10.1016/j.juro.2008.10.074. Epub 2008 Dec 13 [PubMed PMID: 19084853]
Brauner R, Neve M, Allali S, Trivin C, Lottmann H, Bashamboo A, McElreavey K. Clinical, biological and genetic analysis of anorchia in 26 boys. PloS one. 2011:6(8):e23292. doi: 10.1371/journal.pone.0023292. Epub 2011 Aug 10 [PubMed PMID: 21853106]
Abeyaratne MR, Aherne WA, Scott JE. The vanishing testis. Lancet (London, England). 1969 Oct 18:2(7625):822-4 [PubMed PMID: 4186283]
Sarto GE, Opitz JM. The XY gonadal agenesis syndrome. Journal of medical genetics. 1973 Sep:10(3):288-93 [PubMed PMID: 4774539]
Vinci G, Anjot MN, Trivin C, Lottmann H, Brauner R, McElreavey K. An analysis of the genetic factors involved in testicular descent in a cohort of 14 male patients with anorchia. The Journal of clinical endocrinology and metabolism. 2004 Dec:89(12):6282-5 [PubMed PMID: 15579790]
Level 2 (mid-level) evidencePhilibert P, Zenaty D, Lin L, Soskin S, Audran F, Léger J, Achermann JC, Sultan C. Mutational analysis of steroidogenic factor 1 (NR5a1) in 24 boys with bilateral anorchia: a French collaborative study. Human reproduction (Oxford, England). 2007 Dec:22(12):3255-61 [PubMed PMID: 17940071]
Level 3 (low-level) evidenceFouatih K, Belin F, Lambert AS, Bouligand J, Bouvattier C. Pubertal growth spurt in patients with bilateral anorchia after testosterone replacement therapy. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. 2019 Sep:26(6):320-323. doi: 10.1016/j.arcped.2019.06.008. Epub 2019 Jul 25 [PubMed PMID: 31353150]
Smith NM, Byard RW, Bourne AJ. Testicular regression syndrome--a pathological study of 77 cases. Histopathology. 1991 Sep:19(3):269-72 [PubMed PMID: 1916702]
Level 2 (mid-level) evidenceSzmer J, Chrzan R. Decision-making challenges in children with congenital and acquired monorchism: a critical literature review. Folia medica Cracoviensia. 2019:59(1):127-136 [PubMed PMID: 31180081]
Pirgon Ö, Dündar BN. Vanishing testes: a literature review. Journal of clinical research in pediatric endocrinology. 2012 Sep:4(3):116-20. doi: 10.4274/Jcrpe.728. Epub [PubMed PMID: 22985611]
Rozanski TA, Wojno KJ, Bloom DA. The remnant orchiectomy. The Journal of urology. 1996 Feb:155(2):712-3; discussion 714 [PubMed PMID: 8558712]
Level 2 (mid-level) evidenceVelázquez de Cuéllar Paracchi M, Leal Orozco A, Ruíz Serrano C, Aguado Roncero P, Pérez Tejerizo G, Soriano Guillén L. [Micropenis and bilateral cryptorchidism secondary to vanishing testes syndrome]. Anales de pediatria (Barcelona, Spain : 2003). 2009 Feb:70(2):199-200. doi: 10.1016/j.anpedi.2008.09.004. Epub 2008 Dec 5 [PubMed PMID: 19217585]
Level 3 (low-level) evidenceGrinspon RP, Habib C, Bedecarrás P, Gottlieb S, Rey RA. Compensatory function of the remaining testis is dissociated in boys and adolescents with monorchidism. European journal of endocrinology. 2016 Mar:174(3):399-407. doi: 10.1530/EJE-15-0938. Epub 2015 Dec 15 [PubMed PMID: 26671976]
Uğuz S, Gürağaç A, Demirer Z, Yilmaz S, Aydur E. Bilateral polyorchidism with ipsilateral two undescended testes: a rare congenital anomaly. Andrologia. 2017 May:49(4):. doi: 10.1111/and.12643. Epub 2016 Jul 4 [PubMed PMID: 27373456]
Yeniyol CO, Nergiz N, Tuna A. Abdominal polyorchidism: a case report and review of the literature. International urology and nephrology. 2004:36(3):407-8 [PubMed PMID: 15783116]
Level 3 (low-level) evidenceBalasar M, Sönmez MG, Oltulu P, Kandemir A, Kılıç M, Göğer YE, Pişkin MM. Polyorchidism; unilateral, one atrophic undescended double testicles. Urology annals. 2017 Apr-Jun:9(2):208-210. doi: 10.4103/0974-7796.204190. Epub [PubMed PMID: 28479781]
Jakhere SG, Saifi SA, Ranwaka AA. Supernumerary testis: Imaging appearance of a rare entity. Indian journal of urology : IJU : journal of the Urological Society of India. 2014 Apr:30(2):233-4. doi: 10.4103/0970-1591.126918. Epub [PubMed PMID: 24744529]
Leung AK. Polyorchidism. American family physician. 1988 Sep:38(3):153-6 [PubMed PMID: 3046267]
Favorito LA, Cavalcante AG, Costa WS. Anatomic aspects of epididymis and tunica vaginalis in patients with testicular torsion. International braz j urol : official journal of the Brazilian Society of Urology. 2004 Sep-Oct:30(5):420-4 [PubMed PMID: 15610580]
Castilla EE, Sod R, Anzorena O, Texido J. Neonatal testicular torsion in two brothers. Journal of medical genetics. 1975 Mar:12(1):112-3 [PubMed PMID: 1121017]
Collins K, Broecker BH. Familial torsion of the spermatic cord. The Journal of urology. 1989 Jan:141(1):128-9 [PubMed PMID: 2908936]
Level 3 (low-level) evidenceCUNNINGHAM RF. Familial occurrence of testicular torsion. JAMA. 1960 Nov 5:174():1330-1 [PubMed PMID: 13718864]
Martin AD, Rushton HG. The prevalence of bell clapper anomaly in the solitary testis in cases of prior perinatal torsion. The Journal of urology. 2014 May:191(5 Suppl):1573-7. doi: 10.1016/j.juro.2013.09.013. Epub 2014 Mar 26 [PubMed PMID: 24679875]
Level 2 (mid-level) evidenceMonteilh C, Calixte R, Burjonrappa S. Controversies in the management of neonatal testicular torsion: A meta-analysis. Journal of pediatric surgery. 2019 Apr:54(4):815-819. doi: 10.1016/j.jpedsurg.2018.07.006. Epub 2018 Aug 8 [PubMed PMID: 30098810]
Level 1 (high-level) evidenceRiaz-Ul-Haq M, Mahdi DE, Elhassan EU. Neonatal testicular torsion; a review article. Iranian journal of pediatrics. 2012 Sep:22(3):281-9 [PubMed PMID: 23400637]
Da Silva Rios S, Monteiro IC, Braz Dos Santos LG, Caldas NG, Chen AC, Chen JR, Silva HS. A case of swyer syndrome associated with advanced gonadal dysgerminoma involving long survival. Case reports in oncology. 2015 Jan-Apr:8(1):179-84. doi: 10.1159/000381451. Epub 2015 Mar 31 [PubMed PMID: 25960730]
Level 3 (low-level) evidenceLipay MV, Bianco B, Verreschi IT. [Gonadal dysgenesis and tumors: genetic and clinical features]. Arquivos brasileiros de endocrinologia e metabologia. 2005 Feb:49(1):60-70 [PubMed PMID: 16544035]
King TF, Conway GS. Swyer syndrome. Current opinion in endocrinology, diabetes, and obesity. 2014 Dec:21(6):504-10. doi: 10.1097/MED.0000000000000113. Epub [PubMed PMID: 25314337]
Level 3 (low-level) evidenceNieschlag E. Klinefelter syndrome: the commonest form of hypogonadism, but often overlooked or untreated. Deutsches Arzteblatt international. 2013 May:110(20):347-53. doi: 10.3238/arztebl.2013.0347. Epub 2013 May 17 [PubMed PMID: 23825486]
Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: Symptoms and treatment. Journal of advanced pharmaceutical technology & research. 2010 Jul:1(3):297-301. doi: 10.4103/0110-5558.72420. Epub [PubMed PMID: 22247861]
Tüttelmann F, Gromoll J. Novel genetic aspects of Klinefelter's syndrome. Molecular human reproduction. 2010 Jun:16(6):386-95. doi: 10.1093/molehr/gaq019. Epub 2010 Mar 12 [PubMed PMID: 20228051]
Level 3 (low-level) evidenceMaiburg M, Repping S, Giltay J. The genetic origin of Klinefelter syndrome and its effect on spermatogenesis. Fertility and sterility. 2012 Aug:98(2):253-60. doi: 10.1016/j.fertnstert.2012.06.019. Epub 2012 Jun 29 [PubMed PMID: 22749222]
Level 3 (low-level) evidenceBatista RL, Costa EMF, Rodrigues AS, Gomes NL, Faria JA Jr, Nishi MY, Arnhold IJP, Domenice S, Mendonca BB. Androgen insensitivity syndrome: a review. Archives of endocrinology and metabolism. 2018 Mar-Apr:62(2):227-235. doi: 10.20945/2359-3997000000031. Epub [PubMed PMID: 29768628]
Mendonca BB, Costa EM, Belgorosky A, Rivarola MA, Domenice S. 46,XY DSD due to impaired androgen production. Best practice & research. Clinical endocrinology & metabolism. 2010 Apr:24(2):243-62. doi: 10.1016/j.beem.2009.11.003. Epub [PubMed PMID: 20541150]
Melo KF, Mendonça BB, Billerbeck AE, Costa EM, Latronico AC, Arnhold IJ. [Androgen insensitivity syndrome: clinical, hormonal and molecular analysis of 33 cases]. Arquivos brasileiros de endocrinologia e metabologia. 2005 Feb:49(1):87-97 [PubMed PMID: 16544039]
Level 3 (low-level) evidenceHughes IA, Deeb A. Androgen resistance. Best practice & research. Clinical endocrinology & metabolism. 2006 Dec:20(4):577-98 [PubMed PMID: 17161333]
Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta pharmacologica Sinica. 2015 Jan:36(1):3-23. doi: 10.1038/aps.2014.18. Epub 2014 Jun 9 [PubMed PMID: 24909511]
Level 3 (low-level) evidenceMongan NP, Tadokoro-Cuccaro R, Bunch T, Hughes IA. Androgen insensitivity syndrome. Best practice & research. Clinical endocrinology & metabolism. 2015 Aug:29(4):569-80. doi: 10.1016/j.beem.2015.04.005. Epub 2015 Apr 26 [PubMed PMID: 26303084]
Gulía C, Baldassarra S, Zangari A, Briganti V, Gigli S, Gaffi M, Signore F, Vallone C, Nucciotti R, Costantini FM, Pizzuti A, Bernardo S, Porrello A, Piergentili R. Androgen insensitivity syndrome. European review for medical and pharmacological sciences. 2018 Jun:22(12):3873-3887. doi: 10.26355/eurrev_201806_15272. Epub [PubMed PMID: 29949163]
Cheon CK. Practical approach to steroid 5alpha-reductase type 2 deficiency. European journal of pediatrics. 2011 Jan:170(1):1-8. doi: 10.1007/s00431-010-1189-4. Epub 2010 Mar 28 [PubMed PMID: 20349245]
Mendonca BB, Domenice S, Arnhold IJ, Costa EM. 46,XY disorders of sex development (DSD). Clinical endocrinology. 2009 Feb:70(2):173-87. doi: 10.1111/j.1365-2265.2008.03392.x. Epub [PubMed PMID: 18811725]
Speiser PW. Congenital Adrenal Hyperplasia. F1000Research. 2015:4(F1000 Faculty Rev):601. doi: 10.12688/f1000research.6543.1. Epub 2015 Aug 20 [PubMed PMID: 26339484]
Speiser PW, White PC. Congenital adrenal hyperplasia. The New England journal of medicine. 2003 Aug 21:349(8):776-88 [PubMed PMID: 12930931]
Witchel SF. Congenital Adrenal Hyperplasia. Journal of pediatric and adolescent gynecology. 2017 Oct:30(5):520-534. doi: 10.1016/j.jpag.2017.04.001. Epub 2017 Apr 24 [PubMed PMID: 28450075]
Simard J, Moisan AM, Morel Y. Congenital adrenal hyperplasia due to 3beta-hydroxysteroid dehydrogenase/Delta(5)-Delta(4) isomerase deficiency. Seminars in reproductive medicine. 2002 Aug:20(3):255-76 [PubMed PMID: 12428206]
Level 3 (low-level) evidenceKrone N, Dhir V, Ivison HE, Arlt W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clinical endocrinology. 2007 Feb:66(2):162-72 [PubMed PMID: 17223983]
New MI. Male pseudohermaphroditism due to 17 alpha-hydroxylase deficiency. The Journal of clinical investigation. 1970 Oct:49(10):1930-41 [PubMed PMID: 5456802]
Hauffa BP, Miller WL, Grumbach MM, Conte FA, Kaplan SL. Congenital adrenal hyperplasia due to deficient cholesterol side-chain cleavage activity (20, 22-desmolase) in a patient treated for 18 years. Clinical endocrinology. 1985 Nov:23(5):481-93 [PubMed PMID: 3841304]
Level 3 (low-level) evidenceBose HS, Sugawara T, Strauss JF 3rd, Miller WL, International Congenital Lipoid Adrenal Hyperplasia Consortium. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. The New England journal of medicine. 1996 Dec 19:335(25):1870-8 [PubMed PMID: 8948562]
Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. The Journal of clinical endocrinology and metabolism. 2009 Oct:94(10):3865-71. doi: 10.1210/jc.2009-0467. Epub 2009 Sep 22 [PubMed PMID: 19773404]
Primatesta P, Goldacre MJ. Inguinal hernia repair: incidence of elective and emergency surgery, readmission and mortality. International journal of epidemiology. 1996 Aug:25(4):835-9 [PubMed PMID: 8921464]
Level 2 (mid-level) evidenceRuhl CE, Everhart JE. Risk factors for inguinal hernia among adults in the US population. American journal of epidemiology. 2007 May 15:165(10):1154-61 [PubMed PMID: 17374852]
Tanyel FC, Müftüoglu S, Dagdeviren A, Kaymaz FF, Büyükpamukçu N. Myofibroblasts defined by electron microscopy suggest the dedifferentiation of smooth muscle within the sac walls associated with congenital inguinal hernia. BJU international. 2001 Feb:87(3):251-5 [PubMed PMID: 11167652]
Rodprasert W, Virtanen HE, Mäkelä JA, Toppari J. Hypogonadism and Cryptorchidism. Frontiers in endocrinology. 2019:10():906. doi: 10.3389/fendo.2019.00906. Epub 2020 Jan 15 [PubMed PMID: 32010061]
Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocrine reviews. 2008 Aug:29(5):560-80. doi: 10.1210/er.2007-0042. Epub 2008 Apr 24 [PubMed PMID: 18436703]
Ramareddy RS, Alladi A, Siddappa OS. Ectopic testis in children: experience with seven cases. Journal of pediatric surgery. 2013 Mar:48(3):538-41. doi: 10.1016/j.jpedsurg.2012.10.005. Epub [PubMed PMID: 23480908]
Level 2 (mid-level) evidenceFerro F, Lais A, Matarazzo E, Capozza N, Caione P. [Retractile testis and gliding testis. Two distinct clinical entities]. Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 1996 Sep:48(3):145-9 [PubMed PMID: 8966651]
. Cryptorchidism: a prospective study of 7500 consecutive male births, 1984-8. John Radcliffe Hospital Cryptorchidism Study Group. Archives of disease in childhood. 1992 Jul:67(7):892-9 [PubMed PMID: 1355643]
Barteczko KJ, Jacob MI. The testicular descent in human. Origin, development and fate of the gubernaculum Hunteri, processus vaginalis peritonei, and gonadal ligaments. Advances in anatomy, embryology, and cell biology. 2000:156():III-X, 1-98 [PubMed PMID: 11008363]
Level 3 (low-level) evidenceNguyen HT, Coakley F, Hricak H. Cryptorchidism: strategies in detection. European radiology. 1999:9(2):336-43 [PubMed PMID: 10101659]
Borrow M, Gough MH. Bilateral absence of testes. Lancet (London, England). 1970 Feb 14:1(7642):366 [PubMed PMID: 4189631]
Aynsley-Green A, Zachmann M, Illig R, Rampini S, Prader A. Congenital bilateral anorchia in childhood: a clinical, endocrine and therapeutic evaluation of twenty-one cases. Clinical endocrinology. 1976 Jul:5(4):381-91 [PubMed PMID: 786507]
Level 3 (low-level) evidenceZenaty D, Dijoud F, Morel Y, Cabrol S, Mouriquand P, Nicolino M, Bouvatier C, Pinto G, Lecointre C, Pienkowski C, Soskin S, Bost M, Bertrand AM, El-Ghoneimi A, Nihoul-Fekete C, Léger J. Bilateral anorchia in infancy: occurence of micropenis and the effect of testosterone treatment. The Journal of pediatrics. 2006 Nov:149(5):687-91 [PubMed PMID: 17095345]
De Marchi M, Campagnoli C, Ghiringhello B, Ponzio G, Carbonara A. Gonadal agenesis in a phenotypically normal female with positive H-Y antigen. Human genetics. 1981:56(3):417-9 [PubMed PMID: 7239525]
Level 3 (low-level) evidenceShadpour P, Kashi AH, Arvin A. Scrotal testis size in unilateral non-palpable cryptorchidism, what it can and cannot tell: Study of a Middle Eastern population. Journal of pediatric urology. 2017 Jun:13(3):268.e1-268.e6. doi: 10.1016/j.jpurol.2016.12.013. Epub 2017 Jan 21 [PubMed PMID: 28254240]
Hodhod A, Capolicchio JP, Jednak R, El-Sherbiny M. Testicular hypertrophy as a predictor for contralateral monorchism: Retrospective review of prospectively recorded data. Journal of pediatric urology. 2016 Feb:12(1):34.e1-5. doi: 10.1016/j.jpurol.2015.06.010. Epub 2015 Jul 30 [PubMed PMID: 26279100]
Level 2 (mid-level) evidenceKheirandish P, Chinegwundoh F. An unusual case of triorchidism. JRSM short reports. 2010 Nov 25:1(6):55. doi: 10.1258/shorts.2010.010067. Epub 2010 Nov 25 [PubMed PMID: 21234118]
Level 3 (low-level) evidenceArlen AM, Holzman SA, Weiss AD, Garola RE, Cerwinka WH. Functional supernumerary testis in a child with testicular torsion and review of polyorchidism. Pediatric surgery international. 2014 May:30(5):565-8. doi: 10.1007/s00383-014-3485-y. Epub 2014 Feb 21 [PubMed PMID: 24557155]
Level 3 (low-level) evidenceAbduljabbar AH. A Case Report: Triorchidism; is a Rare Mistaken Cause for Extra Testicular Neoplasm. Urology case reports. 2015 May:3(3):89-91. doi: 10.1016/j.eucr.2015.02.002. Epub 2015 Mar 14 [PubMed PMID: 26793513]
Level 3 (low-level) evidenceKharrazi SM, Rahmani MR, Sakipour M, Khoob S. Polyorchidism: a case report and review of literature. Urology journal. 2006 Summer:3(3):180-3 [PubMed PMID: 17559037]
Level 3 (low-level) evidenceBergholz R, Wenke K. Polyorchidism: a meta-analysis. The Journal of urology. 2009 Nov:182(5):2422-7. doi: 10.1016/j.juro.2009.07.063. Epub 2009 Sep 17 [PubMed PMID: 19765760]
Level 1 (high-level) evidenceZhao LC, Lautz TB, Meeks JJ, Maizels M. Pediatric testicular torsion epidemiology using a national database: incidence, risk of orchiectomy and possible measures toward improving the quality of care. The Journal of urology. 2011 Nov:186(5):2009-13. doi: 10.1016/j.juro.2011.07.024. Epub 2011 Sep 23 [PubMed PMID: 21944120]
Level 2 (mid-level) evidenceHuang WY, Chen YF, Chang HC, Yang TK, Hsieh JT, Huang KH. The incidence rate and characteristics in patients with testicular torsion: a nationwide, population-based study. Acta paediatrica (Oslo, Norway : 1992). 2013 Aug:102(8):e363-7. doi: 10.1111/apa.12275. Epub 2013 May 13 [PubMed PMID: 23611668]
Level 2 (mid-level) evidenceLee SM, Huh JS, Baek M, Yoo KH, Min GE, Lee HL, Lee DG. A nationwide epidemiological study of testicular torsion in Korea. Journal of Korean medical science. 2014 Dec:29(12):1684-7. doi: 10.3346/jkms.2014.29.12.1684. Epub 2014 Nov 21 [PubMed PMID: 25469070]
Level 2 (mid-level) evidenceHyun GS. Testicular Torsion. Reviews in urology. 2018:20(2):104-106. doi: 10.3909/riu0800. Epub [PubMed PMID: 30288149]
Yiee JH, Chang L, Kaplan A, Kwan L, Chung PJ, Litwin MS. Patterns of care in testicular torsion: influence of hospital transfer on testicular outcomes. Journal of pediatric urology. 2013 Dec:9(6 Pt A):713-20. doi: 10.1016/j.jpurol.2013.06.003. Epub 2013 Jul 27 [PubMed PMID: 23896260]
Level 2 (mid-level) evidencePogorelić Z, Mrklić I, Jurić I, Biočić M, Furlan D. Testicular torsion in the inguinal canal in children. Journal of pediatric urology. 2013 Dec:9(6 Pt A):793-7. doi: 10.1016/j.jpurol.2012.10.013. Epub 2012 Oct 31 [PubMed PMID: 23123082]
Yazbeck S, Patriquin HB. Accuracy of Doppler sonography in the evaluation of acute conditions of the scrotum in children. Journal of pediatric surgery. 1994 Sep:29(9):1270-2 [PubMed PMID: 7807366]
Wikström AM, Hoei-Hansen CE, Dunkel L, Rajpert-De Meyts E. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: evidence for degeneration of germ cells at the onset of meiosis. The Journal of clinical endocrinology and metabolism. 2007 Feb:92(2):714-9 [PubMed PMID: 17148558]
Avendaño A, Paradisi I, Cammarata-Scalisi F, Callea M. 5-α-Reductase type 2 deficiency: is there a genotype-phenotype correlation? A review. Hormones (Athens, Greece). 2018 Jun:17(2):197-204. doi: 10.1007/s42000-018-0013-9. Epub 2018 Apr 20 [PubMed PMID: 29858846]
Byne W. Developmental endocrine influences on gender identity: implications for management of disorders of sex development. The Mount Sinai journal of medicine, New York. 2006 Nov:73(7):950-9 [PubMed PMID: 17195880]
Thilén A, Larsson A. Congenital adrenal hyperplasia in Sweden 1969-1986. Prevalence, symptoms and age at diagnosis. Acta paediatrica Scandinavica. 1990 Feb:79(2):168-75 [PubMed PMID: 2321478]
Level 2 (mid-level) evidenceBois E, Mornet E, Chompret A, Feingold J, Hochez J, Goulet V. [Congenital adrenal hyperplasia (21-OH) in France. Population genetics]. Archives francaises de pediatrie. 1985 Mar:42(3):175-9 [PubMed PMID: 3873926]
Yanase T, Simpson ER, Waterman MR. 17 alpha-hydroxylase/17,20-lyase deficiency: from clinical investigation to molecular definition. Endocrine reviews. 1991 Feb:12(1):91-108 [PubMed PMID: 2026124]