Introduction
Infection from the varicella-zoster virus (VZV) most commonly occurs in childhood and is spread by airborne, droplet, and contact transmission. Herpes zoster results from reactivation of the latent VZV within a sensory nerve ganglion, often presenting decades after the initial infection. The disease typically presents as a unilateral maculopapular or vesicular rash in a single dermatomal distribution. Herpes zoster ophthalmicus (HZO) is defined as the viral involvement of the ophthalmic division (V1) of the trigeminal cranial nerve (V). While the diagnosis of HZO does not necessarily imply eye involvement, ocular disease occurs in about 50% of HZO cases. Ocular manifestations can include conjunctivitis, uveitis, episcleritis, keratitis, and retinitis. The condition is considered an ophthalmologic emergency due to the risk of vision loss if not quickly identified and treated early in the disease course.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In those with a history of prior infection, the VZV normally lies dormant within a dorsal root ganglion. In a healthy individual, immunity acquired from the initial infection allows suppression of the virus. However, often in the setting of weakened immunity, the virus may reactivate in the form of herpes zoster, also known as shingles. HZO specifically refers to a reactivated VZV infection involving the V1 nerve division after having lied dormant within the trigeminal nerve ganglion (also known as the Gasserian ganglion). V1 is subdivided into three branches: the frontal nerve branch, the nasociliary nerve branch, and the lacrimal nerve branch. Any or all of these nerve branches may be affected in the case of HZO. The disease typically results in ocular and facial lesions with potential progression to more serious complications. The main risk factors for HZO include age over 50 years old and immunocompromised status (e.g., history of HIV, autoimmune diseases requiring corticosteroids or other immunosuppressants, organ or bone marrow transplant, or chemotherapy treatment). Other chronic diseases, acute illnesses, and physical and emotional stressors can also precipitate HZO.[2][3]
Epidemiology
In the United States, herpes zoster affects about 1 per 1000 individuals annually. However, in the over 60-years population, the incidence is closer to 1 per 100 individuals. The incidence in older adults is lower in those who have received either the live attenuated or inactivated recombinant zoster vaccines. Among patients diagnosed with herpes zoster, some epidemiological studies estimate about 8 to 20% are complicated by HZO, with many of those cases resulting in ocular involvement.[2][4][5][6]
Pathophysiology
HZO follows the typical pathogenic process of herpes zoster. VZV is a double-stranded DNA virus that initially invades the upper respiratory tract via virus-laden respiratory droplets, where it replicates and invades adjacent lymph nodes. After about a week, the virus disseminates and progresses to a secondary cutaneous infection, with vesicle eruption occurring several days later. Following the primary infection, the virus follows a retrograde pathway along axons toward sensory nerve ganglia, where it enters the latent phase. In the reactivation phase, the virus moves in an anterograde fashion toward superficial tissue, where it reemerges as herpes zoster.[7]
History and Physical
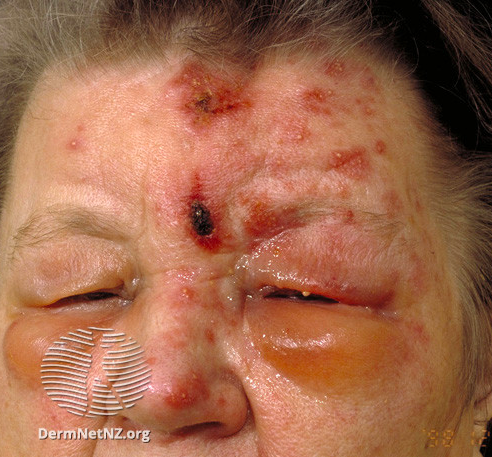
Patients with HZO typically present with prodromal pain in a unilateral V1 dermatomal distribution, followed by an erythematous vesicular or pustular rash to the same area. Pain is neuropathic, and patients may describe the sensation as "burning" or "shooting," sometimes accompanied by paresthesias. Additionally, the herpetic rash may be preceded by constitutional symptoms such as fever, fatigue, malaise, and headaches. The presence of herpetic lesions around the tip of the nose is known as the Hutchinson sign. The presence of Hutchinson's sign indicates nasociliary branch involvement of V1, which confers a higher risk for ocular involvement. The nasociliary branch innervates the tip of the nose and cornea, as well as other ocular structures.[8][9][10][11][12][13]
The physical exam begins with an external evaluation of lesion distribution, including the eyelids and scalp. The rash often appears maculopapular before transitioning to vesicles and pustules that ultimately rupture and scab over. Although the rash is usually limited to a single unilateral dermatome, immunocompromised patients may develop disseminated disease, with a possible bilateral presentation. Particular attention should be paid to eyelid involvement, which is frequently associated with blepharitis, conjunctivitis, and episcleritis. Ptosis is rare but may occur in response to marked inflammation. A thorough ocular exam is required and must include visual acuity, slit-lamp examination with fluorescein staining, and ocular tonometry. A thorough dilated funduscopic exam is also necessary.
- Corneal involvement includes both epithelial keratitis and stromal keratitis.
- Epithelial keratitis may present as punctate and/or pseudodendritic keratitis. Punctate keratitis is characterized by scattered fluorescein-stained swollen lesions on the superficial surface of the cornea. It is thought that these lesions contain the live replicating virus. Pseudodendrites appear as branching lesions similar to the true dendritic lesions seen in herpes simplex virus (HSV) keratitis. Unlike the dendrites of HSV, which appear as brightly staining ulcers with terminal bulbs, HZO pseudodendrites are non-erosive mucous plaques that exhibit minimal staining with an absence of terminal bulbs.
- Stromal keratitis may be classified as anterior or deep and usually develops after epithelial keratitis. Anterior stromal keratitis results from an immune-mediated response to the virus and is characterized by nummular (coin-shaped) granules in the superficial stroma. Deep stromal keratitis usually occurs late in the disease course (often months after the acute phase) and presents with marked corneal edema often associated with anterior uveitis.
- Severe keratitis may eventually lead to neurotrophic keratopathy due to persistent inflammatory injury to the corneal sensory nerves. Assessing corneal sensation can help evaluate for peripheral nerve damage.
- Uveitis may be categorized as anterior, posterior, or panuveitis.
- Anterior uveitis is visualized on slit-lamp examination as floating leukocytes and proteins from vascular damage, commonly referred to as "cell and flare."
- Posterior uveitis presents as leukocytes floating in the vitreous cavity (vitritis) or as areas of retinal whitening representing inflammation and necrosis in severe cases. Optic neuritis may be an associated finding as well.
- Every patient with HZO should undergo ocular tonometry to evaluate for elevated intraocular pressure (IOP). Elevated IOP due to HZO usually occurs secondary to zoster-induced trabeculitis. However, it may also occur due to obstruction of the trabecular meshwork from the inflammatory cell and protein debris or from the formation of synechiae as a consequence of extensive intraocular inflammation. Prolonged elevation in IOP may lead to secondary glaucoma.
Evaluation
HZO is primarily a clinical diagnosis based on history and classic findings on physical and slit-lamp examination. Additional procedures, such as ocular tonometry and corneal esthesiometry, may be performed for a more thorough evaluation to assess for complications. Other diagnostic tests, such as viral cultures, polymerase chain reaction (PCR), and antibody testing, are rarely required to establish a diagnosis of HZO. For patients with disseminated herpes zoster (disease involving multiple dermatomes), severe illness, or those with significant risk factors, it is reasonable to consider HIV testing. Additional laboratory tests and imaging are seldom warranted.[14][15]
Treatment / Management
Treatment for HZO includes prompt initiation of antiviral agents for all patients, as well as supportive care for symptom management. Other adjunct therapies, such as antibiotics, topical or systemic corticosteroids, and corneal epithelial debridement, are considered on a case-by-case basis.[16][17][18][19](A1)
- Supportive care: Artificial tears, cold compresses, and analgesics may be employed.
- Antiviral agents: Ideally, treatment with systemic antiviral agents should begin within 72 hours of disease onset. Initiation should not be delayed while awaiting definitive diagnosis or ophthalmology follow-up. Topical antiviral agents may be considered, but there is limited evidence regarding their utility in managing HZO.
- Immunocompetent adult dosing (choose single agent):
- Acyclovir 800 mg orally five times per day for at least 7 days
- Valacyclovir 1000 mg orally every eight hours for at least 7 days (may require renal dosing)
- Famciclovir 500 mg orally three times per day for at least 7 days (may require renal dosing)
- Immunocompromised adult dosing (choose a single agent):
- Acyclovir 10 mg/kg of ideal body weight (IBW) intravenously (IV) every eight hours for at least 7 days
- Foscarnet 90 mg/kg IV every 12 hours (typically reserved for severe or acyclovir-resistant disease)
- Immunocompetent adult dosing (choose single agent):
- Antibiotics: Topical antibiotics (e.g., erythromycin ophthalmic ointment) are often administered to help prevent secondary bacterial infection.
- Corticosteroids: Both topical and systemic corticosteroids may be used in disease management. Systemic corticosteroids are often used in the treatment of herpes zoster, including HZO. However, clinical trials have shown variable results, and the potential for adverse events must be weighed against any potential benefit. The timing and extent to which topical corticosteroids are used are determined through ophthalmology consultation. Manifestations treated with topical corticosteroids include stromal keratitis, uveitis, and trabeculitis.
- Topical aqueous suppressants: These agents are commonly used in combination with topical corticosteroids in the treatment of elevated IOP secondary to HZO.
- Debridement: Ophthalmology may consider debridement in cases of epithelial keratitis.
Differential Diagnosis
- HSV keratitis
- HSV dermatitis
- Other viral or bacterial conjunctivitis
- Allergic conjunctivitis
- Exposure conjunctivitis/keratitis
- Acute angle-closure glaucoma
- Corneal ulcer
- Corneal abrasion
- Impetigo
- Cellulitis
- Insect bites
- Contact dermatitis
Prognosis
The prognosis of HZO is highly variable and largely dependent on the patient’s risk factors, the timing of treatment, and the aggressiveness of the disease. There is limited data on the morbidity and mortality rates of HZO. However, most immunocompetent patients with herpes zoster who receive early treatment have a resolution of lesions within four weeks and can be managed outpatient.
Complications
As discussed in the history and physical section, a thorough examination of the HZO patient may reveal an array of complications, including blepharitis, conjunctivitis, keratitis, uveitis, and secondary glaucoma. Prolonged inflammation and corneal scarring can lead to serious complications, including permanent vision loss. Neurotrophic keratopathy, resulting from dysfunctional corneal innervation, leaves patients more susceptible to corneal abrasions, corneal ulcers, and persistent corneal epithelial defects, even in the absence of replicating viruses or active inflammation. Necrotizing retinitis is an uncommon complication of HZO that can lead to retinal tears, retinal detachment, and subsequent permanent vision changes. Additionally, patients with HZO commonly develop postherpetic neuralgia (PHN) as a result of a peripheral nerve injury. PHN is a chronic painful condition in which herpes zoster pain persists for more than 90 days after the initial rash outbreak. Older age and severe disease are risk factors for PHN, while prior zoster vaccination appears to be protective. In addition to complications directly related to HZO, patients are also prone to secondary bacterial infections.[10][11][12][13]
Deterrence and Patient Education
As part of their preventative care guidelines, the United States Centers for Disease Control and Prevention (CDC) recommends adults over 50 years old receive herpes zoster vaccination to lower their risk of viral reactivation. There is one vaccine on the market - an inactivated recombinant vaccine. The inactivated vaccine was approved in 2018 and is administered as a 2-dose series. It has demonstrated 97% efficacy in patients aged 50 to 70 and 90% efficacy in patients older than 70 years. Patients can still receive the inactivated vaccine despite having previously received the live vaccine. The safety of the inactivated vaccine in immunocompromised patients is still under investigation. While vaccination does not completely protect against herpes zoster, vaccinated individuals tend to have shorter and less severe disease courses. The spread of disease can be mitigated by advising all patients with an active infection to keep the rash covered and perform frequent handwashing. Those with disseminated disease also require airborne precautions. All patients with herpes zoster should avoid contact with pregnant women, immunologically naïve infants and children, and immunocompromised individuals.[20][21][22][23]
Enhancing Healthcare Team Outcomes
HZO prevention and management involve an interprofessional approach. Preventative efforts often take place in the primary care office, where patients receive education on vaccination. Patients with acute disease may present to their primary care provider, urgent care, or the emergency department. It is important that these first-contact providers initiate antiviral treatment and promptly consult and refer to ophthalmology for further evaluation. Pharmacists can assist with dosing regimens and provide information on pharmacodynamic interactions. Those with severe disease may require inpatient admission under the care of a hospitalist and a consulting ophthalmologist. Patients may go on to develop a prolonged disease course with chronic complications requiring frequent follow-up visits with their healthcare team.
Media
(Click Image to Enlarge)
References
HOPE-SIMPSON RE. THE NATURE OF HERPES ZOSTER: A LONG-TERM STUDY AND A NEW HYPOTHESIS. Proceedings of the Royal Society of Medicine. 1965 Jan:58(1):9-20 [PubMed PMID: 14267505]
Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008 Feb:115(2 Suppl):S3-12. doi: 10.1016/j.ophtha.2007.10.009. Epub [PubMed PMID: 18243930]
Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. Journal of the American Academy of Dermatology. 2007 Dec:57(6 Suppl):S130-5 [PubMed PMID: 18021864]
Borkar DS, Tham VM, Esterberg E, Ray KJ, Vinoya AC, Parker JV, Uchida A, Acharya NR. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013 Mar:120(3):451-456. doi: 10.1016/j.ophtha.2012.09.007. Epub 2012 Dec 1 [PubMed PMID: 23207173]
Level 2 (mid-level) evidenceKong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence Rate of Herpes Zoster Ophthalmicus: A Retrospective Cohort Study from 1994 through 2018. Ophthalmology. 2020 Mar:127(3):324-330. doi: 10.1016/j.ophtha.2019.10.001. Epub 2019 Oct 9 [PubMed PMID: 31668889]
Level 2 (mid-level) evidenceBrisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos LL, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiology and infection. 2001 Oct:127(2):305-14 [PubMed PMID: 11693508]
Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nature reviews. Microbiology. 2014 Mar:12(3):197-210. doi: 10.1038/nrmicro3215. Epub 2014 Feb 10 [PubMed PMID: 24509782]
Level 3 (low-level) evidenceKalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF. Eye and Periocular Skin Involvement in Herpes Zoster Infection. Medical hypothesis, discovery & innovation ophthalmology journal. 2015 Winter:4(4):142-156 [PubMed PMID: 27800502]
Tabery HM. Morphology of epithelial keratitis in herpes zoster ophthalmicus. A non-contact photomicrographic in vivo study in the human cornea. Acta ophthalmologica Scandinavica. 2000 Dec:78(6):651-5 [PubMed PMID: 11167225]
Cobo LM. Corneal complications of herpes zoster ophthalmicus. Prevention and treatment. Cornea. 1988:7(1):50-6 [PubMed PMID: 3258220]
Catron T, Hern HG. Herpes zoster ophthalmicus. The western journal of emergency medicine. 2008 Aug:9(3):174-6 [PubMed PMID: 19561738]
Hoeksema L, Jansonius NM, Los LI. Risk Factors for Secondary Glaucoma in Herpetic Anterior Uveitis. American journal of ophthalmology. 2017 Sep:181():55-60. doi: 10.1016/j.ajo.2017.06.013. Epub 2017 Jun 27 [PubMed PMID: 28666730]
Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurologic clinics. 2008 Aug:26(3):675-97, viii. doi: 10.1016/j.ncl.2008.03.011. Epub [PubMed PMID: 18657721]
Opstelten W, van Loon AM, Schuller M, van Wijck AJ, van Essen GA, Moons KG, Verheij TJ. Clinical diagnosis of herpes zoster in family practice. Annals of family medicine. 2007 Jul-Aug:5(4):305-9 [PubMed PMID: 17664496]
Harbecke R, Oxman MN, Arnold BA, Ip C, Johnson GR, Levin MJ, Gelb LD, Schmader KE, Straus SE, Wang H, Wright PF, Pachucki CT, Gershon AA, Arbeit RD, Davis LE, Simberkoff MS, Weinberg A, Williams HM, Cheney C, Petrukhin L, Abraham KG, Shaw A, Manoff S, Antonello JM, Green T, Wang Y, Tan C, Keller PM, Shingles Prevention Study Group. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. Journal of medical virology. 2009 Jul:81(7):1310-22. doi: 10.1002/jmv.21506. Epub [PubMed PMID: 19475609]
Neoh C, Harding SP, Saunders D, Wallis S, Tullo AB, Nylander A, Nelson ME. Comparison of topical and oral acyclovir in early herpes zoster ophthalmicus. Eye (London, England). 1994:8 ( Pt 6)():688-91 [PubMed PMID: 7867830]
Level 1 (high-level) evidenceMorton P, Thomson AN. Oral acyclovir in the treatment of herpes zoster in general practice. The New Zealand medical journal. 1989 Mar 8:102(863):93-5 [PubMed PMID: 2648213]
Level 1 (high-level) evidenceOrmrod D, Goa K. Valaciclovir: a review of its use in the management of herpes zoster. Drugs. 2000 Jun:59(6):1317-40 [PubMed PMID: 10882165]
Tyring SK. Efficacy of famciclovir in the treatment of herpes zoster. Seminars in dermatology. 1996 Jun:15(2 Suppl 1):27-31 [PubMed PMID: 8840413]
Level 1 (high-level) evidenceTricco AC, Zarin W, Cardoso R, Veroniki AA, Khan PA, Nincic V, Ghassemi M, Warren R, Sharpe JP, Page AV, Straus SE. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ (Clinical research ed.). 2018 Oct 25:363():k4029. doi: 10.1136/bmj.k4029. Epub 2018 Oct 25 [PubMed PMID: 30361202]
Level 1 (high-level) evidenceBharucha T, Ming D, Breuer J. A critical appraisal of 'Shingrix', a novel herpes zoster subunit vaccine (HZ/Su or GSK1437173A) for varicella zoster virus. Human vaccines & immunotherapeutics. 2017 Aug 3:13(8):1789-1797. doi: 10.1080/21645515.2017.1317410. Epub 2017 Apr 20 [PubMed PMID: 28426274]
Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. Journal of internal medicine. 2011 May:269(5):496-506. doi: 10.1111/j.1365-2796.2011.02359.x. Epub 2011 Feb 23 [PubMed PMID: 21294791]
Chua JV, Chen WH. Herpes zoster vaccine for the elderly: boosting immunity. Aging health. 2010 Apr 1:6(2):169-176 [PubMed PMID: 20607105]