Introduction
In 1865, Iwanoff described an epiretinal membrane (ERM) as the proliferation of cellular tissue on the surface of the retina along the inner limiting membrane. Its a commonly occurring condition, affecting the posterior pole of the retina over the macula. It appears as a greyish semi-translucent avascular membrane over the internal limiting membrane (ILM) on the surface of the retina. The other synonyms for ERM are macular pucker, preretinal macular fibrosis, surface wrinkling retinopathy, epimacular proliferations, epiretinal fibrosis or gliosis, and cellophane maculopathy.
Etiology is unknown and can be seen as an idiopathic (IERM) condition or secondary to trauma, post intraocular surgery, chronic ocular diseases, etc. It progressively affects the central vision and causes metamorphopsia. Optical coherence tomography (OCT) is the diagnostic procedure of choice for diagnosing and assessing the extent of the epiretinal membrane and its impact on the retinal morphology. Treatment, when indicated, is pars-plana vitrectomy (PPV) with ERM and ILM peeling.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Etiology is unknown, and the most common cause of the epiretinal membrane (ERM) is idiopathic (IERM). Secondary epiretinal membranes are seen in trauma, intraocular surgery, post-macular lasers, diabetic retinopathy, retinal vein occlusion, chronic macular edema, chronic intraocular inflammation, retinal detachment, and intraocular tumors. Increasing age, ERM in the other eye, and posterior vitreous detachment (PVD) are other risk factors. Several other names for ERM include cellophane maculopathy, macular pucker, preretinal fibrosis, and preretinal membrane. A systemic review involving 49,000 subjects found that greater age and female gender are significant risk factors for ERM.[1]
Epidemiology
The most common type of epiretinal membranes is idiopathic and is common in patients over the age of 50. Approximately 20% of patients over the age of 75 have an epiretinal membrane, with both sexes equally affected. Some reports show a slightly higher incidence in females.
In a multi-ethnic study of the United States population done in 2011 involving 5960 participants aged 45 to 84 years, it was shown that Chinese(39%) show significantly higher prevalence compared to Hispanics (29.3%), Whites (27.5%) and Blacks (26.2%).[2] Increasing age, diabetes, and hypercholesteremia were found to be risk factors for the epiretinal membrane.
In a multicentric meta-analysis of population-based studies involving 40,000 participants, it was shown that 9% had some form of ERM. Epiretinal membranes are classified into two groups: one with retinal folds called preretinal macular fibrosis (PMF) and a second without retinal folds called cellophane macula reflex (CMR). Of the ERM patients, 6.5% had CMR, and 2.6% had an advanced form of PMF. Older and female participants showed a higher risk.[1]
The Blue Mountains study was done over five years to study the incidence and progression of epiretinal membranes in the older Australian population.[3] Three thousand six hundred fifty-four persons, 49 years or older, living in the Blue Mountains area, west of Sydney, Australia, participated in the study from 1992 to 1994. The mean age was 65 years. The incidence of ERM’s in the first eye was 5.3%, and the progression of early ERM to an advanced stage of ERM of 9.3%. Five-year cumulative incidence rates of PMF and CMR were 1.5% and 3.8 %, respectively. Of these, 13.9% developed ERM in their second eye after five years.
Pathophysiology
A fibrocellular proliferation over the internal limiting membrane (ILM) characterizes the epiretinal membrane (ERM). The initiating event in an idiopathic ERM is the posterior vitreous detachment (PVD). Aging vitreous liquefies and detaches from its posterior attachments, leading to posterior vitreous detachment. PVD causes dehiscence in the internal limiting membrane, which allows the microglial cells to migrate to the preretinal surface, where they interact with the hyalocytes and laminocytes of the vitreous cellular membrane. These cells later transdifferentiate into fibroblast-like cells to form a cellophane thin epiretinal membrane.[4] PVD causes 95% of cases of idiopathic ERM.[5] Retinal folds from ERM contract to create a macular pucker. Retinal glial cells, hyalocytes, fibroblasts, and myofibroblasts are predominantly seen in idiopathic ERM. Retinal pigment epithelial cells, macrophages, T, and B cells are seen in secondary ERM as inflammation is the triggering event. A prerequisite for the formation of ERM is dehiscence in the ILM most commonly caused by posterior vitreous detachment. The following are the theories hypothesized for the ERM formation:
- Microglial cells migrate to the retinal surface over micro leaks produced by PVD. These cells later differentiate to form the fibroblasts.[6]
- Following PVD, segments of cellular vitreous remain on the surface. These segments contain hyalocytes that transdifferentiate into myofibroblasts from ERM.[7]
- PVD induced ILM avulsion in the posterior paravascular retina upregulates the cytokines to form the ERM.
History and Physical
Most often, patients with epiretinal membrane (ERM) are asymptomatic clinically and are frequently diagnosed during a routine clinical examination. In some patients, ERM progresses to cause metamorphopsia, micropsia or macropsia, photopsia, decreased visual acuity, diplopia, and loss of central vision. Metamorphopsia especially interferes with the quality of life affecting reading, writing, and most of the regular day-to-day activities of living. All asymptomatic patients should be trained to do self-monitoring periodically with Amsler grid charting. History taking should include previous ocular trauma or episodes of floaters or flashes of light, diabetes, hypertension, and hypercholesteremia. ERM causes retinal striae, which progresses in some patients to retinal folds, progressive contracture, and macular pseudohole. Rarely spontaneous separation of the membrane occurs, causing premacular floaters.
Evaluation
Idiopathic epiretinal membrane (IERM) is commonly seen in the elderly beyond 60 years with no sex predilection. Visual acuity, Amsler grid charting, and dilated fundus examination with scleral depression are mandatory in all patients with visual distortion. Watzke Allen test is an ophthalmoscopic test done to differentiate the full-thickness macular hole from a pseudohole. On the slit-lamp fundus examination, the epiretinal membrane (ERM) appears as an abnormal reflectivity of the macular area with a loss of normal convex contour and fine wrinkling of the surface. ERM is classified as an early milder form called cellophane macular reflex and a severe late form called preretinal macular fibrosis.[4]
Cellophane macular reflex is seen as a thin transparent membrane overlying the macula with no distortion or impairment of vision. Fundoscopy shows a glistening, watery, silky light reflex over the macula. Preretinal macular fibrosis occurs in some cases where a cellophane membrane thickens and contracts, causing retinal folds and traction lines, resulting in impairment of vision and metamorphopsia. Fundoscopy of preretinal macular fibrosis reveals a semi-translucent membrane obscuring the underlying retinal details with retinal fold, traction lines, and vascular tortuosity. Severe cases might show retinal hemorrhages, exudates, macular edema, macula holes, and pseudoholes. A defect in the ERM appears as a pseudohole.
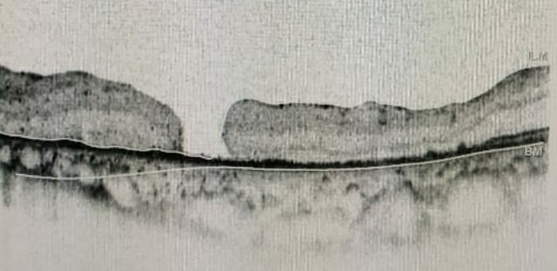
Optical coherence tomography (OCT) is a highly sensitive, routinely done investigation for retinal disorders and is the investigation of choice to diagnose epiretinal membrane and vitreomacular traction (VMT). On OCT, an ERM appears as a hyperreflective layer, often irregular over the inner surface of the retina. It appears corrugated with peg-like attachments to the retina. Elevation of the foveal depression is seen due to traction from ERM. In severe cases, the inner retina shows folds with thickening of the macula along with cystoid spaces. Both ERM and VMT often occur together. Andrea Govetto et al. have studied 194 eyes of 172 patients with ERM with clinical charts and spectral-domain OCT imaging. They found that 63 out of 194 eyes showed the presence of continuous ectopic inner foveal layers, which is associated with lower visual acuity.[8] They proposed the OCT-based grading system of ERMs as below:
- Stage 1: ERMs were mild and thin. Foveal depression is present.
- Stage 2: ERMs with a widening of the outer nuclear layer and loss of the foveal depression.
- Stage 3: ERMs with continuous ectopic inner foveal layers crossing the entire foveal area.
- Stage 4: ERMs were thick with continuous ectopic inner foveal layers and disrupted retinal layers.
In stages 1, 2, and 3, all retinal layers were clearly defined on OCT. Visual acuity progressively declined from stage 1 through stage 4. Hwang classified idiopathic ERM based on spectral-domain OCT.[9]
Group 1: Fovea-involving ERM
- 1a: Outer retinal thickening and minimal inner retinal change
- 1b: Outer retinal inward projection and inner retinal thickening
- 1c: Prominent thickening of the inner retinal layer
Group 2: Fovea-sparing ERM
- 2a: Formation of a macular pseudohole
- 2b: Schisis-like intraretinal splitting
Dr. Altaweel et al. recently proposed a detailed staging based on OCT observations.[10]
- Stage IA: Foveal splitting with a pseudocyst
- Stage IB: Pseudocyst enlargement and extension to the outer retina with roof intact.
- Stage IIA: Full-thickness macular hole (diameter <400 microns) with posterior hyaloid face remaining attached to the roof of the pseudocyst.
- Stage IIB: Full-thickness macular hole (diameter <400 microns) with an operculum.
- Stage III: Full-thickness macular hole (diameter >400 microns) with surrounding thickened retina, including intraretinal cystoid spaces. The perifoveal and prefoveal hyaloid is separated from the macular retina.
- Stage IV: A stage III hole with a complete posterior vitreous detachment. OCT often cannot visualize the posterior hyaloid because it is too anterior.
A fundus fluorescein angiography or an optical coherence angiography (OCT-A) will help in detecting the retinal pathologies causing secondary ERM, such as diabetic retinopathy, choroidal neovascular membrane, retinal telangiectasias, retinal vein occlusions, etc.
Treatment / Management
Most patients with epiretinal membrane (ERM) do not require treatment if it doesn’t affect vision or cause significant metamorphopsia. Blue Mountains study [3] involving 3654 subjects showed that only 20% of epiretinal membranes progressed over five years, 26% regressed, and 39% remained the same. Only 20% of eyes with cellophane maculopathy progressed over a while. The primary treatment for ERM, apart from observation, is surgical. Surgery for ERM has a reasonable success rate, and most patients show improvement in visual acuity and a decrease in metamorphopsia. Hence, the aim of treatment for ERM should be to preserve or improve vision, minimize symptoms like metamorphopsia, diplopia, etc., and enhance the quality of life. Factors that affect the visual outcome include the duration of the condition, the degree of vitreomacular traction, and the cause of ERM. Idiopathic ERM has a better prognosis than ERM secondary to ocular pathology.(B2)
Non-surgical management includes the use of vitreopharmacolysis with ocriplasmin. Ocriplasmin is a recombinant proteolytic enzyme approved by the FDA for intravitreal injection for the treatment of symptomatic VMT. Ocriplasmin can relieve the VMT associated with ERM, which might provide relief from the associated metamorphopsia. It does not affect ERM. Its use in patients with both ERM and VMT is controversial. In patients unfit for lengthy retinal surgical procedures, intravitreal ocriplasmin can treat visual disturbances induced by VMT.
Surgical management involves pars-plana vitrectomy (PPV) with ERM and ILM peeling. PPV with membrane peeling has been used successfully for many decades, with an excellent visual outcome and a reduced recurrence rate. ILM is thought to provide a platform for the proliferation of fibroblasts, glial cells, and astrocytes for the retina to form ERM.[11] ILM peeling, along with membrane peeling, has become a standard procedure following the advent of staining dyes like trypan blue, indocyanine green (ICG), and brilliant blue G (BBG).[12] Triamcinolone is used to stain the vitreous and the membrane. Triamcinolone staining helps to induce posterior vitreous detachment and to ensure an excellent subtotal vitrectomy. Accelerated nuclear sclerosis or a worsening of cataracts is the most common complication of vitrectomy. If a cataract is present, the procedure should be combined with a cataract removal to ensure better visualization of the surgical procedure and also to prevent a subsequent second surgery.(B2)
Dye assisted ILM peeling has resulted in better visual outcomes, fewer recurrence rates, and reduced retinal striae.[13] Although ILM peeling is beneficial, it is also believed that it might cause damage to muller cells as ILM is the basal lamina, which is connected to the end feet of Müller cells.[14][15][16] Due to this uncertainty of the effectiveness versus the damage caused by ILM peeling in idiopathic ERM, many studies have been conducted comparing single peeling (only ERM) and double peeling (ERM and ILM peeling). Vitrectomy with ILM peeling results in visual improvement in long-term follow-ups and lower ERM recurrence rates. While vitrectomy with only ERM peeling is more efficacious in the reduction of a central retinal thickness (CRT) than with vitrectomy with ILM peeling.[17](A1)
Differential Diagnosis
The clinical appearance of the epiretinal membrane (ERM) is clearly remarkable. However, some conditions mimic an epiretinal membrane during their early stages:
- Fine vessel tortuosity: Combined hamartoma of retina and RPE
- Membrane over the retina: Proliferative vitreoretinopathy and tractional retinal detachment.
Prognosis
Epiretinal membrane (ERM) is asymptomatic in its early stage with a good prognosis. Blue Mountains study reveals 39% of the membranes to be non-progressive. Progressive ERM reduces vision and causes annoying metamorphopsia. For worsening ERM, pars plana vitrectomy with membrane and ILM peeling has an excellent prognosis both by improving visual acuity and reducing metamorphopsia. The recurrence of ERM ranges from 1%[18] to 21%.[13]
The recurrence and incomplete recovery of final visual acuity are due to incomplete removal of the membrane. Additional peeling of ILM removes the platform for cellular proliferation. One study has suggested that photoreceptor disruption detected by OCT and P1 implicit time delay on multifocal electroretinogram as significant predictors for poor visual recovery after ERM surgery.[19] To date, the most determining risk factor that predicts post-operative visual recovery is the duration of the ERM.
Complications
Epiretinal membrane (ERM) can cause various ocular complications, and these include:
- Accelerated nuclear sclerosis or cataract formation
- Retinal breaks or detachment
- Macular toxicity (trauma/light)
- Endophthalmitis
- Macular hole
- Vitreous hemorrhage
- Retinal petechiae/hemorrhage
- The dissociated optic nerve fiber layer
Deterrence and Patient Education
Patients should be informed that most epiretinal membranes that do not affect vision or cause metamorphopsia are best left alone. Periodic self-assessment with Amsler grid charts would help one to assess the beginning or progression of metamorphopsia. Besides, self-monitoring monocular vision also helps to detect early central vision changes. Patients should also be made aware that surgical procedures for epiretinal membrane (ERM) are very successful in addressing visual acuity symptoms and improving quality of life. Patient education and appropriate counseling will help patients to have realistic expectations.
Enhancing Healthcare Team Outcomes
Diagnosis and management of the epiretinal membrane (ERM) require appropriate diagnostic and surgical skills in addition to the availability of special equipment. Even though the diagnostic procedures are done by trained personnel, interpretation and diagnosis are made by a trained ophthalmologist. Hence appropriate referral to an ophthalmologist is mandatory for patients who present to primary eye care with decreased visual acuity and metamorphopsia unresolved by refractory correction. Surgical management needs intervention from a retinal surgeon, and hence, a liaison between a general ophthalmologist or optometrist is mandatory in the care of patients with epiretinal membranes.
Media
(Click Image to Enlarge)
References
Xiao W, Chen X, Yan W, Zhu Z, He M. Prevalence and risk factors of epiretinal membranes: a systematic review and meta-analysis of population-based studies. BMJ open. 2017 Sep 25:7(9):e014644. doi: 10.1136/bmjopen-2016-014644. Epub 2017 Sep 25 [PubMed PMID: 28951399]
Level 1 (high-level) evidenceNg CH, Cheung N, Wang JJ, Islam AF, Kawasaki R, Meuer SM, Cotch MF, Klein BE, Klein R, Wong TY. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011 Apr:118(4):694-9. doi: 10.1016/j.ophtha.2010.08.009. Epub 2010 Oct 29 [PubMed PMID: 21035863]
Level 2 (mid-level) evidenceFraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003 Jan:110(1):34-40 [PubMed PMID: 12511343]
Level 2 (mid-level) evidenceStevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clinical ophthalmology (Auckland, N.Z.). 2016:10():527-34. doi: 10.2147/OPTH.S97722. Epub 2016 Mar 29 [PubMed PMID: 27099458]
Wiznia RA. Posterior vitreous detachment and idiopathic preretinal macular gliosis. American journal of ophthalmology. 1986 Aug 15:102(2):196-8 [PubMed PMID: 3740180]
Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Investigative ophthalmology & visual science. 1977 May:16(5):416-22 [PubMed PMID: 852943]
Yamashita T, Uemura A, Sakamoto T. Intraoperative characteristics of the posterior vitreous cortex in patients with epiretinal membrane. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008 Mar:246(3):333-7. doi: 10.1007/s00417-007-0745-8. Epub 2008 Jan 12 [PubMed PMID: 18193261]
Govetto A, Lalane RA 3rd, Sarraf D, Figueroa MS, Hubschman JP. Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. American journal of ophthalmology. 2017 Mar:175():99-113. doi: 10.1016/j.ajo.2016.12.006. Epub 2016 Dec 18 [PubMed PMID: 27993592]
Hwang JU, Sohn J, Moon BG, Joe SG, Lee JY, Kim JG, Yoon YH. Assessment of macular function for idiopathic epiretinal membranes classified by spectral-domain optical coherence tomography. Investigative ophthalmology & visual science. 2012 Jun 14:53(7):3562-9. doi: 10.1167/iovs.12-9762. Epub 2012 Jun 14 [PubMed PMID: 22538422]
Level 2 (mid-level) evidenceAltaweel M, Ip M. Macular hole: improved understanding of pathogenesis, staging, and management based on optical coherence tomography. Seminars in ophthalmology. 2003 Jun:18(2):58-66 [PubMed PMID: 14566624]
Level 3 (low-level) evidencede Bustros S, Thompson JT, Michels RG, Rice TA, Glaser BM. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. The British journal of ophthalmology. 1988 Sep:72(9):692-5 [PubMed PMID: 3179258]
Level 2 (mid-level) evidenceFarah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. American journal of ophthalmology. 2009 Sep:148(3):332-40. doi: 10.1016/j.ajo.2009.04.003. Epub 2009 May 24 [PubMed PMID: 19477708]
Park DW, Dugel PU, Garda J, Sipperley JO, Thach A, Sneed SR, Blaisdell J. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003 Jan:110(1):62-4 [PubMed PMID: 12511347]
Level 3 (low-level) evidenceUemoto R, Yamamoto S, Aoki T, Tsukahara I, Yamamoto T, Takeuchi S. Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. The British journal of ophthalmology. 2002 Nov:86(11):1240-2 [PubMed PMID: 12386079]
Terasaki H, Miyake Y, Nomura R, Piao CH, Hori K, Niwa T, Kondo M. Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Investigative ophthalmology & visual science. 2001 Jan:42(1):229-34 [PubMed PMID: 11133873]
Level 1 (high-level) evidenceUemura A, Kanda S, Sakamoto Y, Kita H. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. American journal of ophthalmology. 2003 Aug:136(2):252-7 [PubMed PMID: 12888046]
Level 3 (low-level) evidenceChang WC, Lin C, Lee CH, Sung TL, Tung TH, Liu JH. Vitrectomy with or without internal limiting membrane peeling for idiopathic epiretinal membrane: A meta-analysis. PloS one. 2017:12(6):e0179105. doi: 10.1371/journal.pone.0179105. Epub 2017 Jun 16 [PubMed PMID: 28622372]
Level 1 (high-level) evidenceGupta OP, Brown GC, Brown MM. A value-based medicine cost-utility analysis of idiopathic epiretinal membrane surgery. American journal of ophthalmology. 2008 May:145(5):923-8. doi: 10.1016/j.ajo.2007.12.037. Epub 2008 Mar 10 [PubMed PMID: 18329000]
Kim JH, Kim YM, Chung EJ, Lee SY, Koh HJ. Structural and functional predictors of visual outcome of epiretinal membrane surgery. American journal of ophthalmology. 2012 Jan:153(1):103-10.e1. doi: 10.1016/j.ajo.2011.06.021. Epub 2011 Sep 19 [PubMed PMID: 21937015]
Level 2 (mid-level) evidence