Definition/Introduction
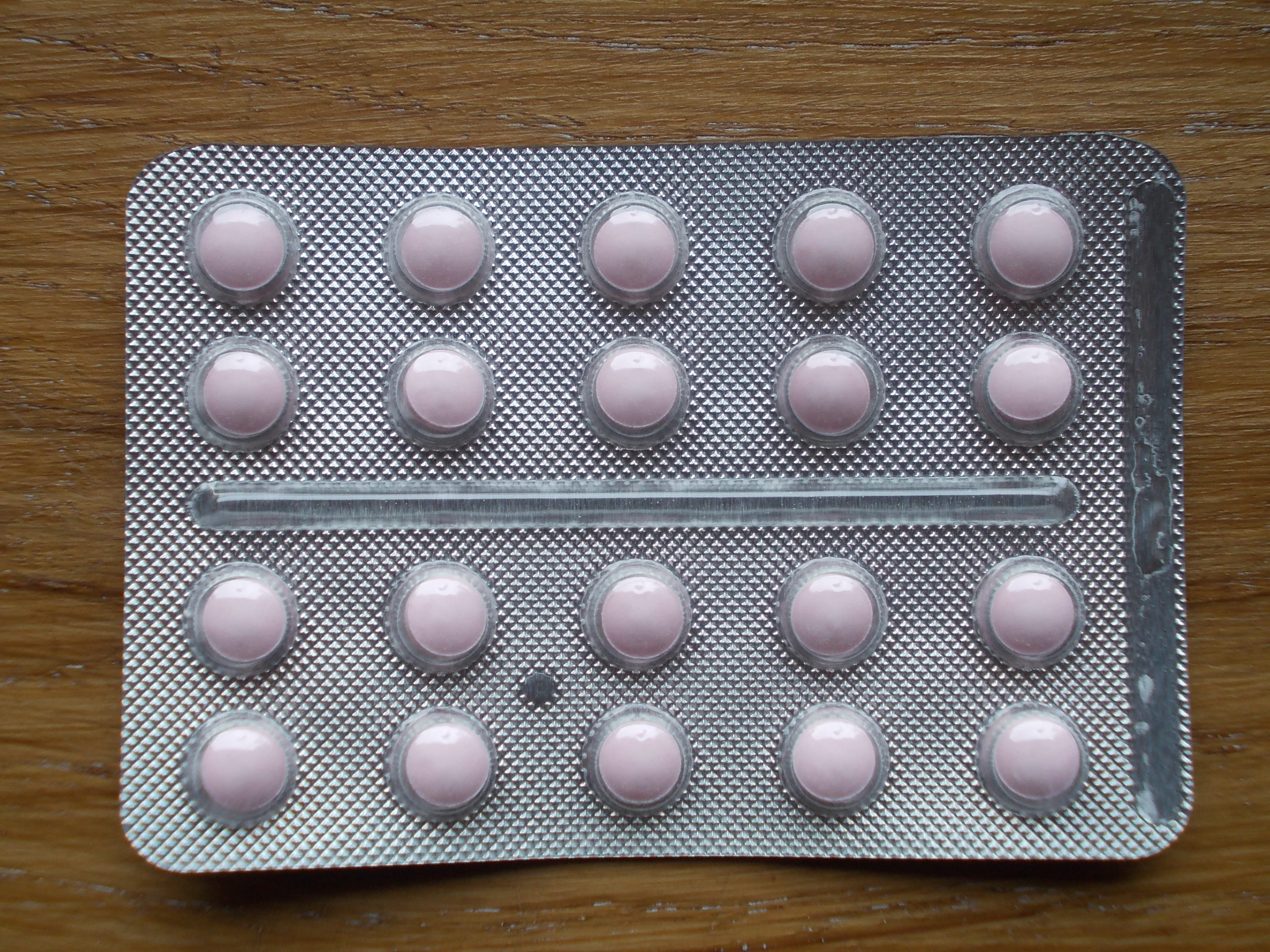
Packaging of pharmaceutical medications may vary depending on the use and type of substance packaged: prescription drugs, non-prescription (over-the-counter) drugs, and herbals and dietary supplements. Variations of pharmaceutical packaging include blister packs, alternative tamper-evident packaging, bottles, vials, ampules, intravenous bags, and calendar packs.[1][2] A blister pack is a form of tamper-evident packaging where an individual pushes individually sealed tablets through the foil in order to take the medication. This form of packaging can also help the individual recall if the previous dose was taken.[1] Calendar blister packaging is a derivation of a blister pack in which the blister is labeled with the date of the month or day of the week. This form of packaging has classically been used for oral contraceptives.[3] Other forms of tamper-evident packaging include seals or specially designed caps.
Child-resistant packaging of oral prescription drugs began with the Poison Prevention Packaging Act in the 1970s.[4] This act mandated packaging specific household chemicals and most oral prescription drugs in child-resistant packaging, ensuring that at least 80% of children cannot open it in a given time frame.[4][5] The enactment of the PPPA resulted in an estimated 45% reduction of child mortality due to the consumption of oral prescription drugs. Exceptions to this mandate include a caveat for the elderly and handicapped. With these populations, the individual or prescriber may request non-child-resistant packaging for ease of opening the container.[5] Some over-the-counter (OTC) drugs are also required to be packaged in child-resistant packaging, as mandated by the Food and Drug Administration. OTC drugs that contain active ingredients that were previously only available by prescription are now required to have child-resistant packaging.[6]
Optimal drug packaging maximizes the physical and chemical stability of the medication, which may be affected by environmental factors such as light, temperature, and humidity. Blister package material may be chosen based on its barrier properties. Common materials include polyvinyl chloride, polyvinyl dichloride, polychlorotrifluoroethylene (Aclar), aluminum foil, and cyclin olefin copolymer. Though aluminum foil offers the least moisture penetrance, polyvinyl chloride is often the preferred blister material due to its low cost and ease of production; however, polyvinyl chloride has many disadvantages. Polyvinyl chloride is a minimal moisture barrier linked to environmental issues associated with its disposal. Aclar provides better moisture protection than polyvinyl chloride but does not offer as much protection as an aluminum foil.[2]
A pharmacy insert is a written piece intended to inform the reader about various aspects of the medication. In the United States, there are three forms of written informational material associated with medicines: medication guides, patient package inserts, and consumer medication information packages. The Food and Drug Administration regulates medication guides and patient package inserts. On the other hand, consumer medication information packages are developed by commercial providers with the intent of distribution within pharmacies.[7] Whereas the goal of medication guides is to guide the health care professional, patient package inserts provide information intended for patient use.[8][9] Across the globe, pharmaceutical regulatory agencies regulate the contents of the package inserts. In Europe and the United States, the European Medicine Agency and the Food and Drug Administration have approved specific illustrations and symbols to be used in packaging inserts and must accurately portray the information without ambiguity.[10]
Medication packaging inserts provide unbiased prescribing and safety information for the health care provider. In June 2006, the FDA officially instated a new format of package inserts. The new format has three sections—the highlights of prescribing information, a table of contents for prescribing information, and the complete prescribing information.[9]
Highlights of Prescribing: This section is a bulleted list style summary of pertinent clinical information and is able to be cross-referenced to the third section, the prescribing information. In this section of every medication package insert, there must be a universal disclaimer written verbatim except for the medication name. The section also contains the drug name bolded, followed by the year of approval in the United States. Other sub-sections of this first section includes the black box warning, recent significant changes, indications and usage, dosage forms and strengths, dosage and administration, drug interactions, contraindications, warnings, adverse drug reactions, and use in specific populations.[9] The black box warning is an FDA mandated box warning of potential risks and serious adverse effects associated with a medication, such as an injury or death.[11] The indications and usage sub-section details conditions the drug may treat. The dosage forms and strengths sub-section details information regarding the amount of drug to be given to individuals the administration sub-section indicates how the drug may be given to the patient (i.e., intravenous, subcutaneous, orally, etc.). Interactions between the medication and other drugs are also detailed in this section as specific patient population contraindications. The adverse drug reactions sub-section makes the provider aware of the potential side effects of a drug. The final sub-section reviews the use of the drug in special populations such as pregnant women.
Full Prescribing Information Table of Contents: this section references all the sub-sections and sections in the full prescribing information.[9]
Full Prescribing Information: The final section begins with pertinent dosing information and warnings. This section also details all risks associated with taking the drug. Additional sub-sections include drug abuse and dependence, toxicity, clinical pharmacology, nonclinical toxicology, clinical studies, patient counseling information, how the drug is supplied, stored, and handled, and references.[9]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Although intended to act as safeguards for the patient, pharmaceutical packaging may hinder medical adherence. With child-resistant and tamper-evident packaging, many individuals face difficulties in opening the prescription packaging, discouraging them from taking their medications. Difficulties opening pharmacy packaging may include individuals using sharp objects in an attempt to open the packaging, breaking tablets during efforts to push them out of blister packs, and simply being unable to open the drug container due to tamper-evident packaging.[12] This problem is exacerbated in the elderly population, many of whom experience a decline in dexterity affecting their ability to open these containers. Alternatively, there are issues of concern with pharmacy packaging that is not tamper-evident or child-resistant, as this increases the risk of a child accidentally obtaining and consuming the medication.[1]
Also, with some forms of packaging, it may be difficult for individuals to be able to ascertain if they have taken the previous dose. Whereas blister packs and pill organizers aim to mitigate this problem, some individuals have difficulty removing the pill from blister packs without breaking the drug itself. Individuals also must accurately separate medications into the pill organizer in order for it to be beneficial.[1]
There are also concerns regarding differences in how drug information is conveyed to the general public versus the health care providers. While the Food and Drug Administration regulates both the medication guide insert and patient package inserts in the U.S., the patient targeted pharmacy brochures are not regulated. This lack of regulation on patient targeted brochures is of particular concern because unregulated pharmacy leaflets may address adverse drug reactions in a manner that may undermine their severity.[8] In addition, the accessibility of drug information is also of concern due to differential health literacy across the population. A lack of understanding or misunderstanding with pharmacy inserts may cause a patient to handle or consume a drug incorrectly, leading to possible adverse drug reactions or changes in bioavailability of the drug. Research has shown that illustrations augment individual comprehension of drug inserts; however, comprehension diminishes with pharmacy inserts solely comprising of text or illustrations.[3]
Clinical Significance
Medication packaging mostly affects the shelf life of a drug, which is determined by the physical and chemical stabilities of the product. Chemical stability is determined by how long the drug will continue to have 100 ± 5 % of the label claim potency and contain sufficiently low amounts of any degradation products. This stability is often the basis of the drug expiration date. Physical stability is associated with the drug’s appearance and action (i.e., dissolution). Moisture is a significant destabilizer of drugs; thus, care must be taken in package design to minimize moisture exposure to extend the shelf life of the pharmaceutical product.[13] Without adequate packaging to maintain the drug’s physical and chemical properties, the medication’s efficacy may be affected, thereby subjecting the patient to a lower than intended dose or unintended toxicity.
Pharmaceutical packaging comprised of polymers to preserve a drug may have unintended consequences should the polymers from the package leach into the medication.[14] This inadvertent additive to the drug may affect its safety, efficacy, or be associated with unintended toxicity. An additional clinical concern associated with drug packaging is its role as a barrier to medication adherence. As elderly, handicapped, and other individuals may have difficulty with child-resistant or tamper-evident packaging, they are more likely to forgo their medications and compromise their health.
Pharmacy packaging inserts yield further obstacles in delivering quality patient care and obtaining medical adherence. With low health literacy rates and confusing jargon often used in patient pharmaceutical packing, many individuals have difficulty comprehending the patient prescription inserts. This lack of comprehension subsequently increases the risk of adverse events associated with taking the medication incorrectly or forgoing treatment due to misunderstanding.[10] Patients are not alone in making errors due to misunderstandings of pharmacy inserts. The new format of package inserts in the U.S. was brought to fruition in June 2006 making it easier for health care providers to locate pertinent clinical information. It was previously estimated that preventable adverse drug reactions and medication errors cost the U.S. health care system $3.5 billion in preventable expenses.[9]
Nursing, Allied Health, and Interprofessional Team Interventions
Pharmacy packaging interventions are now at the forefront of the attempt to decrease medical noncompliance. The FDA and EMA have guidelines for the design of medications to optimize medical adherence; however, there are no guidelines for the design of pharmacy packaging to optimize patient usability.[12] A meta-analysis of 48 RCT studies found that groups receiving pharmacy packaging interventions via blister packs or pillboxes had significantly better medication adherence in comparison to the controls.[1] [Level 1] There are clear barriers to generalizing the findings of these studies to a broader population. This pharmaceutical intervention is not appropriate for individuals with dexterity issues and those who cannot accurately fill their pillboxes. Despite these exceptions, the studies show this may be a promising method to increase medication compliance for some individuals.
Additional studies examining the effects of calendar pill organizers have shown inconclusive results. Whereas an epilepsy study showed a significant improvement to medication adherence as measured by a reduction in seizure frequency, two studies on hypertensives and diabetics failed to show a significant decrease in medication adherence as measured by reductions in health care expenditure.[3] [Level 2] With such inconsistent results, the effectiveness of increasing compliance via pharmaceutical packaging may be augmented with patient education. Reports of patient confusion regarding how to open the blister packs and changes to the packaging identify potential areas of education. Health care professionals ranging from nurses to pharmacists to physicians should educate patients on the proper use of such blister packs to minimize user error.
Nursing, Allied Health, and Interprofessional Team Monitoring
Calendar pillboxes offer a feasible, tangible way for the individual, his/her caretaker, or other health care professionals to monitor medication adherence. In Japan, there have been successful attempts to virtually monitor patient medication compliance using a programmed calendar pill organizer. With the use of specially designed pill organizers, physicians were able to determine when the organizer was opened and the number of drugs removed from the organizer.[15] With this real-time monitoring, physicians were able to intervene and contact the patient if the individual was being noncompliant with the medication. Such innovation allows for a more hands-on approach to manipulating pharmaceutical packaging in order to improve patient medication adherence. Even in the absence of a monitoring system on calendar pill boxes, pill organizers offer a feasible vehicle for medication monitoring whether by a health care professional or a caretaker. In addition to the technologically enhanced pill organizers, researchers have turned to digital medicine to monitor and increase patients’ adherence to treatment.
A digital medicine program (DMP) analyzing the efficacy of digital medicine on Hepatitis C infected patients found higher rates of treatment adherence and sustained virologic response among those using the digital medicine program. This particular DMP was comprised of an oral drug encapsulated with an ingestible sensor, a patch to detect real-time medication consumption, a mobile app for patients to log their information and a health care provider portal for the team to coordinate patient management. If the software detected the patient was not wearing the patch, did not ingest the drug while wearing the patch, or did not consume the correct dose of the drug, the patient and the patient-care team was notified and the provider or a member of the research team reached out to the patient to discuss the noncompliance.
A post-engagement survey showed that many participants felt more involved in their healthcare through the DMP due to the personal mobile app tracking and the ability to connect with their patient-care team through the app.[16] With the improved adherence using a DMP, the study highlights the potential for advancements in pharmaceuticals to improve patient medication adherence.
Media
(Click Image to Enlarge)
References
Conn VS, Ruppar TM, Chan KC, Dunbar-Jacob J, Pepper GA, De Geest S. Packaging interventions to increase medication adherence: systematic review and meta-analysis. Current medical research and opinion. 2015 Jan:31(1):145-60. doi: 10.1185/03007995.2014.978939. Epub 2014 Nov 4 [PubMed PMID: 25333709]
Level 1 (high-level) evidenceAllinson JG, Dansereau RJ, Sakr A. The effects of packaging on the stability of a moisture sensitive compound. International journal of pharmaceutics. 2001 Jun 19:221(1-2):49-56 [PubMed PMID: 11397566]
Zedler BK, Kakad P, Colilla S, Murrelle L, Shah NR. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clinical therapeutics. 2011 Jan:33(1):62-73. doi: 10.1016/j.clinthera.2011.02.003. Epub [PubMed PMID: 21397775]
Level 1 (high-level) evidenceAgarwal M, Lovegrove MC, Geller RJ, Pomerleau AC, Sapiano MRP, Weidle NJ, Morgan BW, Budnitz DS. Circumstances Involved in Unsupervised Solid Dose Medication Exposures among Young Children. The Journal of pediatrics. 2020 Apr:219():188-195.e6. doi: 10.1016/j.jpeds.2019.12.027. Epub 2020 Jan 28 [PubMed PMID: 32005542]
Rodgers GB. The safety effects of child-resistant packaging for oral prescription drugs. Two decades of experience. JAMA. 1996 Jun 5:275(21):1661-5 [PubMed PMID: 8637140]
Consumer Product Safety Commission. Child-resistant packaging for certain over-the-counter drug products. Final rule. Federal register. 2001 Aug 2:66(149):40111-6 [PubMed PMID: 11732555]
Pizzol TDSD, Moraes CG, Arrais PSD, Bertoldi AD, Ramos LR, Farias MR, Oliveira MA, Tavares NUL, Luiza VL, Mengue SS. Medicine package inserts from the users' perspective: are they read and understood? Revista brasileira de epidemiologia = Brazilian journal of epidemiology. 2019 Mar 14:22():e190009. doi: 10.1590/1980-549720190009. Epub 2019 Mar 14 [PubMed PMID: 30892472]
Level 3 (low-level) evidenceDoshi P, Sieluk J, Hung A. The possible harms of statins: What do product labels, patient package inserts, and pharmacy leaflets tell us? Journal of the American Pharmacists Association : JAPhA. 2019 Mar-Apr:59(2):195-201. doi: 10.1016/j.japh.2018.12.003. Epub 2019 Jan 17 [PubMed PMID: 30661956]
Watson KT, Barash PG. The new Food and Drug Administration drug package insert: implications for patient safety and clinical care. Anesthesia and analgesia. 2009 Jan:108(1):211-8. doi: 10.1213/ane.0b013e31818c1b27. Epub [PubMed PMID: 19095852]
Pires C, Vigário M, Cavaco A. Graphical content of medicinal package inserts: an exploratory study to evaluate potential legibility issues. Health information and libraries journal. 2016 Jun:33(2):121-39. doi: 10.1111/hir.12128. Epub 2015 Dec 7 [PubMed PMID: 26640041]
Kafali N, Progovac A, Hou SS, Cook BL. Long-Run Trends in Antidepressant Use Among Youths After the FDA Black Box Warning. Psychiatric services (Washington, D.C.). 2018 Apr 1:69(4):389-395. doi: 10.1176/appi.ps.201700089. Epub 2017 Dec 15 [PubMed PMID: 29241433]
Notenboom K, Leufkens HG, Vromans H, Bouvy ML. Learning from patients: Identifying design features of medicines that cause medication use problems. International journal of pharmaceutics. 2017 Jan 30:517(1-2):128-134. doi: 10.1016/j.ijpharm.2016.12.004. Epub 2016 Dec 5 [PubMed PMID: 27931784]
Waterman KC, MacDonald BC. Package selection for moisture protection for solid, oral drug products. Journal of pharmaceutical sciences. 2010 Nov:99(11):4437-52. doi: 10.1002/jps.22161. Epub [PubMed PMID: 20845442]
Singh G, Gollapalli R, Blinder A, Patel M. Identification of leachable impurities in an ophthalmic drug product originating from a polymer additive Irganox 1010 using mass spectroscopy. Journal of pharmaceutical and biomedical analysis. 2018 Apr 15:152():197-203. doi: 10.1016/j.jpba.2018.01.053. Epub 2018 Feb 2 [PubMed PMID: 29414013]
Hoshi K, Kawakami J, Aoki S, Hamada K, Sato K. Real-time wireless compliance monitoring system using calendar-type pill organizer. Technology and health care : official journal of the European Society for Engineering and Medicine. 2013:21(5):455-67. doi: 10.3233/THC-130747. Epub [PubMed PMID: 24029048]
Sulkowski M, Luetkemeyer AF, Wyles DL, Martorell C, Muir A, Weisberg I, Gordon SC, McLain R, Huhn G. Impact of a digital medicine programme on hepatitis C treatment adherence and efficacy in adults at high risk for non-adherence. Alimentary pharmacology & therapeutics. 2020 Jun:51(12):1384-1396. doi: 10.1111/apt.15707. Epub 2020 Apr 30 [PubMed PMID: 32352586]