Introduction
An abdominal aortic aneurysm (AAA) is defined as a permanent dilation of the abdominal aorta, with a diameter greater than 3 cm or a diameter greater than 50% of the aortic diameter at the level of the diaphragm. If left untreated, progressive vessel wall degeneration leads to dilation and thinning of the vessel. Eventually, these changes can result in the rupture of the AAA. AAA prevalence and incidence rates have decreased over the last 20 years, both in developed and in developing countries. This decrease has been attributed partially to the decline in smoking. Prevalence is negligible before the age of 55 to 60 years, and after that, the prevalence increases with age. AAA prevalence is up to fourfold more in men (between 1.3% and 12.5%) than women (between 0.0% and 5.2%).[1] The risk of rupture increases with the size of the aneurysm: the 5-year risk for aneurysms less than 5 cm is 1% to 2%, whereas it is 20% to 40% for aneurysms greater than 5 cm in diameter. Abdominal aortic aneurysm represents about 1% of deaths in males over the age of 65 and is the tenth leading cause of death in men 65 years of age or older. The mortality rate of ruptured abdominal aortic aneurysm is over 80%.[2] Early diagnosis and treatment, therefore, is very important before its rupture.
To this day, treatment for AAA relies on two different surgical methods: Endovascular placement of an aortic stent graft (EVAR) and open surgical repair of AAA (OSR). Open surgical repair is a major operation involving the excision of dilated area and placement of a sutured woven graft. The surgery may be performed electively or under emergent situations. Unlike OSR, the EVAR is meant to seal the sac from the inside of the aneurysm, while the aneurysm wall is left untouched. The paradigm is therefore changed from replacing the aneurysm to excluding it from the systemic circulation.[3][4]
A serial noninvasive follow-up of small aneurysms (less than 5 cm) is an alternative to surgery. Ultrasonography is the recommended modality for surveillance; it should be performed every three years for aneurysms 3 to 3.9 cm in diameter, or annually for aneurysms 4.0 to 4.9 cm. There is no specific medication or other therapy that can be recommended to reduce the rate of aneurysm growth. Moreover, beta-adrenergic blockers and renin-angiotensin inhibitors, have not proven effective in reducing the rate of aneurysm growth. Lifestyle changes, such as exercise, also have not demonstrated a reduction in the aneurysmal growth rate. However, smoking cessation leads to a reduction in aneurysmal growth rate, as well as the risk of aneurysm rupture. Abdominal aortic aneurysm patients’ have a significant risk for future cardiovascular events that should be addressed. Recommendation of a healthy lifestyle (including exercise and a healthy diet) and blood pressure control, statins, and antiplatelet therapy, should be considered in all patients with abdominal aortic aneurysms.[3]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Abdominal aortic aneurysms are typically categorized based on the location of the dilation. In suprarenal or para-visceral aneurysms, the dilation involves the visceral arteries, whereas if para-renal, it includes the origins of the renal arteries. The majority of abdominal aortic aneurysms are infrarenal in extent, infrarenal if it begins below the renal arteries.[4]
Indications
Elective abdominal aortic aneurysm repair is indicated under any one of the following conditions:
- The AAA diameter is greater than 5.5 cm in diameter in men. In women, it might be considered from a smaller diameter (5.0 cm).[3]
- The patient with AAA is symptomatic[3]
- The AAA is rapidly expanding (more than 1 cm/year) irrespective of the absolute diameter.[3]
The patient should also undergo assessment for physiological fitness, cardiac and respiratory function, including cardiopulmonary exercise testing echocardiogram, and lung function tests. Moreover, coronary revascularization before aneurysm repair should be considered in patients. There are a significant number of patients with AAA who are not considered to be suitable for repair (including endovascular approach) because of other comorbidities or limited life expectancy. For these patients, strategies to reduce cardiovascular risk and continued surveillance, are recommended.[3][4]
In the case of a ruptured aortic aneurysm, attention is directed to stabilizing the patient for immediate open surgery. Often the diagnosis is made clinically and can be supported by Doppler ultrasound. If the patient is too unstable, a CT scan should not be performed, but proceed directly to the operating room. If the patient is stable, a CT aortogram confirms the diagnosis and assesses suitability for emergency EVAR.[5]
Contraindications
While the contraindications for OSR are loosely defined, under elective setting, it is mainly used for patients unfit for EVAR.[5] The main limitation of EVAR is morphologic and anatomic.
- Aortic neck length is the distance from the lowest renal artery to the top of the aneurysm. It is the most important factor in successful EVAR. The aortic neck angle is defined as the angle between the flow axis of the supra- and infrarenal aorta. These two anatomic factors greatly affect device delivery, aneurysm exclusion, and stent-graft proximal fixation. longer aortic neck greater than 1.5 cm, a wider aortic angle greater than 150 degrees, and absence of calcification or thrombus are considered favorable.[6]
- Aneurysm morphology is determined by different parameters, such as the angle of the aneurysm with respect to the aortic long axis, presence of thrombus, and branching vessels from the aneurysm. A small aneurysmal angle makes stent-graft delivery difficult. Moreover, a thrombus found in the aneurysm (intraluminal or mural) may lead to distal emboli. Branching vessels from the aneurysm such as an inferior mesenteric artery, lumbar arteries, and median sacral artery may lead to endoleak after EVAR.[6]
- Anatomy of iliac and common femoral arteries also affects stent-graft delivery and distal sealing. Presence of atherosclerotic plaque or torsion of this vessel's association with an increased risk for graft limb occlusion.[6]
Technique or Treatment
The open aortic repair was graded as a high-risk intervention (defined as carrying a risk of cardiovascular death or myocardial infarction of 5% or more within 30 days), whereas EVAR is graded as an intermediate risk intervention with a cardiac risk between 1% and 5%. Nearly all the evidence suggests a significant short-term survival benefit for EVAR over OSR, with a similar long- term outcome up to 15 years of follow up. Higher safety profile and efficiency, along with a lower rate of perioperative morbidity and mortality, have been associated with EVAR, compared to the OSR. Hence, the European society of vascular surgery recommends in most patients with suitable anatomy and reasonable life expectancy, endovascular abdominal aortic aneurysm repair as the preferred treatment modality.[3]
Open aortic repair:
The open surgical repair aim is replacing the aneurysmal sac with a synthetic graft tube. The procedure is carried out in a sterile environment under general anesthesia. First, the abdominal aorta is exposed most commonly via a midline incision from the xiphoid to the pubis. Alternatively, a transverse subcostal incision below the ribcage can be used, or less commonly, a retroperitoneal approach is chosen. While the lower postoperative incidence of hernia is associated with transverse subcostal incision, midline incision enables access to all abdominal organs with relative ease. Hence the decision about the incision should be driven by surgeon preference and patient factors. Second, a proximal clamp is placed just below the renal arteries. Later, distal clamps are placed on the common iliac arteries. Third, the aneurysmal segment is removed and replaced with a tube graft. Most commonly, textile polyester material, specifically polyethylene terephthalate, is used. It is recommended to anastomose the aortic graft as close as possible to the renal arteries to prevent later aneurysm development in the remaining infrarenal aortic segment. Only in some selected cases, reimplantation of the inferior mesenteric artery into the graft may be performed. It should be considered if insufficient perfusion of pelvic organs or risk for colonic ischemia is suspected. Finally, abdominal wall closure is performed.
Endovascular placement of an aortic stent graft:
The endovascular approach aim is excluding the aneurysmal sac from the systemic circulation by placing a stent graft inside the aorta. The procedure is carried out under fluoroscopic guidance and can be carried under general anesthesia or local anesthesia. First, access via the patient's common femoral artery established, utilizing a surgical incision or percutaneously, bilaterally, or unilaterally. Then, vascular sheaths are introduced into the femoral arteries, through which guidewires, catheters, and the endograft are passed. Second, angiography images are performed to determine: aneurysmal sac extent, graft landing zone, and renal arteries location. Third, the "main body" of the endograft is placed over the aneurysmal sac, followed by the "limbs" which join the main body and extend to the iliac arteries. Fourth, the graft is molded into place with a balloon, and an angiogram is used to confirm position and absence leaks. Finally, the wires, sheaths, and delivery systems are withdrawn, and femoral artery hemostasis achieved.
Complications
Postoperative mortality of OSR for AAAs has decreased over the years. While the mortality rate is highly variable among studies, it is considered to be less than 50%. Furthermore, EVAR has a lower short-term mortality rate than OSR.
The complication rate following OSR varies among studies. Post-operative complications are pulmonary (42%), cardiac (18%), renal (17%), ischaemic colitis (9%), and wound complications (7%). Postoperative end-organ ischemia, including postoperative colonic ischemia, acute lower limb ischemia, or spinal ischemia, are infrequent but serious complications. Therefore patients should be closely monitored for these conditions. EVAR carries similar risk as OSR but with a lower incident rate. Unique complications of EVAR are graft migration and endoleaks.[3]
Migration, a condition in which due to loss of fixation, the graft moves from the original location. Hooks and barbs assist aortic attachment, increasing the friction to prevent migration from occurring. An endoleak is a condition in which the graft fails to exclude blood from entering the aneurysmal sac. Endoleaks can subdivide into; type I endoleak- due to improper fixation or sealing between the proximal graft and the blood vessel at the proximal or distal zone of the stent. Type II endoleak-blood flow from patent collateral arteries (lumbar or inferior mesenteric arteries), to the aneurysmal sac. Type III endoleak-separation of stent-graft components with possible overlapping of stent material allowing leakage. Type IV and V endoleak- type IV endoleak-blood flow through the pores of the stent-graft. Type V endoleak is blood flow into the aneurysmal sac from an unknown source. In the past, EVAR associated with access site complications such as dissection, perforation, hematoma, and fistula. But with technical advancement, these complications are less frequent.[6][5]
From a didactical point of view, it might be important to classify common postoperative complications as immediate, early, and late;
- OSR immediate complication: hemorrhage and myocardial infarction.
- OSR early complication: hemorrhage, ileus, ischemic colitis, myocardial infarction, pneumonia, renal failure, wound infection.
- OSR late complication: incisional hernia
- EVAR immediate complication: aneurysm rupture with conversion to open, stent misplacement, myocardial infarction
- EVAR early complication: contrast nephropathy, endoleaks
- EVAR late complication: endoleaks, stent migration
Clinical Significance
Abdominal aortic aneurysm repair is a life-saving procedure. The risk of death from ruptured AAA is as high as 90%.[2] Furthermore, elective abdominal aortic repair also plays a crucial role. In one study, following patients who declined abdominal aortic aneurysm elective repair for a period of 10-years, demonstrated that rupture of the aortic aneurysm was the cause of death in 36% to 55% of these patients.[7] Following patients who received abdominal aortic aneurysm elective repair for a similar period of time, demonstrated that mortality associated with aneurysm rupture after repair (secondary) was 8% and 2% for EVAR and OSR respectively.[8]
Enhancing Healthcare Team Outcomes
Unruptured abdominal aortic aneurysms are detected more than ever before primarily because of more frequent use of accessible imaging tools and asymptomatic patient screening. The majority of patients with an abdominal aortic aneurysm are asymptomatic or present with nonspecific symptoms. Screening of asymptomatic patients has shown reduced mortality. Hence, educating first primary care physicians on the criteria for screening and followup for an abdominal aortic aneurysm is highly important.
Screening Recommendation:
- All men above age 65 years should be screened with ultrasound, at least once in a lifetime.[3]
- Women above age 50 with a first-degree family member with a history of AAA should be considered for AAA screening in 10 years intervals.[3]
- Men or women with a peripheral aortic aneurysm should be considered for AAA screening, every 5 to 10 years.[3]
Referral to a vascular surgeon at the time of initial diagnosis of aortic aneurysm, of any diameter is recommended. Furthermore, abdominal aortic aneurysm patients’ significant risk for another cardiovascular event. Hence, lifestyle changes, smoking cessation, and referral for cardiologists can improve patients' outcomes.[3]
The biggest risk of abdominal aortic aneurysm is the risk of rupture. However, less than half of the patient presents with the classical triad of hypotension, pulsatile mass, and abdominal and back pain. Moreover, it has been shown that more than 30% of ruptured AAA are misdiagnosed.[3] Therefore, education of first responders including the nurse practitioner, primary care physicians, physician assistants, and emergency department physicians can facilitate diagnosis in the emergency scenario. Establishing a protocol for urgent management has also shown to improve outcomes.[5]
The mortality rate of emergency surgical intervention following rupture is ten-fold higher than the mortality of patients undergoing planned intervention under specialist vascular surgeons. Hence it is important to educate patients regarding the importance of elective surgery. Moreover, once the decision for repair has been made, a multidisciplinary approach involving a cardiologist and pulmonologist should be utilized for risk stratification and medical optimization before repair to reduce negative outcomes.[3][5]
Media
(Click Image to Enlarge)
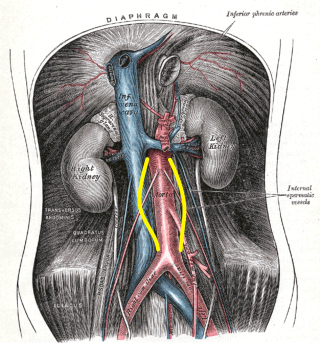
Abdominal Aortic Aneurysm, Illustration. Distention is shown by the yellow markings. This illustration also shows the aorta, inferior vena cava, and aneurysms.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Video to Play)
AAA. Large abdominal aortic aneurysm (AAA) shown with vertebral bone shadow and intramural thrombus.
Contributed by Meghan Herbst, MD
References
Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. International journal of environmental research and public health. 2018 Dec 10:15(12):. doi: 10.3390/ijerph15122805. Epub 2018 Dec 10 [PubMed PMID: 30544688]
Level 1 (high-level) evidenceMedical Advisory Secretariat. Endovascular repair of abdominal aortic aneurysm: an evidence-based analysis. Ontario health technology assessment series. 2002:2(1):1-46 [PubMed PMID: 23074438]
Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z, Esvs Guidelines Committee, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F, Document Reviewers, Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein HH, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco JB, Verhagen H. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2019 Jan:57(1):8-93. doi: 10.1016/j.ejvs.2018.09.020. Epub 2018 Dec 5 [PubMed PMID: 30528142]
Level 1 (high-level) evidenceKuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert review of cardiovascular therapy. 2015:13(9):975-87. doi: 10.1586/14779072.2015.1074861. Epub [PubMed PMID: 26308600]
Level 3 (low-level) evidenceChaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of vascular surgery. 2018 Jan:67(1):2-77.e2. doi: 10.1016/j.jvs.2017.10.044. Epub [PubMed PMID: 29268916]
Level 1 (high-level) evidenceKim HO, Yim NY, Kim JK, Kang YJ, Lee BC. Endovascular Aneurysm Repair for Abdominal Aortic Aneurysm: A Comprehensive Review. Korean journal of radiology. 2019 Aug:20(8):1247-1265. doi: 10.3348/kjr.2018.0927. Epub [PubMed PMID: 31339013]
Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? Journal of vascular surgery. 2001 Apr:33(4):752-7 [PubMed PMID: 11296328]
Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet (London, England). 2016 Nov 12:388(10058):2366-2374. doi: 10.1016/S0140-6736(16)31135-7. Epub 2016 Oct 12 [PubMed PMID: 27743617]
Level 1 (high-level) evidence