Introduction
The mammary glands are one of the distinguishing features found on mammals. Although male and female mammary glands develop similarly from birth to pubescence, they undergo different changes at the onset of puberty. In females, the previously quiescent mammary epithelium invades the mammary fat pad, leading to epithelial proliferation, breast enlargement, and increased fatty tissue deposition. In males, this process is inhibited by increasing testosterone levels, which act on the mesenchymal cells of the mammary stroma to induce regression and necrosis.[1][2][3][4][5]
Mammary gland pathophysiology has been studied primarily in females, as they are considered vestigial organs in males. Nevertheless, both male and female mammary glands can be affected by pathologic entities, and thus, both have clinical relevance.[6][7]
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
The mammary glands are modified tubuloalveolar apocrine sweat glands located on the anterior thoracic wall. They consist of an epithelial bilayer contained within active adipose tissue. These cells have the support of a loose framework of dense fibrous connective tissue known as Cooper ligaments.[3][4][5]
Two distinct types of cells comprise the epithelial bilayer. Luminal cuboidal cells form the inner portion and line the lactiferous ducts. These ducts radiate from the nipple and dilate into the lactiferous sinuses just beneath the areola. Further subdivision of the lactiferous ducts leads to lobes and lobules, of which the adult female mammary gland has 15 to 20 and 20 to 40, respectively. Each lobule ends in small bulb-like glands known as terminal ductal lobular units, wherein milk gets produced in response to prolactin. The outer portion of the bilayer is constituted by myoepithelial cells. These spindle-shaped cells have smooth muscle cell properties and participate in the process of milk ejection during lactation. Together, the epithelial components of the mammary gland constitute 10 to 15% of its overall volume.[1][2][3][5][8]
Transplantation and lineage tracing studies have shown that the epithelial components of the mammary gland originate from the stem and progenitor cells found in the ductular and basal epithelia. However, there is controversy since more than one cell population with stem cell properties has been found on the mammary epithelium. The known stem cell populations include mammary-reconstituting units, bipotent mammary stem cells (MaSCs), and unipotent mammary epithelial progenitors. The bipotent MaSCs can differentiate into both luminal and myoepithelial cells and are characterized by the profile CD49f/CD29/CD24. Stem cell expansion is mediated by progesterone, which induces RANK-L secretion in progesterone positive mammary epithelial cells. Secreted RANK-L acts on the RANK receptor found on hormone receptor-negative progenitor cells, which induces their proliferation.[9][10][11]
Each lactiferous duct connects to the exterior through orifices found in the nipple. These 0.5 mm orifices have tiny sphincters that prevent leakage during breastfeeding. A circular, pigmented area known as the areola surrounds the nipple. The pigment of the areola varies among individuals, as it can range from pink to nearly black. This pigment is also subject to darkening during puberty, pregnancy, and sexual arousal and orgasm. The areola is covered by stratified squamous epithelium, which is continuous with the nipple and surrounding skin. Additionally, the areola contains in its periphery some nodular elevations known as tubercles of Morgagni, which represent the openings of Montgomery glands. These are modified glands that represent an intermediate stage between sweat and true mammary glands. They secrete a substance that provides lubrication during breastfeeding. Deep to both areola and the nipple, smooth muscle bundle fibers are responsible for nipple erection secondary to various sensory stimuli, including cold, arousal, and breastfeeding.[1][5][12][13]
The adipose component of the mammary gland forms a large portion of the breast’s stromal fat pad. The variation in breast size amongst women is related to the adipose tissue volume rather than the epithelial component itself. Adipose tissue is abundant in the interlobular spaces, while its presence in the intralobular stroma is scarce. Adipose tissue actively regulates mammary gland homeostasis, as it participates in epithelial growth, intercellular communication, angiogenesis, and milk production. Additionally, it serves as a reservoir of interstitial fluids that includes locally-derived molecules (for example, prolactin) and those synthesized elsewhere. The intimate and unique relationship between the adipose microenvironment and the mammary epithelium raises the question as to whether these adipocytes are functionally different from those in other body depots.[1][2][10][14]
Fibroblasts are also important components of the mammary stroma. These cells most often neighbor the basal side of the epithelial branching tree. They have various functions, including growth factor synthesis, metalloproteinase (MMP) production, and extracellular matrix (ECM) deposition. The ECM is made up of collagen types I and III, proteoglycans, hyaluronic acid, fibronectin, and tenascins. It actively supports epithelial survival by suppressing apoptosis, a process mediated by MMP-2. It also participates in secondary and tertiary branching of mammary ducts, a process mediated by MMP-3. The ECM also participates in tumor suppression via virtue of the intact basement membrane. The basement membranes are thin, 100-nm thick sheets of glycoproteins and proteoglycans, laminins, and collagen IV fibrils.[2][15][16][17]
The vascular and lymphatic networks develop in close association with the mammary epithelial tree. Myoepithelial cells synthesize VEGF-C and VEGF-D, which likely drive lymphangiogenesis. Adipocytes can also regulate angiogenesis, as they too secrete VEGF.[2][14][18]
Immune cells regulate invasion into the fat pad, and thus, are necessary for ductal morphogenesis; this is especially true for macrophages, mast cells, and eosinophils. Mast cells are also implicated in mammary involution during the later stages of life, as they are capable of activating plasma kallikrein and, thus, the plasminogen cascade.[2][19]
Function
The primary function of the female mammary gland is to produce and secrete milk for the nourishment of newborns. Additionally, an erogenous function exists for both the adult male and female mammary glands.[6]
The mammary gland undergoes changes during both the menstrual cycle and pregnancy in preparation for lactation.
- Menstrual cycle
- Follicular phase: high estrogen levels stimulate ductal proliferation. The intralobular stroma remains dense, appearing more collagenous and less cellular. Additionally, this phase demonstrates an increase in Treg number.[20]
- Mid-luteal phase: there is an increase in the size and height of epithelial cells. The high levels of progesterone trigger the formation of epithelial alveolar buds. Moreover, secretions start to accumulate in the ducts, forming lumina. The number of macrophages also increases when compared to the follicular phase. Mammary tactile sensitivity is at its maximum during this phase.[20]
- Late-luteal phase: before the onset of menstruation, the glandular epithelium undergoes regression via apoptosis.[20]
- Pregnancy – the observed changes are modulated by an increase in the lactogenic hormone complex (estrogen, progesterone, and prolactin). Other hormones, such as placental lactogen, are also involved.[5]
- First trimester: the proliferation of MaSCs and progenitor cells leads to elongation and branching of ducts. The process of alveolar morphogenesis also begins. Each alveolus forms from a mesh-like bilayer of myoepithelial and epithelial cells, which encircles the alveolar lumen. Clinically, superficial veins dilate, and the areola darkens and grows.[1][5]
- Second trimester: increases in prolactin levels stimulate epithelial differentiation at the alveolar lumen, forming lactocytes (secretory differentiation). Colostrum starts accumulating in the alveoli. The intralobular connective tissue becomes infiltrated by plasma cells, lymphocytes, and eosinophils.[5][19][21]
- Third trimester: this phase characteristically demonstrates the maturation of lactocytes, which start to accumulate fat globules within their cytoplasm.[1][5]
After the placenta is delivered, progesterone levels decrease, lifting its inhibitory effect over prolactin. This allows for the initiation of copious milk secretion (secretory activation). The basement membrane that separates the mammary stroma from the epithelium becomes tight, with reduced permeability. This occurs in order to regulate the movement of milk components from the systemic circulation into the alveolar lumen. Oxytocin gets released in response to suckling, which then stimulates myoepithelial cell contraction in the alveoli and ducts, causing the ejection of milk.[1][5]
The first secretion (3 to 5 days post-parturition) is known as colostrum, which is initially secreted during the twelfth to sixteenth week of pregnancy (a process known as lactogenesis I). This phase is followed by transitional milk for 2 to 3 weeks postpartum (lactogenesis II), after which milk is considered mature (lactogenesis III). Various mammary cell types are present in breastmilk, including human breastmilk stem cells, bipotent MaSCs, myoepithelial cells, and lactocytes.[5]
After weaning, breastmilk starts accumulating in the ducts. The resulting breastmilk stasis initiates the process of mammary involution, although the involved mechanisms are not fully understood. The first phase of involution demonstrates the regression of epithelial and stromal tissues. An increase in hydrolytic enzymes clears the residual milk. This phase lasts up to two weeks and is reversible. The second phase is irreversible and is characterized by luminal cell loss and stromal remodeling. The apoptotic cells get shed into the alveolar lumen, where they are thought to be cleared by macrophages. However, murine studies suggest that a subpopulation of mammary epithelial cells may also phagocytize apoptotic epithelial cells, which brings the role of macrophages into question. After involution, the breast is similar but not identical to its pregestational state. Some epithelial cells escape the clearing process and remain as memory precursor cells that fuel mammary remodeling in subsequent pregnancies.[1][5]
Following menopause, the ductal and glandular elements of the breast begin to involute. This process gets triggered by declining ovarian function and reduced levels of estrogen and progesterone. Eventually, the fat and stromal elements begin to regress as well, leading to breast shrinkage and loss of contour. Breast ptosis may develop as the suspensory ligaments of Cooper relax.[1][5]
Tissue Preparation
The mammary gland is frequently biopsied to confirm the diagnosis of breast cancer. Various stains can be used to determine certain tumor characteristics, such as grade and histological type. For the initial tissue preparation, the biopsy gets fixed in 10% neutral-buffered formalin for 48 hours at 4ºC. It should also be dehydrated in 70% ethanol to minimize adipose distortion. Following the initial 48 hours, the fixated specimens are transferred to xylene, embedded in paraffin, sectioned at 2 to 5 mm, and dyed with the desired stain.[22][23]
Histochemistry and Cytochemistry
Various stains are useful for the study of the mammary glands. The hematoxylin and eosin combination is useful for the visualization of various structures. The hematoxylin component stains the nuclei in blue/purple, while the eosin gives a pinkish hue to eosinophilic structures (e.g., cytoplasm, collagen, and muscle fibers). Masson’s trichrome stains collagen fibers and is mostly used to evaluate tumor fibrosis. Moreover, immunostaining can be used to identify antigens that serve as important diagnostic markers. Some of the most relevant examples include antibodies against the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). These markers help classify malignant tumors into molecular subtypes (luminal-A, luminal-B, HER-2 enriched, and triple-negative). Other antibodies used in immunohistochemistry include those against myoepithelial antigens (a-SMA, myosin, calponin, p63, CD10, S100) and luminal antigens (cytokeratins [CK] 7, 8, 18, and 19). Differentiation between cell types is generally done using CK5 for bipotent MaSCs, CK19 for ductal luminal cells, CK18 for alveolar luminal cells, and CK14 for myoepithelial cells. CK8 and E-cadherin are especially useful for distinguishing between ductal and lobular carcinomas. Although CK8 is positive for both, it usually stains the peripheral cytoplasm in ductal carcinoma, while it has a perinuclear staining pattern in lobular carcinoma. The E-cadherin antigen is mainly expressed in ductal carcinomas and absent in lobular ones.[5][24][25][26][27]
Clinical Significance
Breast cancer is the most clinically relevant pathology of the mammary gland. Among females, it represents the most common malignancy, as well as the leading cause of cancer death. In males, it is a rare entity, representing 1% of cancer diagnoses and less than 0.1% cancer-related deaths. Risk factors for female breast cancer include advanced age, positive family history, early menarche, late menopause, nulliparity, oral contraceptive use, obesity, and full-term pregnancy after the age of 30. In addition, around 5 to 10% of breast cancer cases are considered to be hereditary, with most cases linked to germline BRCA1/2 mutations. On the other hand, risk factors for male breast cancer include obesity, positive family history, Klinefelter syndrome, gynecomastia, orchitis, testicular injury, cryptorchidism, alcoholism, liver disease, radiation exposure, and germline BRCA1/2 mutations. The most common histological type of breast cancer is invasive ductal carcinoma, which represents about 80% of all breast cancers. Other less common histological types include lobular, tubular, medullary, mucinous, neuroendocrine, papillary, metaplastic, and inflammatory carcinoma.[27][28][29][30][31]
Regarding molecular subtypes, the most prevalent ones vary with age. For example, in young women with breast cancer (40 years of age or less), the triple-negative subtype has been found to be the most common. However, as age increases, luminal-A and luminal-B tumors become the predominant subtypes.[32]
Other than breast cancer, the mammary glands can also be affected by some benign pathologies. These include fibroadenoma, papillomatosis, fibrocystic disease, lactating adenoma, gynecomastia, phyllodes tumor, mammary duct ectasia, mastitis, and mastalgia. Of these, the phyllodes tumor stands out because of its potential for malignancy.[5][12][21]
Media
(Click Image to Enlarge)
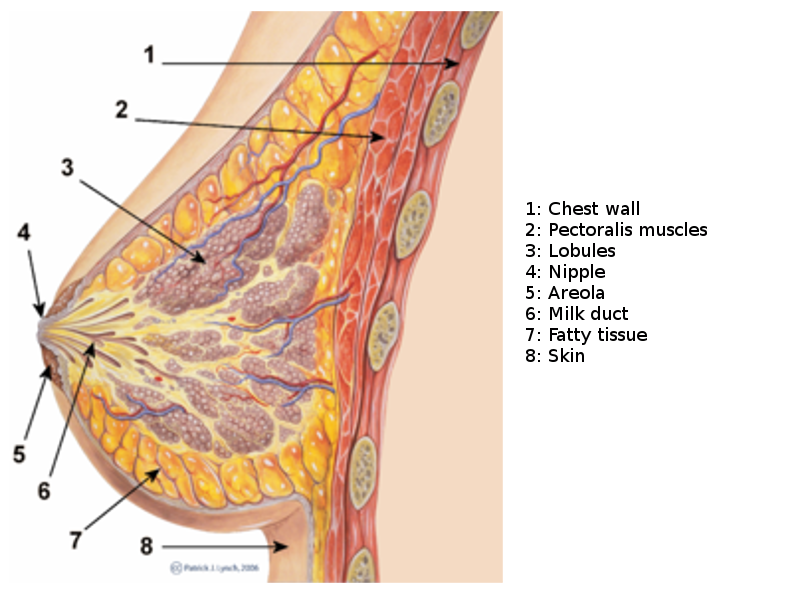
Breast Sagittal View. This illustration shows the chest wall, pectoralis, lobules, nipple, areola, milk duct, fatty tissue, and skin.
PJ Lynch and Morgoth666, Public Domain, via Wikimedia Commons.
References
Pandya S, Moore RG. Breast development and anatomy. Clinical obstetrics and gynecology. 2011 Mar:54(1):91-5. doi: 10.1097/GRF.0b013e318207ffe9. Epub [PubMed PMID: 21278507]
Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development (Cambridge, England). 2015 Mar 15:142(6):1028-42. doi: 10.1242/dev.087643. Epub [PubMed PMID: 25758218]
Level 3 (low-level) evidenceMacias H, Hinck L. Mammary gland development. Wiley interdisciplinary reviews. Developmental biology. 2012 Jul-Aug:1(4):533-57 [PubMed PMID: 22844349]
Level 3 (low-level) evidenceMusumeci G, Castrogiovanni P, Szychlinska MA, Aiello FC, Vecchio GM, Salvatorelli L, Magro G, Imbesi R. Mammary gland: From embryogenesis to adult life. Acta histochemica. 2015 May-Jun:117(4-5):379-85. doi: 10.1016/j.acthis.2015.02.013. Epub 2015 Mar 20 [PubMed PMID: 25800977]
Hassiotou F, Geddes D. Anatomy of the human mammary gland: Current status of knowledge. Clinical anatomy (New York, N.Y.). 2013 Jan:26(1):29-48. doi: 10.1002/ca.22165. Epub 2012 Sep 19 [PubMed PMID: 22997014]
Misery L, Talagas M. Innervation of the Male Breast: Psychological and Physiological Consequences. Journal of mammary gland biology and neoplasia. 2017 Jun:22(2):109-115. doi: 10.1007/s10911-017-9380-0. Epub 2017 May 27 [PubMed PMID: 28551701]
Anderson WF, Devesa SS. In situ male breast carcinoma in the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. Cancer. 2005 Oct 15:104(8):1733-41 [PubMed PMID: 16138363]
Duivenvoorden HM, Rautela J, Edgington-Mitchell LE, Spurling A, Greening DW, Nowell CJ, Molloy TJ, Robbins E, Brockwell NK, Lee CS, Chen M, Holliday A, Selinger CI, Hu M, Britt KL, Stroud DA, Bogyo M, Möller A, Polyak K, Sloane BF, O'Toole SA, Parker BS. Myoepithelial cell-specific expression of stefin A as a suppressor of early breast cancer invasion. The Journal of pathology. 2017 Dec:243(4):496-509. doi: 10.1002/path.4990. Epub 2017 Oct 31 [PubMed PMID: 29086922]
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes & development. 2014 Jun 1:28(11):1143-58. doi: 10.1101/gad.242511.114. Epub [PubMed PMID: 24888586]
Level 3 (low-level) evidenceZhu W, Nelson CM. Adipose and mammary epithelial tissue engineering. Biomatter. 2013 Jul-Sep:3(3):. doi: 10.4161/biom.24630. Epub 2013 Apr 1 [PubMed PMID: 23628872]
Sigl V, Jones LP, Penninger JM. RANKL/RANK: from bone loss to the prevention of breast cancer. Open biology. 2016 Nov:6(11): [PubMed PMID: 27881737]
Stone K, Wheeler A. A Review of Anatomy, Physiology, and Benign Pathology of the Nipple. Annals of surgical oncology. 2015 Oct:22(10):3236-40. doi: 10.1245/s10434-015-4760-4. Epub 2015 Aug 5 [PubMed PMID: 26242366]
Val-Bernal JF, Diego C, Rodriguez-Villar D, Garijo MF. The nipple-areola complex epidermis: a prospective systematic study in adult autopsies. The American Journal of dermatopathology. 2010 Dec:32(8):787-93. doi: 10.1097/DAD.0b013e3181ddbec5. Epub [PubMed PMID: 20802299]
Level 3 (low-level) evidenceHovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. Journal of mammary gland biology and neoplasia. 2010 Sep:15(3):279-90. doi: 10.1007/s10911-010-9187-8. Epub 2010 Aug 19 [PubMed PMID: 20717712]
Level 3 (low-level) evidenceMuschler J, Streuli CH. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harbor perspectives in biology. 2010 Oct:2(10):a003202. doi: 10.1101/cshperspect.a003202. Epub 2010 Aug 11 [PubMed PMID: 20702598]
Level 3 (low-level) evidenceSchedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Molecular carcinogenesis. 2004 Dec:41(4):207-20 [PubMed PMID: 15468292]
Level 3 (low-level) evidenceBrinkman RJ, Hage JJ. Andreas Vesalius' 500th Anniversary: First Description of the Mammary Suspensory Ligaments. World journal of surgery. 2016 Sep:40(9):2144-8. doi: 10.1007/s00268-016-3481-6. Epub [PubMed PMID: 26943658]
Betterman KL, Paquet-Fifield S, Asselin-Labat ML, Visvader JE, Butler LM, Stacker SA, Achen MG, Harvey NL. Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli. The American journal of pathology. 2012 Dec:181(6):2225-38. doi: 10.1016/j.ajpath.2012.08.035. Epub 2012 Oct 11 [PubMed PMID: 23063660]
Level 3 (low-level) evidenceNeed EF, Atashgaran V, Ingman WV, Dasari P. Hormonal regulation of the immune microenvironment in the mammary gland. Journal of mammary gland biology and neoplasia. 2014 Jul:19(2):229-39. doi: 10.1007/s10911-014-9324-x. Epub 2014 Jul 4 [PubMed PMID: 24993978]
Level 3 (low-level) evidenceAtashgaran V, Wrin J, Barry SC, Dasari P, Ingman WV. Dissecting the Biology of Menstrual Cycle-Associated Breast Cancer Risk. Frontiers in oncology. 2016:6():267. doi: 10.3389/fonc.2016.00267. Epub 2016 Dec 26 [PubMed PMID: 28083513]
Onstad M, Stuckey A. Benign breast disorders. Obstetrics and gynecology clinics of North America. 2013 Sep:40(3):459-73. doi: 10.1016/j.ogc.2013.05.004. Epub 2013 Jul 24 [PubMed PMID: 24021252]
Tucker DK, Foley JF, Hayes-Bouknight SA, Fenton SE. Preparation of High-quality Hematoxylin and Eosin-stained Sections from Rodent Mammary Gland Whole Mounts for Histopathologic Review. Toxicologic pathology. 2016 Oct:44(7):1059-64. doi: 10.1177/0192623316660769. Epub 2016 Jul 29 [PubMed PMID: 27474221]
Level 2 (mid-level) evidenceHvid H, Thorup I, Oleksiewicz MB, Sjögren I, Jensen HE. An alternative method for preparation of tissue sections from the rat mammary gland. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2011 May:63(4):317-24. doi: 10.1016/j.etp.2010.02.005. Epub 2010 Mar 2 [PubMed PMID: 20199854]
Level 3 (low-level) evidenceJanowczyk A, Basavanhally A, Madabhushi A. Stain Normalization using Sparse AutoEncoders (StaNoSA): Application to digital pathology. Computerized medical imaging and graphics : the official journal of the Computerized Medical Imaging Society. 2017 Apr:57():50-61. doi: 10.1016/j.compmedimag.2016.05.003. Epub 2016 May 16 [PubMed PMID: 27373749]
Sun C, Wang B, Li J, Shangguan J, Figini M, Zhou K, Pan L, Ma Q, Zhang Z. Quantitative measurement of breast carcinoma fibrosis for the prediction in the risk of bone metastasis. American journal of translational research. 2018:10(6):1852-1859 [PubMed PMID: 30018725]
Zaha DC. Significance of immunohistochemistry in breast cancer. World journal of clinical oncology. 2014 Aug 10:5(3):382-92. doi: 10.5306/wjco.v5.i3.382. Epub [PubMed PMID: 25114853]
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biological research. 2017 Oct 2:50(1):33. doi: 10.1186/s40659-017-0140-9. Epub 2017 Oct 2 [PubMed PMID: 28969709]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018 Nov:68(6):394-424. doi: 10.3322/caac.21492. Epub 2018 Sep 12 [PubMed PMID: 30207593]
Serdy KM, Leone JP, Dabbs DJ, Bhargava R. Male Breast Cancer. American journal of clinical pathology. 2017 Jan 1:147(1):110-119. doi: 10.1093/ajcp/aqw207. Epub [PubMed PMID: 28171879]
Ferzoco RM, Ruddy KJ. The Epidemiology of Male Breast Cancer. Current oncology reports. 2016 Jan:18(1):1. doi: 10.1007/s11912-015-0487-4. Epub [PubMed PMID: 26694922]
Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Molecular oncology. 2010 Jun:4(3):192-208. doi: 10.1016/j.molonc.2010.04.004. Epub 2010 Apr 18 [PubMed PMID: 20452298]
Level 3 (low-level) evidenceAzim HA Jr, Partridge AH. Biology of breast cancer in young women. Breast cancer research : BCR. 2014 Aug 27:16(4):427. doi: 10.1186/s13058-014-0427-5. Epub 2014 Aug 27 [PubMed PMID: 25436920]
