Introduction
In most primates, nails are unique features consisting of compact, translucent, keratinized cells. They protect the distal dorsal area of digits and participate in multiple functions, including grip, fine motor movements, and scratching.[1] Like hair, nails are ectodermal appendages whose histology shares similarities and differences with the skin.[2]
Nail abnormalities can result from local nail pathology or manifest underlying systemic disease.[3] A nail biopsy may be warranted to provide a definitive diagnosis, and understanding the macroscopic and microscopic nail histology is an essential aid to the clinicopathological diagnosis of nail diseases.
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
The nail unit includes the nail plate and supporting structures. This section explains the anatomy of the nail unit and histologic findings of each region.
Nail Plate
The nail plate is the nail itself. It is a rigid, keratinized structure composed of around 196 rows of compact, well-differentiated keratinocytes called onychocytes.[4] The curvature of the nail plate along both the transverse and longitudinal axes contributes to its strength and allows for a snug fit within the proximal nail fold (PNF) and lateral nail folds (LNFs). Histologically, the nail plate resembles a modified stratum corneum.[5] Like the stratum corneum, the nail plate contains keratinocytes with lost nuclei. The lack of nuclei in keratinocytes results in the transparency of the nail plate.[5] In contrast to the stratum corneum, the nail plate has a lower percentage of total fat and water, a more significant percentage of cysteine, and thus, many strong disulfide bonds.[6][7] Additionally, onychocytes are flatter and do not desquamate like corneocytes.[4]
Nail Matrix
The nail matrix is the origin of the nail plate. Its proximal end resides halfway between the distal interphalangeal (DIP) joint and the PNF.[8][9] An opaque half-moon shape can be seen when its distal end extends past the PNF, which is the lunula. The lunula is most prominent on the thumb and gets concealed by the PNF as one progresses to the digits on the ulnar side. The contour of the distal margin of the nail plate follows the shape of the lunula as observed in neonates and following nail avulsion.[5] The nail matrix has a relatively thick stratified squamous epithelium that lacks a granular layer in histology. It has long rete ridges whose tips point distally.[4][5]
At the edge of the lunula, an abrupt thinning of the epidermis is visible as the epithelium transitions to the nail bed.[10] When forming the nail plate, matrical keratinocytes flatten and lose their nuclei; this occurs in the eosinophilic keratogenous zone. The prekeratogenous zone and basal layer are below the keratogenous zone.[11] The nail unit has a lower concentration of melanocytes than the skin; however, compared to other nail components, the matrix contains the highest concentration of melanocytes at around 217 cells per square millimeter.[12] Melanocytes in the matrix have a basal and suprabasal distribution, occasionally being found as isolated in the prekeratogenous zone.[12] This suprabasal distribution of melanocytes can cause confusion when interpreting the histology in cases of suspected melanoma. Other cells in the matrix include Langerhans cells and Merkel cells.[11]
Two elements constitute the mesenchymal layer under the nail matrix epithelium: a matrical dermis and a matrical hypoderm.[13] The matrical dermis contains a thin papillary and a thick reticular layer. The matrical hypoderm is continuous with the hypodermis of the PNF. It includes clusters of adipocytes interweaving with loose connective tissue, large vessels, and nerves. The density of adipose tissue below the epithelium diminishes distally and becomes scarce at the level of the nail bed.[13]
Nail Bed
The nail bed sits underneath the nail plate, spanning between the lunula and the hyponychium. The nail bed epithelium is composed of a monocellular basal layer, a spinous layer, and, like the nail matrix, is devoid of a stratum granulosum.[14] Longitudinal epidermal ridging complementary with grooves in the overlying nail plate contributes to a firm attachment between the bed and the plate.[14][15] On transverse slices, rete ridges are discernable, but they are lost when cutting longitudinally.[4] There are scant melanocytes with a basal distribution.
The nail bed dermis has one uniform compartment of collagen bundles and elastic fibers. There is a rich vascular network that is oriented longitudinally.[16] Glomus bodies are present in the underlying dermis, which are arteriovenous anastomoses involved in thermoregulation.[5]
Nail Folds
The nail folds help secure the proximal and lateral margins of the nail plate.
- Proximal - The PNF is a skin wedge covering the proximal nail plate and matrix. Its dorsal surface is an extension of the normal finger epidermis, which reflects proximally to form a ventral surface. It overlies the proximal nail plate and divides into a distal and proximal zone.[15] The distal ventral surface is the eponychium. At the angle between the dorsal and ventral surfaces of the PNF, the eponychium produces the cuticle, a thick stratum corneum layer that firmly adheres to the nail plate.[5][15] The cuticle forms a seal to protect the matrix from toxins and microbes. As commonly practiced during manicures, removing the cuticle can leave nails vulnerable to contamination and paronychia.[9] The proximal ventral surface of the PNF may contribute to the formation of the nail plate, which would be considered a dorsal extension of the matrix.[15] The dorsal PNF resembles normal skin, and the ventral PNF has a thin epidermis.
- Lateral – The LNFs line the sides of the nail. Histologically, apart from their lack of pilosebaceous units, they resemble normal skin.[11]
Hyponychium
The hyponychium refers to the epidermis underlying the free margin of a nail. Melanocytes here have a basal distribution.[5] The crevice between the hyponychium and the nail plate harbors pathogens and can contribute to infection transmission as microbes remain despite thorough hand washing.[9][17][18] Histologically, the hyponychium characteristically demonstrates the reappearance of the stratum granulosum, and it has an epithelial thickness that rivals the matrix.[4][19]
Isthmus
The isthmus is the transitional zone between the nail bed and the hyponychium.[11] As a transition zone, it expresses a keratin profile that contains a mixture of keratins produced by the nail bed and the hyponychium.[14] It has a discontinuous stratum granulosum and a layer of parakeratotic corneocytes firmly affixed to the nail plate.[14] This zone helps seal the undersurface of the nail plate, preventing onycholysis.
Function
The nails have multiple functions, including protective, mechanical, thermoregulatory, and cosmetic. Nails provide a rigid barrier for the distal digits and prevent hypertrophy that has been shown to ensue after great toe avulsions.[5] The nail's free edge is a useful tool for grasping small objects and scratching.[20] The nail contributes to tactile perception by providing counter-pressure to the fingertips.[5] The glomus bodies help regulate the body's temperature by diverting blood flow from the capillaries.
Tissue Preparation
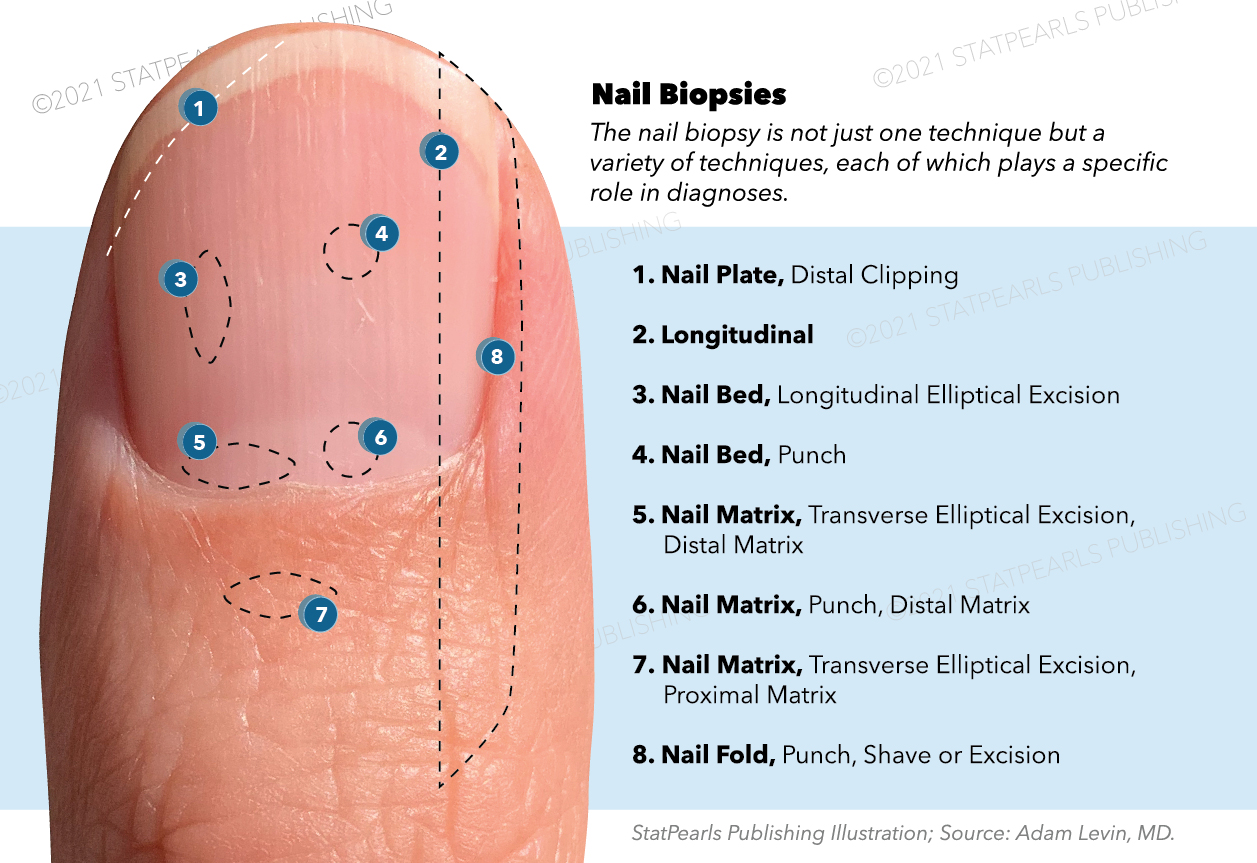
A nail biopsy should be conducted to diagnose inflammatory, infectious, or tumorous conditions. It is the most sensitive technique for diagnosing onychomycosis and is especially useful for pigmented nail lesions.[21][22][23] An apprehension of performing nail biopsies exists due to fear of pain and scarring. Other than the fleeting pain of the anesthetic injection, the procedure should be pain-free, and a biopsy of a site other than the proximal nail matrix has a minimal risk of scarring.[24] Nail biopsy techniques include elliptical excision, punch biopsy, shave biopsy, and longitudinal biopsy. The anatomic site guides the biopsy method.
Nail Plate
A nail plate biopsy is the easiest to perform and has the lowest risk of scarring. If separation of the nail bed and nail plate is necessary or a proximal nail plate punch biopsy is needed, such as with proximal subungual onychomycosis, then anesthesia is required. Otherwise, a nail plate biopsy can be clipped from the free margin as is commonly utilized for distal-lateral subungual onychomycosis. An alternative method for diagnosing distal-lateral subungual and superficial onychomycosis does not require the removal of the nail plate. This modified nail plate biopsy involves a simple scraping of the subungual or superficial debris for direct microscopic examination with potassium hydroxide and sent for a fungal culture. Nail plate biopsies can also be useful for psoriasis, gout, and distinguishing melanin versus blood deposits.[25][26] With spectrometry, chromatography, and various other analytic techniques, nail clippings can detect methamphetamine, cocaine, opioids, tetrahydrocannabinol, and many other constituents.[5][27]
Nail Matrix
Contrary to the nail plate biopsy, a nail matrix biopsy is the most difficult to perform and carries the highest risk of permanent nail dystrophy because of its role as the origin of the nail plate. The proximal matrix produces 81% of the cells in the nail plate; therefore, if possible, the biopsy should be confined to the distal matrix to decrease scarring potential.[28] The following clinical findings suggest nail matrix pathology and could warrant a nail matrix biopsy: melanonychia, erythronychia, leukonychia, nail matrix tumors, onychorrhexis, and nail pitting. A nail matrix biopsy can be acquired as a punch biopsy, shave biopsy, or transverse elliptical excision. The PNF may need to be retracted or reflected to expose the underlying matrix, which is possible with skin hooks or lateral release incisions at the angle of the PNF and LNFs. Nail plate avulsion may or may not be used depending on surgeon preference and clinical judgment.[4][29]
Some clinicians prefer to replace the avulsed nail plate following the procedure.[10] A double-punch technique exists in which there is no retraction of the PNF. Instead, a 2 mm punch biopsy is pushed through the PNF, nail plate, and nail matrix at the expected level of the proximal nail matrix.[30] Biopsies of lesions wider than 3 mm have a higher risk of leaving permanent dystrophy,[29] and it has been proposed to shave biopsy such lesions.[23]
Nail Bed
A nail bed biopsy is necessary for dyschromia, onycholysis, tumorous growths, atypical-appearing subungual warts, and to differentiate psoriasis from onychomycosis.[31][32] A longitudinal elliptical excision or punch biopsy are both options.[8] The elliptical excision necessitates avulsion of the nail plate, while the punch biopsy does not.[25] Avulsion techniques should only be for pathologies isolated to the dermis because removing the plate displaces part of the firmly attached nail bed epithelium compromising its histologic architecture.[25] A partial avulsion is preferred over a complete nail avulsion whenever possible. For a punch biopsy without nail plate avulsion, the nail plate can be softened by soaking the digit in warm water for a few minutes.[25] Nail bed biopsies can create onycholysis, but they generally heal without permanent dystrophy.
Nail Fold
Nail fold biopsies are useful for paronychia and nail fold tumors.[29] If the Hutchinson sign, an extension of pigment to the nail fold in the setting of longitudinal melanonychia, is present, a nail fold biopsy is not sufficient because its histopathologic findings can be misleading.[4] To protect the underlying nail bed or matrix, place a spatula or nail elevator underneath the fold. Biopsy techniques can be of the shave, punch, or excisional varieties. Nail folds heal well with secondary intention.[8]
Another biopsy technique is the longitudinal biopsy, which incorporates all components of the nail apparatus. The incisions penetrate the periosteum with a straight medial incision and a curved lateral one.[29] This biopsy method provides the most information to the pathologist. It can be used for large lesions on the lateral third of the nail but is otherwise not routinely performed because of its high risk of scarring.[25] It is suggested to choose a starting point of 75% of the distance from the cuticle to the DIP joint to ensure the inclusion of the matrical horns (the proximal corners of the matrix).[29][33]
Detailed information should be provided to the dermatopathologist, including pertinent patient history, differential diagnosis, and the exact location of the sample. Communicating the orientation of the sample is vital to optimizing histopathologic assessment because, as mentioned above, the histologic features greatly vary depending on the region of the nail unit. Ink, suture, or an accompanying diagram can facilitate communication of the orientation.[34][35]
Processing a nail biopsy is more challenging than a standard skin biopsy. The specimen first undergoes fixation in a 10% formalin solution, and incubation time is proportionate to the tissue thickness. Because of the rigid nature of the nail plate, a softening step should be performed to reduce chattering, which may impair the quality of the slides and compromise the diagnostic potential. Many softening agents are available, including a combination of ethanol, methanol, acetone, glycerin, 4-hexylresorcinol, 10% to 30% potassium hydroxide, 10% potassium thioglycolate, polyoxyethylenesorbitan monopalmitate, 5% trichloroacetic acid, 4% phenol, 10% formalin, chitin-softening agent, 4 to 10% sodium hydroxide, and water.[24][34]
Certain common household agents have even been shown to be effective softening agents.[36][37] Decalcifying agents such as nitric acid have been used but are not recommended anymore because they may alter the morphology and disrupt molecular analysis.[34][38] Children tend to have thinner nail plates; thus, softening is often not required in the pediatric population.[38] Alternatively, the plastic embedding of the nail specimen has been suggested, a process that eliminates the need for the softening step.[39] A potential downside of this technique is that it requires specialized equipment and takes over two weeks to perform. Following softening, the specimen is embedded, the most common medium being paraffin wax.[34] The samples are then sliced longitudinally, mounted on slides, routinely stained with hematoxylin and eosin, and evaluated using light microscopy.
Histochemistry and Cytochemistry
In addition to hematoxylin and eosin, other stains that are useful for interpreting nail biopsies include, but are not limited to, the following:
- Periodic acid Schiff (PAS) and Grocott methenamine silver (GMS) stains are used to highlight fungi. They adhere to the fungal cell wall elements and emit a magenta and brown color.[5][24][40]
- Diaminobenzidine is used to identify blood.[24][32] It helps differentiate subungual hemorrhage from pigmented lesions. Of note, Prussian blue and Perl iron stains are commonly used to identify blood; however, they are not recommended in examining nail plates because they stain blood breakdown products from macrophages, which have difficulty navigating to the nail plate.[41]
- HMB-45, Melan-A, S100, Fontana-Masson, and PLN-2 are useful for lesions suspicious of melanoma.[32][41][42] They help visualize melanin or other components of melanocytes.[5] S100 should not be used alone because many nail melanocytes lack expression of the antigen, and other cells of neural crest origin, such as Meissner’s corpuscles, can produce false positives. S100 still has utility since it may be the only positive marker in the case of desmoplastic melanoma.[43]
Pathophysiology
The nail matrix is responsible for nail plate growth.[15] The proximal nail matrix produces the dorsal nail plate, and the distal matrix makes the ventral nail plate, possibly with a minor contribution from the nail bed.[44] Thus, in the case of melanonychia, the depth of the pigment suggests the biopsy site. A distal matrix biopsy is sufficient if the melanin remains confined to the ventral nail plate, and a proximal matrix biopsy is required if the dorsal nail plate is involved. The distal matrix is more often engaged in longitudinal melanonychia because its melanocyte population possesses more potential activity.[12] Fingernails grow at approximately 3 mm per month, while toenails grow slightly slower at around 1 mm per month.[45]
Several factors influence the rate of growth. Lowered temperatures, female sex, non-dominant hand, first and fifth digits, certain disease states, and many medications are associated with a slower growth rate.[45] A variety of changes can appear on the nail plate due to nail matrix injury. The dystrophy of the nail plate depends on the severity, duration, and location of the nail matrix insult.[46] A mild, transient, diffuse injury to the nail matrix manifests in a transverse depression across the nail plate, known as Beau’s line. Along the same spectrum, onychomadesis, a transverse separation of the nail plate, can ensue if the diffuse insult to the matrix is more severe. In comparison, a focal, prolonged abuse can produce longitudinal leukonychia.[46]
Clinical Significance
Often underutilized, nails can offer an important clue in many clinical scenarios. Not only can they reveal a recent health history, but they can also act as a window to internal pathology. For instance, in the case of hypoxia, under-perfusion, iron-deficiency anemia, and endocarditis, the nails can display clubbing, delayed capillary refill, spooning, and splinter hemorrhages, respectively. Nail dystrophy can be the first sign of inflammatory diseases such as lichen planus and psoriasis.[11][47]
The nails can be a site of aggressive conditions like melanoma. A histopathologic analysis is mandatory in the setting of suspicious melanoma. A helpful mnemonic that highlights clinical features associated with subungual melanoma is the ABCDEF rule, which stands for[48]:
- Age of 50 to 70 years old, Asian, African American, Native American
- Borders irregular, Brown or Black, Breadth over 3 mm
- Changing on own or not Changing with treatment
- Digit affected is most commonly the thumb, great toe, or index finger
- Extension to nail fold (Hutchinson sign)
- Family/personal history of melanoma or a dysplastic nevus
In addition to pigmented lesions, nail biopsies can help differentiate other similar-appearing entities, such as subungual verruca and subungual squamous cell carcinoma, as well as psoriasis and onychomycosis.[32] Now fully equipped with an understanding of normal nail histology, abnormal histologic findings can be explored and linked to clinical diagnoses.
Histopathologic Findings
- Spongiosis: intercellular edema in the epidermis
- Acantholysis: loss of intercellular cohesion
- Parakeratosis: retention of nuclei in the stratum corneum
- Hyperkeratosis: thickened stratum corneum (orthokeratosis specifically defines a thickened stratum corneum without parakeratosis)
- Hypergranulosis: thickened stratum granulosum or presence of granular layer where it is normally absent
- Hypogranulosis: thinning or loss of stratum granulosum
- Acanthosis: thickened stratum spinosum
- Exocytosis: migration of cells (inflammatory or blood cells) into the epidermis
- Papillomatosis: the irregular fluctuation of epithelial surface overlying dermal papillae
- Atrophy: thinning of the dermis or epidermis
- Pagetoid migration: abnormal cells spreading upward in the epidermis
- Koilocyte: a suprabasal cell with a condensed nucleus and surrounding halo[32]
Diseases of the Nails and Associated Histopathologic Findings
- Subungual verruca: Hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, koilocytes[4]
- Subungual squamous cell carcinoma: Mitotic figures, full-thickness keratinocyte atypia, parakeratosis, dermal invasion, koilocytes[11]
- Onychomycosis: Hyphae, pseudohyphae, spores, neutrophil exocytosis, hyperkeratosis, parakeratosis, spongiosis[47]
- Psoriasis: Hypergranulosis in nail matrix and bed, hypogranulosis in hyponychium, neutrophil exocytosis (Munro microabscesses), hyperkeratosis, parakeratosis, spongiosis
- Lichen planus: Hypergranulosis, acanthosis, dermal fibrosis, lymphocytic infiltrate in the papillary dermis, hyperkeratosis, necrotic keratinocytes (Civatte bodies), spongiosis[47]
- Melanocytic activation: No increase in melanocyte density, increase in pigment[49]
- Lentigo: Melanocytic proliferation, solitary melanocytes located in the basal or suprabasal layer, absent or mild cytologic atypia[49]
- Nevus: Melanocytic proliferation, nests predominate over individual melanocytes[50]
- Melanoma: Melanocytic proliferation with atypical melanocytes, isolated melanocytes predominate over nests, pagetoid migration, dermal invasion[51]
Media
References
Martin B. Nail histopathology. Actas dermo-sifiliograficas. 2013 Sep:104(7):564-78. doi: 10.1016/j.adengl.2013.06.001. Epub 2013 Jul 17 [PubMed PMID: 23871460]
Fernandez-Guerrero M, Yakushiji-Kaminatsui N, Lopez-Delisle L, Zdral S, Darbellay F, Perez-Gomez R, Bolt CC, Sanchez-Martin MA, Duboule D, Ros MA. Mammalian-specific ectodermal enhancers control the expression of Hoxc genes in developing nails and hair follicles. Proceedings of the National Academy of Sciences of the United States of America. 2020 Dec 1:117(48):30509-30519. doi: 10.1073/pnas.2011078117. Epub 2020 Nov 16 [PubMed PMID: 33199643]
Patel LM, Lambert PJ, Gagna CE, Maghari A, Lambert WC. Cutaneous signs of systemic disease. Clinics in dermatology. 2011 Sep-Oct:29(5):511-22. doi: 10.1016/j.clindermatol.2011.01.019. Epub [PubMed PMID: 21855727]
André J, Sass U, Richert B, Theunis A. Nail pathology. Clinics in dermatology. 2013 Sep-Oct:31(5):526-39. doi: 10.1016/j.clindermatol.2013.06.005. Epub [PubMed PMID: 24079581]
de Berker DA, André J, Baran R. Nail biology and nail science. International journal of cosmetic science. 2007 Aug:29(4):241-75. doi: 10.1111/j.1467-2494.2007.00372.x. Epub [PubMed PMID: 18489354]
Gniadecka M, Faurskov Nielsen O, Christensen DH, Wulf HC. Structure of water, proteins, and lipids in intact human skin, hair, and nail. The Journal of investigative dermatology. 1998 Apr:110(4):393-8 [PubMed PMID: 9540981]
Helmdach M, Thielitz A, Röpke EM, Gollnick H. Age and sex variation in lipid composition of human fingernail plates. Skin pharmacology and applied skin physiology. 2000 Mar-Apr:13(2):111-9 [PubMed PMID: 10754459]
Rich P. Nail biopsy: indications and methods. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2001 Mar:27(3):229-34 [PubMed PMID: 11277887]
de Berker D. Nail anatomy. Clinics in dermatology. 2013 Sep-Oct:31(5):509-15. doi: 10.1016/j.clindermatol.2013.06.006. Epub [PubMed PMID: 24079579]
Fleckman P, Allan C. Surgical anatomy of the nail unit. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2001 Mar:27(3):257-60 [PubMed PMID: 11277893]
Fernandez-Flores A, Saeb-Lima M, Martínez-Nova A. Histopathology of the nail unit. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2014:55(2):235-56 [PubMed PMID: 24969971]
Perrin C, Michiels JF, Pisani A, Ortonne JP. Anatomic distribution of melanocytes in normal nail unit: an immunohistochemical investigation. The American Journal of dermatopathology. 1997 Oct:19(5):462-7 [PubMed PMID: 9335239]
Perrin C. The nail dermis: from microanatomy to constitutive modelling. Histopathology. 2015 May:66(6):864-72. doi: 10.1111/his.12608. Epub 2015 Jan 26 [PubMed PMID: 25387989]
Perrin C. Expression of follicular sheath keratins in the normal nail with special reference to the morphological analysis of the distal nail unit. The American Journal of dermatopathology. 2007 Dec:29(6):543-50 [PubMed PMID: 18032949]
Perrin C, Langbein L, Schweizer J. Expression of hair keratins in the adult nail unit: an immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. The British journal of dermatology. 2004 Aug:151(2):362-71 [PubMed PMID: 15327543]
Sangiorgi S, Manelli A, Congiu T, Bini A, Pilato G, Reguzzoni M, Raspanti M. Microvascularization of the human digit as studied by corrosion casting. Journal of anatomy. 2004 Feb:204(2):123-31 [PubMed PMID: 15032919]
Rayan GM, Flournoy DJ. Microbiologic flora of human fingernails. The Journal of hand surgery. 1987 Jul:12(4):605-7 [PubMed PMID: 3611661]
Tanner J, Khan D, Walsh S, Chernova J, Lamont S, Laurent T. Brushes and picks used on nails during the surgical scrub to reduce bacteria: a randomised trial. The Journal of hospital infection. 2009 Mar:71(3):234-8. doi: 10.1016/j.jhin.2008.11.023. Epub 2009 Jan 21 [PubMed PMID: 19162371]
Level 1 (high-level) evidenceBaswan S, Kasting GB, Li SK, Wickett R, Adams B, Eurich S, Schamper R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses. 2017 May:60(5):284-295. doi: 10.1111/myc.12592. Epub 2017 Jan 18 [PubMed PMID: 28098391]
Level 3 (low-level) evidenceZook EG. Anatomy and physiology of the perionychium. Hand clinics. 2002 Nov:18(4):553-9, v [PubMed PMID: 12516972]
Lawry MA, Haneke E, Strobeck K, Martin S, Zimmer B, Romano PS. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Archives of dermatology. 2000 Sep:136(9):1112-6 [PubMed PMID: 10987866]
Level 2 (mid-level) evidenceBlake N, Zhu J, Hernandez G, Juliano PJ. A Retrospective Review of Diagnostic Testing for Onychomycosis of the Foot. Journal of the American Podiatric Medical Association. 2015 Nov:105(6):503-8. doi: 10.7547/14-063.1. Epub [PubMed PMID: 26667504]
Level 2 (mid-level) evidenceJellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. Journal of the American Academy of Dermatology. 2007 May:56(5):803-10 [PubMed PMID: 17437887]
Stewart CL, Rubin AI. Update: nail unit dermatopathology. Dermatologic therapy. 2012 Nov-Dec:25(6):551-68. doi: 10.1111/j.1529-8019.2012.01510.x. Epub [PubMed PMID: 23210754]
Grover C, Bansal S. Nail Biopsy: A User's Manual. Indian dermatology online journal. 2018 Jan-Feb:9(1):3-15. doi: 10.4103/idoj.IDOJ_268_17. Epub [PubMed PMID: 29441291]
Tirado-González M, González-Serva A. The nail plate biopsy may pick up gout crystals and other crystals. The American Journal of dermatopathology. 2011 Jun:33(4):351-3. doi: 10.1097/DAD.0b013e3181fe4b86. Epub [PubMed PMID: 21430503]
Level 3 (low-level) evidenceShu I, Jones J, Jones M, Lewis D, Negrusz A. Detection of Drugs in Nails: Three Year Experience. Journal of analytical toxicology. 2015 Oct:39(8):624-8. doi: 10.1093/jat/bkv067. Epub [PubMed PMID: 26378136]
De Berker D, Mawhinney B, Sviland L. Quantification of regional matrix nail production. The British journal of dermatology. 1996 Jun:134(6):1083-6 [PubMed PMID: 8763429]
Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatologic therapy. 2007 Jan-Feb:20(1):68-74 [PubMed PMID: 17403262]
Kim JE, Ahn HS, Cheon MS, Lee KJ, Cho BK, Park HJ. Proximal nail fold-lunula double punch technique: a less invasive method for sampling nail matrix without nail avulsion. Indian journal of dermatology, venereology and leprology. 2011 May-Jun:77(3):346-8. doi: 10.4103/0378-6323.79727. Epub [PubMed PMID: 21508584]
Level 3 (low-level) evidenceGrover C, Chaturvedi UK, Reddy BS. Role of nail biopsy as a diagnostic tool. Indian journal of dermatology, venereology and leprology. 2012 May-Jun:78(3):290-8. doi: 10.4103/0378-6323.95443. Epub [PubMed PMID: 22565428]
Kovich OI, Soldano AC. Clinical pathologic correlations for diagnosis and treatment of nail disorders. Dermatologic therapy. 2007 Jan-Feb:20(1):11-6 [PubMed PMID: 17403256]
Reardon CM, McArthur PA, Survana SK, Brotherston TM. The surface anatomy of the germinal matrix of the nail bed in the finger. Journal of hand surgery (Edinburgh, Scotland). 1999 Oct:24(5):531-3 [PubMed PMID: 10597925]
Wlodek C, Lecerf P, Andre J, Ruben BS, de Berker D. An international survey about nail histology processing techniques. Journal of cutaneous pathology. 2017 Sep:44(9):749-756. doi: 10.1111/cup.12976. Epub 2017 Jul 10 [PubMed PMID: 28589672]
Level 3 (low-level) evidenceOcampo-Garza J, Di Chiacchio NG, Dominguez-Cherit J, Fonseca Noriega L, Di Chiacchio N. Submitting tangential nail-matrix specimens. Journal of the American Academy of Dermatology. 2017 Nov:77(5):e133-e134. doi: 10.1016/j.jaad.2017.05.029. Epub [PubMed PMID: 29029922]
Orchard GE, Torres J, Sounthararajah R. Use of softening agents to improve the production of formalin-fixed, paraffin-embedded sections of nail tissue: an assessment. British journal of biomedical science. 2008:65(2):68-70 [PubMed PMID: 19055107]
Grammer-West NY, Corvette DM, Giandoni MB, Fitzpatrick JE. Clinical Pearl: Nail plate biopsy for the diagnosis of psoriatic nails. Journal of the American Academy of Dermatology. 1998 Feb:38(2 Pt 1):260-2 [PubMed PMID: 9486684]
Level 3 (low-level) evidenceRubin AI. Nail histology processing techniques: What is happening around the world. Journal of cutaneous pathology. 2017 Sep:44(9):727-728. doi: 10.1111/cup.13007. Epub [PubMed PMID: 28715085]
Chang A, Wharton J, Tam S, Kovich OI, Kamino H. A modified approach to the histologic diagnosis of onychomycosis. Journal of the American Academy of Dermatology. 2007 Nov:57(5):849-53 [PubMed PMID: 17939937]
D'Hue Z, Perkins SM, Billings SD. GMS is superior to PAS for diagnosis of onychomycosis. Journal of cutaneous pathology. 2008 Aug:35(8):745-7. doi: 10.1111/j.1600-0560.2007.00890.x. Epub 2008 Mar 10 [PubMed PMID: 18331569]
Level 2 (mid-level) evidenceBaran R, Haneke E. [Diagnosis and therapy of streaked nail pigmentation]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 1984 Jul:35(7):359-65 [PubMed PMID: 6469639]
Stephen S, Tosti A, Rubin AI. Diagnostic applications of nail clippings. Dermatologic clinics. 2015 Apr:33(2):289-301. doi: 10.1016/j.det.2014.12.011. Epub 2015 Feb 24 [PubMed PMID: 25828720]
Theunis A, Richert B, Sass U, Lateur N, Sales F, André J. Immunohistochemical study of 40 cases of longitudinal melanonychia. The American Journal of dermatopathology. 2011 Feb:33(1):27-34. doi: 10.1097/DAD.0b013e3181e67c87. Epub [PubMed PMID: 20940616]
Level 3 (low-level) evidenceJohnson M, Comaish JS, Shuster S. Nail is produced by the normal nail bed: a controversy resolved. The British journal of dermatology. 1991 Jul:125(1):27-9 [PubMed PMID: 1873199]
Level 3 (low-level) evidenceGeyer AS, Onumah N, Uyttendaele H, Scher RK. Modulation of linear nail growth to treat diseases of the nail. Journal of the American Academy of Dermatology. 2004 Feb:50(2):229-34 [PubMed PMID: 14726877]
Nicolopoulos J, Goodman GJ, Howard A. Diseases of the generative nail apparatus. Part I: Nail matrix. The Australasian journal of dermatology. 2002 May:43(2):81-90; quiz, 91-2 [PubMed PMID: 11982563]
Grover C, Reddy BS, Uma Chaturvedi K. Diagnosis of nail psoriasis: importance of biopsy and histopathology. The British journal of dermatology. 2005 Dec:153(6):1153-8 [PubMed PMID: 16307651]
Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. Journal of the American Academy of Dermatology. 2000 Feb:42(2 Pt 1):269-74. doi: 10.1016/S0190-9622(00)90137-3. Epub [PubMed PMID: 10642684]
Level 3 (low-level) evidenceHanno R, Mathes BM, Krull EA. Longitudinal nail biopsy in evaluation of acquired nail dystrophies. Journal of the American Academy of Dermatology. 1986 May:14(5 Pt 1):803-9 [PubMed PMID: 3711384]
Kaul S, Singal A, Grover C, Sharma S. Clinical and histological spectrum of nail psoriasis: A cross-sectional study. Journal of cutaneous pathology. 2018 Nov:45(11):824-830. doi: 10.1111/cup.13334. Epub 2018 Aug 30 [PubMed PMID: 30073694]
Level 2 (mid-level) evidencePerrin C. Tumors of the nail unit. A review. Part I: acquired localized longitudinal melanonychia and erythronychia. The American Journal of dermatopathology. 2013 Aug:35(6):621-36. doi: 10.1097/DAD.0b013e31826b74b8. Epub [PubMed PMID: 23872872]