Introduction
The heart becomes the first functional organ in the human embryo. By the end of the fourth week of development, the heart can beat spontaneously. Development of the atrioventricular (AV) septum begins in the third week. Failure of proper development of the atrioventricular septum can result in abnormalities that affect the normal physiology of the heart. These defects can range in severity.[1][2][3]
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
The developing heart undergoes many processes as it develops from a primitive tube to a functional organ. During embryonic development, there are various stages the heart undergoes to become functional. Around 18 days after fertilization, the primitive heart begins developing from 2 bilateral tubes that are found on the immature, disc-like embryo. Next, the embryo folds, allowing the 2 tubes to fuse in the midline forming a single heart tube. The primitive atria and ventricles are structurally found between the sinus venosus and the bulbus cordis. During the fourth week of development, the heart tube begins looping to form a shape that more closely resembles the adult heart.
The major septa of the heart are formed between days 27 and 37 of development. Around this time, endocardial cushions are formed by deposition of extracellular matrices and cell proliferation and migration. The cellular composition of the cushions depends on where it is deposited in the heart. For example, the atrioventricular cushions are derived from adjacent endocardial cells; conotruncal cushions are derived from neural crest cells. The atrioventricular endocardial cushions are formed at the ventral and dorsal borders of the atrioventricular (AV) canal. The primitive atrioventricular canal only has access to the primitive left ventricle with a bulboventricular flange to separate it from the bulbus cordis. With time, the posterior flange shrinks and becomes less prominent. The atrioventricular canal enlarges to the right, allowing blood flow to both primitive right and left ventricles. The dorsal and ventral endocardial cushions, along with 2 lateral AV cushions, grow and eventually fuse, forming a septum that divides the right and left AV canals.
Endocardial cushions that are involved in the development of the AV canal also play a role in the development of the interventricular septum and the closure of the ostium primum. Because of this, it gives off a cross-like appearance. This cross-like appearance is crucial to visualize on ultrasound to confirm the integrity of the heart during pregnancy.
Endocardial cushion development is crucial in the formation of atrial and ventricular septa, atrioventricular canals and valves, and aortic and pulmonary channels. Failure of fusion can result in several medical anomalies.
Within the primitive atrium forms the septum primum, which separates the right from the left. This septum extends down toward the endocardial cushions. A space in this septum, known as the foramen primum, maintains blood flow within the heart. As the foramen primum decreases in size, the foramen secundum forms. To the right of the septum primum, the septum secundum forms and eventually expands to cover a majority of the foramen secundum. The remaining foramen is maintained as the foramen ovale in the fetus to maintain a right to left shunt. The atrial septum is then formed by a fusion of the septum primum and secundum. A flap covering the foramen ovale is formed from the septum primum. Shortly after birth, the increase in left atrial pressure causes the flap to close. A patent foramen ovale is able to form if there is a failure of the septa to fuse.
By the end of the fourth week, the ventricular septum also begins to form. The 2 primitive ventricles begin to expand via continuous growth of the myocardium on the outside and trabeculae formation inside the ventricle. With time, the medial wall of the ventricles fuses forming a muscular interventricular septum. Above this septum, the interventricular foramen exists. Next, the aorticopulmonary septum rotates and binds with the muscular interventricular septum, together making up the membranous portion of the interventricular septum.[4][5][6]
Clinical Significance
Atrioventricular septal defects (AVSDs) account for 4% to 5% of all congenital heart defects and approximately 0.5% of live births. AVSDs are found to have a strong association with Trisomy 21. Nonsyndromic AV canal defects appear to be associated with maternal diabetes and obesity.[7][8][9]
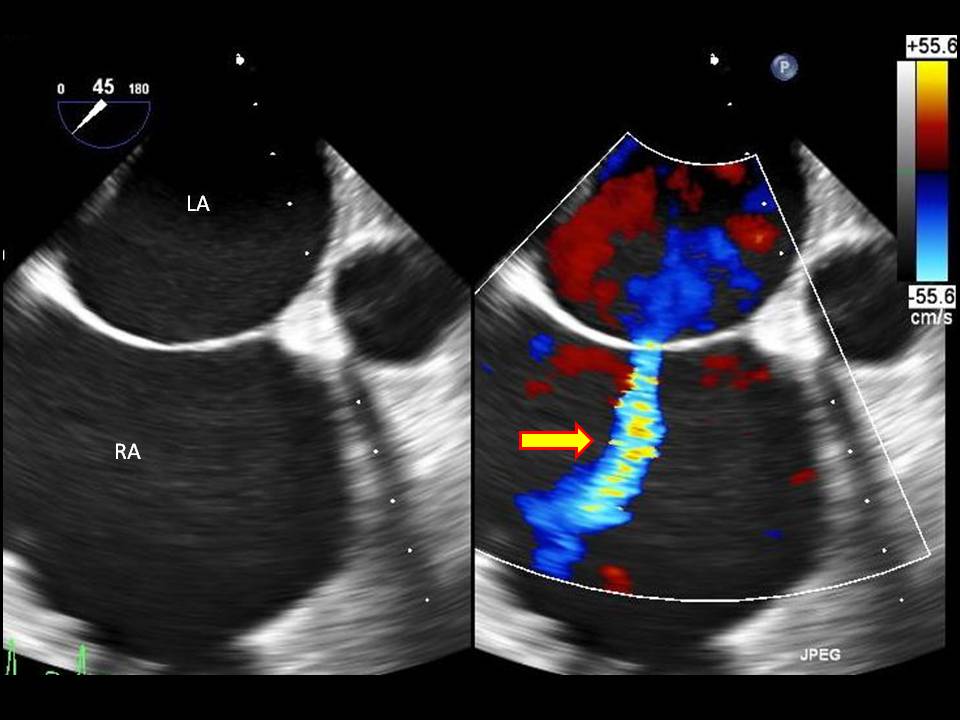
AVSDs occur when there is an inadequate fusion of the endocardial cushions with the middle atrial septum and the muscular portion of the ventricular septum. There are two common types of AVSDs, complete and partial. Approximately half of these AV canal defects are complete AV defects involving both atrial and ventricle septal defects with a common AV valve.
Atrial septal defects, which occur in approximately 6.5 out of 10,000 births, are congenital heart abnormalities due to an ostium secundum defect. This defect results in an unwanted opening between the left and right atrium. The proposed mechanisms which this may occur could be from the insufficient formation of the septum secundum or through excessive cell death and resorption of the septum primum. Another serious and rare abnormality which may develop includes cor triloculare, which includes triloculare biventriculare (common atrium) and triloculare biatrium. Triloculare biventricular is defined as a complete absence of the atrial septum and is the more common of the 2. Triloculare biatrium is the complete absence of a ventricular septum.
If there is a failure of the AV septum to fuse with the septum premium of the atria, the foramen premium remains open, resulting in a foramen premium defect. Generally, this occurs with a malformation of the mitral valve.
When the ventral and dorsal endocardial cushions fail to fuse, a large opening in the center of the heart develops. This defect is called a persistent common atrioventricular canal. This malformation results in the tricuspid and mitral valves to be unified as one common AV valve. Due to this error, there is left to right shunting in the atrium, resulting in an enlarged right atrium and ventricles.
When there is a defect in the atrioventricular septum, Ebstein's anomaly can develop. Ebstein anomaly may occur when posterior and septal leaflets of the tricuspid fail to attach as they are supposed to, displacing the leaflets into the right ventricle. This causes the right ventricle to “atrialize.”
Media
(Click Image to Enlarge)
References
Anderson RH, Spicer DE, Mohun TJ, Hikspoors JPJM, Lamers WH. Remodeling of the Embryonic Interventricular Communication in Regard to the Description and Classification of Ventricular Septal Defects. Anatomical record (Hoboken, N.J. : 2007). 2019 Jan:302(1):19-31. doi: 10.1002/ar.24020. Epub 2018 Nov 29 [PubMed PMID: 30408340]
Kohnken R, Schober K, Godman J, Gardner A, Jenkins T, Schroeder E, Baker P, Dunbar L. Double outlet right ventricle with subpulmonary ventricular septal defect (Taussig-Bing anomaly) and other complex congenital cardiac malformations in an American Quarter Horse foal. Journal of veterinary cardiology : the official journal of the European Society of Veterinary Cardiology. 2018 Feb:20(1):64-72. doi: 10.1016/j.jvc.2017.10.005. Epub 2017 Nov 22 [PubMed PMID: 29174590]
Gopalakrishnan A, Sasidharan B, Tharakan J, Valaparambil A. Left atrial outflow obstruction in double-outlet right atrium. Asian cardiovascular & thoracic annals. 2018 Jan:26(1):50-53. doi: 10.1177/0218492317736962. Epub 2017 Oct 8 [PubMed PMID: 28988491]
Saremi F, Sánchez-Quintana D, Mori S, Muresian H, Spicer DE, Hassani C, Anderson RH. Fibrous Skeleton of the Heart: Anatomic Overview and Evaluation of Pathologic Conditions with CT and MR Imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Sep-Oct:37(5):1330-1351. doi: 10.1148/rg.2017170004. Epub 2017 Aug 18 [PubMed PMID: 28820653]
Level 3 (low-level) evidencePatrick WL, Mainwaring RD, Reinhartz O, Punn R, Tacy T, Hanley FL. Major Aortopulmonary Collateral Arteries With Anatomy Other Than Pulmonary Atresia/Ventricular Septal Defect. The Annals of thoracic surgery. 2017 Sep:104(3):907-916. doi: 10.1016/j.athoracsur.2017.02.029. Epub 2017 May 17 [PubMed PMID: 28527961]
Jensen B, Spicer DE, Sheppard MN, Anderson RH. Development of the atrial septum in relation to postnatal anatomy and interatrial communications. Heart (British Cardiac Society). 2017 Mar:103(6):456-462. doi: 10.1136/heartjnl-2016-310660. Epub 2016 Dec 21 [PubMed PMID: 28003417]
Anderson RH, Mohun TJ, Brown NA. Clarifying the morphology of the ostium primum defect. Journal of anatomy. 2015 Mar:226(3):244-57. doi: 10.1111/joa.12272. Epub 2015 Feb 9 [PubMed PMID: 25676858]
Level 3 (low-level) evidenceAnderson RH, Spicer DE, Brown NA, Mohun TJ. The development of septation in the four-chambered heart. Anatomical record (Hoboken, N.J. : 2007). 2014 Aug:297(8):1414-29. doi: 10.1002/ar.22949. Epub 2014 May 27 [PubMed PMID: 24863187]
Level 3 (low-level) evidenceTardy MM, Galvaing G, Sakka L, Garcier JM, Chazal J, Filaire M. [Embryology of the heart walls]. Morphologie : bulletin de l'Association des anatomistes. 2013 Mar:97(316):2-11. doi: 10.1016/j.morpho.2012.11.001. Epub 2013 Feb 12 [PubMed PMID: 23414788]
Level 3 (low-level) evidence