Introduction
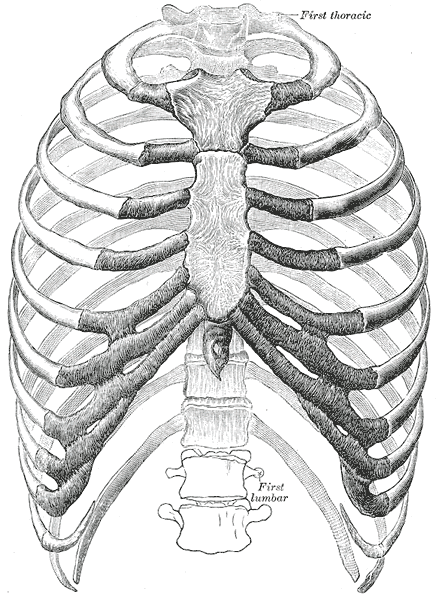
The thoracic wall consists of a bony framework held together by twelve thoracic vertebrae posteriorly, giving rise to ribs that encircle the lateral and anterior thoracic cavity. The first nine ribs curve around the lateral thoracic wall and connect to the manubrium and sternum. Ribs 10 to 12 are relatively short and attach to the costal margins of the ribs just above them. Ribs 10 to 12, due to their short course, do not reach the sternum.
The first seven ribs are called true ribs and attach to the manubrium and directly attach to the body of the sternum. Ribs eight to ten only attach to the inferior part of the sternum via the costal cartilage. Ribs 11 to 12 are termed floating ribs because they do not attach directly to the sternum. Ribs eight to ten are known as false ribs because they lack direct attachment to the sternum. At the level of the spine, the ribs articulate with the costal facet of two opposing vertebrae. An articular capsule surrounds the head of each rib, and the attachment to the transverse process is made with the help of the radiate ligament. Once the ribs leave the vertebrae, they gently curve around the lateral thoracic wall and approach the anterior wall of the thoracic cavity.[1]
The vertical bone of the chest, the sternum, defines the anterior chest wall. The three separate bone segments of different sizes and shape that make up the sternum include 1) the thick manubrium, 2) the long body of the sternum, and 3) the xiphoid process. The sternum develops independently of the ribs. In sporadic cases, the sternum may not fully form, and the underlying heart may be exposed.
The most superior portion of the sternum is the manubrium, and it is also the first to form during embryogenesis. The sternal body and xiphoid process soon follow the manubrium in development. Anatomically, the manubrium is located at the level of thoracic vertebral bodies T3 and T4. The manubrium is also the widest and thickest segment of the sternum. During a physical exam of the chest, one noticeable feature of the manubrium is the presence of the suprasternal notch. On either side of this notch, one will feel the thick attachment from the clavicles. Some thoracic surgeons will only make a midline incision in the manubrito access to the superior mediastinum, suprasternal goiter, or thymus.
The sternal body is located at the level of vertebral bodies T5 to T9, covers a significant portion of the mid-chest, and is very strong. To access the chest cavity, surgeons usually cut through the sternum with a mechanical saw.
The xiphoid process is a thin and very small bone. The bone's size may vary from 2 to 5 cm, and its shape is also variable. The xiphoid may appear bifid, oval, or curve inward or outward. The xiphoid is mostly cartilaginous in younger individuals but is nearly wholly ossified by age 40. By the age of 60 and over, the xiphoid is almost certainly completely calcified. To perform pericardiocentesis safely, the needle has to be placed directly underneath the xiphoid because the heart is just a few fingerbreadths below.[1][2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The thoracic cavity subdivides into three compartments; the mediastinum and two pleural cavities, one on each side. The mediastinum is the median compartment containing the heart and great vessels, whereas the pleural cavities house the lungs. The thoracic cage protects the lungs and the heart and provides attachments for the muscles of the thorax, upper extremities, back, and abdomen. The thoracic cage communicates superiorly with the neck via the thoracic outlet and inferiorly separates the abdomen by the respiratory diaphragm.[1]
The boundaries of the thoracic wall are important landmarks used by clinicians and surgeons for various procedures, including sternotomy, pericardiocentesis in patients with cardiac tamponade, and thoracentesis for pleural effusion. The thoracic wall is bounded anteriorly by the sternum and costal cartilages; laterally by the ribs and intercostal spaces; posteriorly by the thoracic vertebrae and intervertebral discs; superiorly by the suprapleural membrane and inferiorly by the respiratory diaphragm.[1][2]
Embryology
Somite formation begins as the paraxial mesoderm starts to spiral into an organized cell called somitomere. These somitomeres cluster together by epithelium and separate from the presomitic paraxial mesoderm to form individual somites. The differentiation in somite forms the cartilage of the vertebrae, ribs, the muscle of the rib cage, limb, and even the dermis of the skin.
Blood Supply and Lymphatics
Three arteries supply each intercostal space; the posterior intercostal artery and two branches of anterior intercostal arteries. These intercostal blood vessels run along with the nerves between the internal intercostal muscle and the innermost intercostal muscles in the costal groove. They are ordered from superior to inferior: vein, artery, and nerve.
The posterior intercostal artery for the first two intercostal spaces is fed from the superior (supreme) intercostal artery. This artery arises from the costocervical trunk of the subclavian artery. The remaining pair of posterior intercostal arteries from the 3rd to the 11th intercostal spaces and a pair of subcostal arteries emerge directly from the descending thoracic aorta.[1]
The anterior intercostal arteries of the 1st through 6th intercostal spaces are branches of the internal thoracic artery originating from the first portion of the subclavian artery. The anterior intercostal arteries of the 7th through 9th intercostal spaces are branches of the musculophrenic artery, a terminal tributary of the internal thoracic artery. The anterior and posterior intercostal arteries anastomose laterally in the costal groove.[3]
The corresponding posterior intercostal vein drains into azygos or hemiazygos veins, and the corresponding anterior intercostal veins drain into internal thoracic or musculophrenic veins. The lymphatics of the thoracic wall drain into parasternal lymph nodes and intercostal lymph nodes. The parasternal lymph nodes and intercostal lymph nodes from the upper thorax drain into the bronchomediastinal trunk, whereas the intercostal nodes from the lower thorax drain into the thoracic duct.[4]
Nerves
The thoracic wall is primarily innervated by the intercostal nerves, which are the anterior rami of spinal nerves of T1-T11, and the anterior ramus of T12 is a subcostal nerve. Each intercostal nerve supplies a dermatome and a myotome. Only the anterior ramus of T1 forms the lower trunk of the brachial plexus; the remaining intercostals do not form a plexus.[1][2]
Muscles
There are three intercostal muscles; external intercostal, internal intercostal, and innermost intercostal muscles. These muscles are present in the intercostal spaces, and the intercostal nerves and blood vessels run between them. The most superficial layer is the external intercostal muscle. The external intercostal muscles extend posteriorly from the rib tubercle to the costochondral junction anteriorly, where the anterior (external) intercostal membrane takes the place of the muscle fibers.[2]
The internal intercostal muscle forms the intermediate layer. This muscle extends anteriorly from the sternum to the rib cage posteriorly, where the muscle fibers are replaced by the posterior (internal) intercostal membrane. The innermost intercostal muscle forms the deepest layer and is lined internally by the endothoracic fascia, which in turn is lined internally by the parietal pleura.[2][5]
Physiologic Variants
The difference in the size of the sternum in both genders may provide essential clues in determining the skeletal remains during forensic evaluation.[1]
Surgical Considerations
Understanding the anatomy of the thorax is vital, as it provides access to the heart, great vessels, lungs, diaphragm, and mediastinum.
- The intercostal nerve block is a procedure through which a local anesthetic agent is injected around the intercostal nerve between the paravertebral line and the area of required anesthesia for different surgical procedures. However, the surgeon must also anesthetize the adjacent nerve because there is considerable overlapping of contiguous dermatomes.[6]
- Median sternotomies are the most commonly performed osteotomy in the world and a standard incision for thoracic and cardiac surgery. The median sternotomy is a critical procedure in which the surgeon splits the sternum in the median plane to gain access to the heart, great vessels, as well as the lungs.[7]
- Flail chest is an extremely painful injury affecting respiration, often caused by blunt chest trauma, resulting in multiple successive rib fractures. The fractured ribs of the anterior and/or lateral chest wall move inward on inspiration and outward on expiration (paradoxical) and may be associated with pneumothorax and/or contusion of the heart and lung. Today, flail chest management is through pain control, pulmonary toilet, and early ambulation. Surgical repair of the ribs is not common.[8]
Clinical Significance
The vitality of the organs, vessels, and nerves located within the thoracic cavity predisposes it to be a location of high clinical significance.
- The sternum is a commonly used site for bone marrow aspiration because it possesses hematopoietic marrow throughout life. However, the surgeon needs to exercise great care because if the sternal puncture is improperly executed, the needle can pierce the structures related to the posterior surface of the manubrium, such as the left brachiocephalic vein in the upper part and aortic arch in the lower part.[9]
- Thoracentesis is a diagnostic procedure that the surgeon performs to remove excess fluid from the thoracic cavity for both diagnostic and therapeutic purposes. This procedure can be performed in 2 positions depending on patient comfort. If the patient is lying supine, the needle placement is typically in the midaxillary line between the 6th and 8th ribs. If the patient is upright or seated, the needle is placed posteriorly between the 9th and 10th rib in the midscapular line.[10] However, in both cases, a too-low needle insertion can result in a puncture of the liver or the spleen
- Pericardiocentesis is also necessary as it can be life-saving in a patient with cardiac tamponade. Pericardiocentesis may be guided using surface landmarks ("blindly") in emergencies, or electrocardiogram/echocardiography may be used to advance the needle to avoid complications. The needle is inserted 1-2cm lateral to the apex beat within the fifth, sixth, or seventh intercostal space to remove excess fluid from the pericardial sac. The needle is advanced over the superior border of the rib to avoid intercostal nerves and vessels. If performed improperly, the needle can puncture the left ventricle or causes pneumothorax.[11]
Thoracic Outlet Syndrome
The thoracic outlet syndrome involves compression of the lower roots of the brachial plexus, the subclavian and/or axillary artery, and the subclavian and/or axillary vein.
Subtypes of Thoracic Outlet Syndrome
The thoracic outlet syndrome (TOS) has three subtypes.[12][13] Neurogenic thoracic outlet syndrome exhibits damage typically involving C8 and T1. In this variant of thoracic outlet syndrome, the T1 nerve root is more commonly damaged than the C8 nerve root.[14] Neurogenic thoracic outlet syndrome can cause atrophy of the small intrinsic muscles of the hand (Gilliat-Sumner hand). Pain when the arms move above the shoulder can be a key sign of this type of TOS.[14]
The arterial thoracic outlet syndrome involves compression of the subclavian artery in the interscalene triangle. One symptom of this type is a difference in the blood pressure in the affected arm compared to the normal arm. This pressure differential can be as high as 20 millimeters of mercury.[15] Venous thoracic outlet syndrome can cause differences in hair distribution and skin color, including shoulder cyanosis and hand pallor. Venous and arterial thoracic outlet syndromes are diagnosed by imaging and ultrasound with consideration of the clinical aspects.[15]
Three spaces are involved in thoracic outlet syndrome. (1) in the scalene triangle between the first rib, scalenus anterior, scalenus medius, and clavicle. (2) The costoclavicular space is located in the middle third of the clavicle, the first rib, and the aponeurosis of the subclavius muscle. (3) The subpectoral space lies between the tendon of the pectoralis minor, the coracoid process, and the thoracic wall. The entire brachial plexus can be involved here.[13]
Causes of Thoracic Outlet Syndrome
The subtypes of thoracic outlet syndrome are the neurogenic, arterial, and venous thoracic outlet syndromes. The causes of thoracic outlet syndrome can be congenital (cervical rib or abnormal first rib) or traumatic (as in the case of sports injuries, falls, or whiplash injuries). The incidence ranges from 3 per thousand to 80 per thousand.[13]
In a case series involving 324 cases from the University of Padua, 90 to 95% were due to neurogenic thoracic outlet syndrome; 3 to 5% were due to venous thoracic outlet syndrome, and 1% to 2% to arterial thoracic outlet syndrome. [13] The incidence of neurogenic thoracic outlet syndrome is greater in females than males. Arterial thoracic outlet syndrome has an equal gender distribution. The venous thoracic outlet syndrome is more common in men. Venous thoracic outlet syndrome is common in athletic patients who practice swimming and baseball.[13]
Factors that lead to abnormalities have been tabulated.[13] A cervical rib can lead to pain with postural changes. Abnormalities of the first rib can cause injuries in patients who fall, particularly older females, especially those with significant osteoporosis. A cervical rib (1% to 2%, especially in females) is a risk factor in 20% of neurogenic thoracic outlet syndrome cases. A cervical rib Is also a risk factor for arterial thoracic outlet syndrome.
Tests for Thoracic Outlet Syndrome
There are several stress tests for thoracic outlet syndrome. These are especially diagnostic for neurogenic cases.
The Elevated Arm Stress Test
This test is performed with the arm abducted ninety degrees with external rotation. The hands are opened and closed for up to three minutes. Pain is usually elicited with this maneuver. Patients commonly cannot continue for more than a minute due to pain.[16]
The Upper Limb Tension Test
To perform this test, the patient places the arms at 90 degrees of abduction. The forearms are extended at the elbows, and the palms are flat. The symptoms can be reproduced by extension at the wrist. The patient may experience some relief of pain during wrist flexion.[13]
The Adson Test
The affected arm is abducted thirty degrees at the shoulder with maximal extension. The patient inhales deeply while extending the head towards the ipsilateral shoulder. This maneuver causes a decrease or absence of the radial pulse.[15]
Treatment of Thoracic Outlet Syndrome
For neurogenic thoracic outlet syndrome cases, conservative management and physical therapy can be appropriate for as long as 6 months.[13] Neurogenic thoracic outlet syndrome can be diagnosed with electrophysiological studies and should generally be treated surgically if conservative treatment does not improve the condition.[12][15]
Surgery for neurogenic thoracic outlet syndrome is appropriate for patients who have muscular atrophy or persistent neurological dysfunction. Surgical treatment can be accomplished by supraclavicular, infraclavicular, and transaxillary approaches.[15] There is considerable disagreement among surgeons concerning the preferred surgical approach. The transaxillary approach is often utilized when the goal is performing a resection of the costoclavicular ligament and the first rib. Scalenectomy and neurolysis of C8-T1 are also reasons for making this choice. Other surgeons may prefer the supraclavicular approach, especially when reoperating on the patient.[17] There is data that the supraclavicular approach results in superior results to the transaxillary approach.[17]
The treatment for arterial and venous thoracic outlet syndromes is usually surgical, involving resection of the first rib.
The functional postoperative outcomes for arterial and venous thoracic outlet syndrome are much better than for neurogenic thoracic outlet syndrome.[17]
Pancoast Tumor and Thoracic Outlet Syndrome
A Pancoast tumor is a malignant tumor of the apex of the lung. The tumor causes Horner syndrome and may be involved in the genesis of thoracic outlet syndrome.[18][19][20][21]
Other Issues
Chest wall deformities, including pectus excavatum and pectus carinatum, are among the most common congenital chest wall defects in young people. Surgical correction is needed in some people to avoid complications that may lead to heart and lung dysfunction. However, these techniques require aggressive cartilage and rib cage resection, leading to severe post-operational complications such as infections and delayed healing.[22]
Media
(Click Image to Enlarge)
References
Donley ER, Holme MR, Loyd JW. Anatomy, Thorax, Wall Movements. StatPearls. 2023 Jan:(): [PubMed PMID: 30252279]
Clemens MW, Evans KK, Mardini S, Arnold PG. Introduction to chest wall reconstruction: anatomy and physiology of the chest and indications for chest wall reconstruction. Seminars in plastic surgery. 2011 Feb:25(1):5-15. doi: 10.1055/s-0031-1275166. Epub [PubMed PMID: 22294938]
Berdajs D, Zünd G, Turina MI, Genoni M. Blood supply of the sternum and its importance in internal thoracic artery harvesting. The Annals of thoracic surgery. 2006 Jun:81(6):2155-9 [PubMed PMID: 16731146]
Riquet M, Mordant P, Pricopi C, Achour K, Le Pimpec Barthes F. [Anatomy, micro-anatomy and physiology of the lymphatics of the lungs and chest wall]. Revue de pneumologie clinique. 2013 Apr:69(2):102-10. doi: 10.1016/j.pneumo.2012.12.007. Epub 2013 Mar 21 [PubMed PMID: 23523433]
Level 3 (low-level) evidenceMiller JI Jr. Muscles of the chest wall. Thoracic surgery clinics. 2007 Nov:17(4):463-72. doi: 10.1016/j.thorsurg.2006.12.007. Epub [PubMed PMID: 18271161]
Marchetti-Filho MA, Leão LE, Costa-Junior Ada S. The role of intercostal nerve preservation in acute pain control after thoracotomy. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2014 Mar-Apr:40(2):164-70 [PubMed PMID: 24831401]
Level 2 (mid-level) evidenceKüçükdurmaz F, Ağır I, Bezer M. Comparison of straight median sternotomy and interlocking sternotomy with respect to biomechanical stability. World journal of orthopedics. 2013 Jul 18:4(3):134-8. doi: 10.5312/wjo.v4.i3.134. Epub 2013 Jul 18 [PubMed PMID: 23878782]
Yasuda R, Okada H, Shirai K, Yoshida S, Nagaya S, Ikeshoji H, Suzuki K, Kitagawa Y, Tanaka T, Nakano S, Nachi S, Kato H, Yoshida T, Kumada K, Ushikoshi H, Toyoda I, Ogura S. Comparison of two pediatric flail chest cases. Scandinavian journal of trauma, resuscitation and emergency medicine. 2015 Sep 25:23():73. doi: 10.1186/s13049-015-0156-5. Epub 2015 Sep 25 [PubMed PMID: 26408024]
Level 3 (low-level) evidenceArnáiz-García ME, González-Santos JM, Arnáiz-García AM, López-Rodríguez J, Arnáiz J. Acute Type A Aortic Dissection After Sternal Bone Marrow Puncture. The Annals of thoracic surgery. 2017 Dec:104(6):e455. doi: 10.1016/j.athoracsur.2017.07.039. Epub [PubMed PMID: 29153817]
Wiederhold BD, Amr O, Modi P, O'Rourke MC. Thoracentesis. StatPearls. 2023 Jan:(): [PubMed PMID: 28722896]
Halabi M, Faranesh AZ, Schenke WH, Wright VJ, Hansen MS, Saikus CE, Kocaturk O, Lederman RJ, Ratnayaka K. Real-time cardiovascular magnetic resonance subxiphoid pericardial access and pericardiocentesis using off-the-shelf devices in swine. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013 Jul 20:15(1):61. doi: 10.1186/1532-429X-15-61. Epub 2013 Jul 20 [PubMed PMID: 23870697]
Level 3 (low-level) evidenceOhman JW, Thompson RW. Thoracic Outlet Syndrome in the Overhead Athlete: Diagnosis and Treatment Recommendations. Current reviews in musculoskeletal medicine. 2020 Aug:13(4):457-471. doi: 10.1007/s12178-020-09643-x. Epub [PubMed PMID: 32514995]
Camporese G, Bernardi E, Venturin A, Pellizzaro A, Schiavon A, Caneva F, Strullato A, Toninato D, Forcato B, Zuin A, Squizzato F, Piazza M, Stramare R, Tonello C, Di Micco P, Masiero S, Rea F, Grego F, Simioni P. Diagnostic and Therapeutic Management of the Thoracic Outlet Syndrome. Review of the Literature and Report of an Italian Experience. Frontiers in cardiovascular medicine. 2022:9():802183. doi: 10.3389/fcvm.2022.802183. Epub 2022 Mar 22 [PubMed PMID: 35391849]
Chang MC, Kim DH. Essentials of thoracic outlet syndrome: A narrative review. World journal of clinical cases. 2021 Jul 26:9(21):5804-5811. doi: 10.12998/wjcc.v9.i21.5804. Epub [PubMed PMID: 34368299]
Level 3 (low-level) evidenceJones MR,Prabhakar A,Viswanath O,Urits I,Green JB,Kendrick JB,Brunk AJ,Eng MR,Orhurhu V,Cornett EM,Kaye AD, Thoracic Outlet Syndrome: A Comprehensive Review of Pathophysiology, Diagnosis, and Treatment. Pain and therapy. 2019 Jun; [PubMed PMID: 31037504]
Teijink SBJ, Pesser N, Goeteyn J, Barnhoorn RJ, van Sambeek MRHM, van Nuenen BFL, Gelabert HA, Teijink JAW. General Overview and Diagnostic (Imaging) Techniques for Neurogenic Thoracic Outlet Syndrome. Diagnostics (Basel, Switzerland). 2023 May 4:13(9):. doi: 10.3390/diagnostics13091625. Epub 2023 May 4 [PubMed PMID: 37175016]
Level 3 (low-level) evidenceKhabyeh-Hasbani N, Connors K, Buksbaum JR, Koehler SK. Current Concepts in the Management of Neurogenic Thoracic Outlet Syndrome: A Review. Plastic and reconstructive surgery. Global open. 2023 Mar:11(3):e4829. doi: 10.1097/GOX.0000000000004829. Epub 2023 Mar 3 [PubMed PMID: 36875924]
Munir M, Jamil SB, Rehmani S, Borz-Baba C. Pancoast-Tobias Syndrome: A Unique Presentation of Lung Cancer. Cureus. 2021 Feb 3:13(2):e13112. doi: 10.7759/cureus.13112. Epub 2021 Feb 3 [PubMed PMID: 33728131]
Parissis H, Young V. Treatment of pancoast tumors from the surgeons prospective: re-appraisal of the anterior-manubrial sternal approach. Journal of cardiothoracic surgery. 2010 Nov 4:5():102. doi: 10.1186/1749-8090-5-102. Epub 2010 Nov 4 [PubMed PMID: 21050456]
Jammeh ML, Yang A, Abuirqeba AA, Ohman JW, Thompson RW. Reoperative Brachial Plexus Neurolysis After Previous Anatomically Complete Supraclavicular Decompression for Neurogenic Thoracic Outlet Syndrome: A 10-Year Single-Center Case Series. Operative neurosurgery (Hagerstown, Md.). 2022 Aug 1:23(2):125-132. doi: 10.1227/ons.0000000000000252. Epub 2022 May 9 [PubMed PMID: 35838452]
Level 2 (mid-level) evidenceChu EC, Trager RJ, Shum JSF, Lai CR. Pancoast Tumor Presenting as Neck Pain in the Chiropractic Office: A Case Report and Literature Review. The American journal of case reports. 2022 Jul 7:23():e937052. doi: 10.12659/AJCR.937052. Epub 2022 Jul 7 [PubMed PMID: 35797264]
Level 3 (low-level) evidenceSharma G, Carter YM. Pectus Excavatum. StatPearls. 2023 Jan:(): [PubMed PMID: 28613668]