 Anatomy, Abdomen and Pelvis: Female Internal Genitals
Anatomy, Abdomen and Pelvis: Female Internal Genitals
Introduction
The female reproductive system is an intricate arrangement of structures that can be categorized into the external and the internal genitalia (see Image. The Female Reproductive System). The external genitalia comprises the structures outside of the true pelvis, including the labia majora and minora, vestibule, Bartholin glands, Skene glands, clitoris, mons pubis, perineum, urethral meatus, and periurethral area. The internal genitalia are the structures within the true pelvis, including the vagina, cervix, uterus, fallopian tubes, and ovaries (see Image. The Female Genital Organs). These latter structures are the focus of this activity. A comprehensive understanding of the female reproductive system is essential for healthcare professionals involved in women's healthcare to gain in-depth knowledge of female anatomy and its functions.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The uterus is the central anatomical landmark of the female internal genitalia and pelvic anatomy. The highly muscular, childbearing organ measures approximately 3 x 2 x 1 inches in size in a nulliparous woman. Even though the uterus is primarily a pelvic organ, during later stages of pregnancy, it can reach up to the epigastric region due to hypertrophy and hyperplasia of the myometrium.
The most common anatomical position of the uterus is anteverted-anteflexed. Anteversion is the angulation between the long axis of the cervix and the vagina, which nears a right angle.[1][2] Anteflexion is the angulation between the body's long axis and the cervix, an obtuse angle of around 120° to 125°. In an anteverted-anteflexed position, the uterine body sits within the vesicouterine space. In rare cases, the uterus may be retroverted-retroflexed, causing the uterine body to be within the pouch of Douglas.
The shape of the uterus has been described as "pyriform" on examination in a sagittal plane. The uterus has 4 parts: the fundus, body, isthmus, and cervix.[3][4] The uterine body is the largest portion, while the uterine isthmus is where the uterine body meets the cervix.[4] The fundus is the part of the uterus above the opening of the fallopian tubes into the uterine cavity. The uterine cavity has a lining membrane called the endometrium. The endometrium will shed during menstruation; inflammation of this membrane is endometritis, which, when chronic, can lead to infertility.[5]
The cervix is the lower, short, broad cylindrical part of the uterus extending from the internal to the external os and has a narrow central lumen, which opens into the uterine cavity as the internal os and into the vagina as the external os. The external os is seen as a circular opening in nulliparous females and as a horizontal slit in multiparous females. This horizontal slit divides the cervix into an anterior and posterior lip. The narrow upper third of the cervix is the isthmus, which defines the lower uterine segment and is the site for the lower segment cesarean sections.[6]
The ovaries are the primary organs of the female reproductive system. They are oval-shaped gonadal structures, approximately 3 x 1.5 x 1 cm in size, and homologous with the testis in males. They are present in pairs with the long axis oriented downward and forward. The ovaries are within the pelvis, just lateral to the uterus. They lie within the ovarian fossa, in front of the ureters, and behind the external iliac vessels.[3] In nulliparous females, they are pinkish with a smooth outer surface, but in multiparous women, they are more greyish with a puckered surface due to repeated ovulations.
The ovaries are entirely covered with peritoneum except for the mesovarian (anterior) border through which all the blood vessels, nerves, and lymphatics pass. They contain thousands of follicles. Each month, 1 follicle will develop into the dominant (Graafian) follicle, which releases an ovum during ovulation.[4] On ultrasound, the ovary appears to have an echoic central area and a peripheral region, with the former corresponding to the stroma and the latter corresponding to the cortex.[3] The ovaries secrete 2 steroid hormones, estrogen and progesterone, under the direct control of follicle-stimulating hormone and luteinizing hormone secreted by the pituitary. These hormones play a significant role in many aspects of the female life.[7][8][9]
The ovaries link to the uterus through fallopian tubes, which carry the zygote to the uterine cavity for implantation. The fallopian tubes (oviducts or uterine tubes) divide into the fimbriated infundibulum, ampulla, isthmus, and intramural parts.[10] The ampullary part is the usual site for fertilization.[11] The fallopian tube also helps bring the sperm and ovum to this site of fertilization. The infundibulum is the later part, funnel-shaped, with finger-like projections hanging from the sides, called fimbriae. This part opens into the peritoneal cavity and is in contact with the ovaries through some of its fimbriae. The intramural section is the narrow and medial part of the fallopian tube, which is present within the uterine wall. Each region represents characteristic physiologic features.
Salpingitis is a bacterial infection of the fallopian tube. The inflammation may be acute or chronic, most commonly resulting from Neisseria gonorrhoeae or Chlamydia trachomatis. These conditions can lead to fallopian tube scarring and predispose one to infertility and ectopic tubal pregnancy. A tubal factor caused by disruption of the integrity of the fallopian tubes by infusion, endometriosis, or surgical complication is an important consideration when evaluating women with unexplained infertility.[10] Ectopic tubal pregnancy most commonly occurs in the ampulla of the fallopian tube. Common predisposing factors include salpingitis, pelvic surgery, or pelvic inflammatory disease. This diagnosis may be confused with appendicitis in a young woman, and early differentiation between the 2 diagnoses is crucial.
The vagina is a fibromuscular canal ranging from 6 to 8 cm in length and anatomically is located anteriorly to the rectum and posteriorly to the wall of the urinary bladder and the urethra.[12] This canal directs downwards and forwards. Because of the oblique nature of the vagina, the anterior wall is slightly shorter, ie, about 6 cm, compared to the posterior wall, which is about 8 cm. The upper segment of the vagina forms a vaginal vault by being enclosed within the vaginal portion of the cervix.[13]
The cervix extends into the vagina, forming a channel between the 2 structures. Thus the cervix is divided into a supravaginal and a vaginal part. The narrow space between the cervix and the wall of the vaginal is the fornix. The recess between the anterior lip of the cervix and the anterior wall of the vagina is the anterior fornix. Similarly, the recess between the posterior lip of the cervix and the wall of the vagina is the posterior fornix. The spaces on either side between the cervix and the lateral walls of the vagina are known as the lateral fornices. The posterior fornix is the deepest and is a common site for ruptures, foreign bodies, and malignancies.[14][15][16][17]
Embryology
Before the determination of phenotypic sex, the gonads are classified as "unisex" and have the potential to develop into testes or ovaries.[18] The embryologic development of the female internal genitals has been a topic of research in recent years. The determination of female sexual differentiation is by the absence of the SRY (sex-determining region on Y) gene, a gene on the short arm of the Y chromosome (Yp11). When present, the SRY gene will cause the indifferent embryo to develop into the male phenotype.
The belief is that the ovaries derive from the gonadal ridge while the primordial germ cells develop in the epiblast and migrate to endoderm cells in the yolk sac.[19] They finally appear in the genital ridges in the sixth week of gestation. The ovaries initially develop within the abdomen and migrate to the pelvis later in fetal life. Even though they migrate into the pelvis, they still retain their blood supply, nerve supply, and lymphatic drainage back to their site of origin, the upper abdomen.[20]
The gubernaculum forms the ovarian ligament and the round ligament of the uterus. In the recent decade, there has been ongoing research to identify a female-determining sex gene. So far, the "Z" gene hypothesis, which names FoxL2 as a possible ovary-determining factor, has been postulated.[21] Other genes believed to be involved, including Dax1 and Wnt4, may have similar effects; however, many unanswered questions remain regarding this pathway.[18]
The paramesonephric (Mullerian) duct is the embryologic derivative of the genital ducts. The cranial portions form the uterine tubes, while the caudal parts form the uterovaginal primordium. The uterovaginal primordium gives rise to the uterus, cervix, and superior third of the vagina. The vaginal plate is a derivative of the sinovaginal bulbs, which canalize to form the lower two-thirds of the vagina.[22] These derivatives are surrounded by a layer of myometrium that develops from the mesenchyme. The outermost covering is the perimetrium, which develops from folds of the peritoneum.
Embryologic defects that are important to consider include unicornuate uterus anomalies, where 1 paramesonephric duct fails to develop; didelphys anomalies, where there is a complete lack of fusion of the paramesonephric ducts; and bicornuate uterine anomalies, where there is a partial fusion of the paramesonephric ducts.[23][24] Bicornuate uterine anomalies are associated with the highest risk of infertility. The mesonephric (Wolffian) duct disappears in the case of females, except for a small cranial part that remains as the epoophoron and paroophoron. The remnant of the mesonephric duct is called the Gartner duct.[25] A small caudal portion of this duct can be visualized and is referred to as a Gartner duct cyst within the wall of the uterus or vagina.[26]
Blood Supply and Lymphatics
The ovarian artery is a branch of the abdominal aorta; it reaches the ovary through the suspensory ligament of the ovary. Although ovaries are pelvic organs, they derive their blood supply from the abdominal aorta, indicating their site of origin. The uterine artery, the main arterial supply of the uterus, originates from the anterior division of the internal iliac artery and the umbilical artery in a common trunk.[27] The uterine artery is highly tortuous and travels across the pelvic floor in the cardinal ligament and runs anteriorly and superiorly to the ureter close to the lateral fornix of the vagina, ending by anastomosing with the ovarian artery within the broad ligament of the uterus (see Image. Female Reproductive System Blood Supply). Ligation of the uterine artery during a hysterectomy is typically at the level of the internal cervical os.[27] The small, inferior branch of the uterine artery becomes the vaginal artery and supplies the cervix and vagina. The vaginal artery may also arise from the internal iliac artery.
The veins of the female internal genitals typically correspond to the arteries. The uterine vein follows the course of the uterine artery and is also contained within the cardinal ligament; it drains into the internal iliac vein. The ovarian veins receive blood from the ovaries; the right ovarian vein drains directly into the inferior vena cava, while the left ovarian vein drains into the left renal vein. Malignancies in the pelvis may metastasize to the vertebral column or spinal cord via connections of the pelvic veins. The lymphatic flow from the uterus, cervix, and vagina drains into the internal and external iliac nodes.[28] Lymphatic vessels from the ovary and fallopian tube drain directly into the paraaortic nodes.
Nerves
The nerve fibers that feed the pelvis are both parasympathetic and sympathetic. The preganglionic fibers are in the sacral parasympathetic (S2-S4) region of the spinal cord. Their processes run into pelvic splanchnic nerves and travel inside the pelvic organs within the intramural plexus (the postganglionic neuron). The sensory fibers from S2-S4 dorsal root ganglia travel with parasympathetic fibers and carry pain sensations from the organs. Sympathetic fibers of preganglionic neurons (T12-L2) form the sacral splanchnic nerves. They contribute to the inferior hypogastric plexus, which is the location of the postganglionic neurons.
The pudendal nerve provides sensory innervation to the lower vagina. A pudendal nerve block is an option during early labor to relieve maternal pain and may be achieved by piercing the vaginal wall posterolaterally, using the ischial spine as a landmark.[29] The block may also be performed percutaneously along the medial side of the ischial tuberosity. However, this method does not affect pain from uterine contractions and, thus, is no longer commonly used.
Muscles
Several pelvic diaphragm muscles support the uterus, the urogenital diaphragm, and the round, broad, lateral, and cardinal ligaments. The pelvic diaphragm includes the following muscles: piriformis, coccygeus, iliococcygeus, pubococcygeus, and puborectalis. The pubococcygeus, puborectalis, and iliococcygeus muscles are levator ani muscles.
Vaginal delivery is a major cause of levator ani defects, genital organ prolapse, and urinary incontinence.[30] Results from several studies have shown that during the second stage of labor, the pubococcygeus muscle bears the largest tissue strain compared to other pelvic floor muscles.[30] The upper portion of the posterior vagina is suspended and held in place by several lateral connections. Prolapse of this area can occur if there are defects in the perineal body, which most commonly occur following vaginal delivery. The role of episiotomy in the prevention of perineal body tears is controversial.[31][32]
The major ligaments that support the uterus include the round ligament, transverse cervical ligament (cardinal ligament), uterosacral ligament, and broad ligament. The cardinal ligament reaches from the cervix to the sidewall of the pelvis and appears at the base of the broad ligament. The uterosacral ligament plays a significant role in keeping the uterus in the anteverted position. This ligament extends from the cervix to the sacrum and supports the uterus posteriorly. The pubocervical ligament provides anterior support to the uterus, helping to prevent a cystocele. The broad ligament is a double folding of the peritoneum, divided into 4 regions composed of the mesosalpinx, mesovarium, mesometrium, and the suspensory ligament of the ovary. This ligament overlies the ovaries, uterus, and fallopian tubes and secures the uterus laterally to the sidewalls of the pelvis.[30]
When the supportive structures of the pelvis fail, pelvic organ prolapse results; this is a common condition among women and is usually treated with conservative management, mechanical support using pessaries, or surgical repair. Laparoscopic promontofixation is a surgical option that has excellent long-term results.[33] Additionally, laparoscopic promontofixation is associated with low recurrence and morbidity rates and improved quality of life in women with pelvic organ prolapse.[33]
Physiologic Variants
A common physiologic variant is the retroverted uterus. The presence of a retroverted uterus is not associated with a higher incidence of infertility, urinary tract infection, or hyperemesis gravidarum. However, study results have demonstrated that the incidence of early pregnancy bleeding and spontaneous abortion is significantly higher in those with a retroverted gravid uterus.[34] These patients require careful observation, and the clinician should not perform bimanual manipulation in gravid women with a retroverted uterus.[34]
Additionally, the origin of the uterine artery may vary in up to 1 in 5 cases.[27] The uterine artery originates directly from the internal iliac, superior gluteal, obturator, or internal pudendal artery.[27] A particularly challenging variation occurs when the uterine artery adopts a C-shaped configuration, with a secondary branch arising directly from the internal iliac artery. Healthcare professionals must anticipate these variants during surgery for successful ligation.
Surgical Considerations
A surgeon must be aware of anatomical relationships when operating on a female. Postpartum hemorrhage is a serious complication, especially after cesarean section. Typically, postpartum hemorrhage management involves clamping or embolization of the uterine artery. If bleeding persists, the surgeon should clamp the internal iliac artery below the origin of the superior gluteal artery. Some study results have suggested that several arteries, including the uterine artery, are recruited to respond to the demand from the gravid uterus.[35] In these cases, treatment of recurrent or persistent postpartum hemorrhage after bilateral uterine artery embolization may be successfully treated with round ligament artery embolization.[35]
Being aware of the position of the ureter during a hysterectomy is crucial; it is especially prone to being ligated because of its proximity to the uterine artery and cervix. The ureter travels under the cardinal ligament and is a vital structure to be mindful of. Furthermore, during a hysterectomy, the uterine artery is usually isolated to prevent unintentional ligation and massive bleeding. After clamping, the uterine artery is ligated at the level of the internal cervical os during a hysterectomy.[27] Femoral hernias may occur in females, but the herniated tissue is usually omentum or bowel. However, there have been rare, reported cases where the ovary, fallopian tube, and/or uterus were contained within the hernia.[36] This possibility should be carefully considered in the management of such cases.
Clinical Significance
Significant clinical correlations to consider when considering the female internal genitalia are as follows:
- The uterus typically sits in an anteverted, anteflexed position. A retroverted uterus is associated with an increased risk of early bleeding in pregnancy and spontaneous abortion. Cesarean section is correlated with a retroverted uterus.[37]
- Embryologic defects in the shape of the uterus, such as bicornuate uterine anomalies, may also lead to infertility and first-trimester spontaneous miscarriages.
- The uterus receives its vascular supply from the uterine artery, an anterior division of the internal iliac artery, which travels within the cardinal ligament. Physiologic variants of the uterine artery may be present in many cases, and surgeons must anticipate these cases for appropriate ligation of the artery.[38]
- There must be careful consideration of the ureter during surgeries involving the internal female genitals to avoid unintentional ligation.
- The ovaries are the gonadal structures of females; they derive from the gonadal ridge. The “Z” gene hypothesis names FoxL2, Dax1, and Wnt4 as possible ovary-determining factors.[39]
- The ovaries obtain their blood supply from the ovarian arteries, and their lymphatic drainage is directed to the paraaortic nodes.
- The fallopian tubes are the connections from the ovary to the uterus. Salpingitis may lead to scarring of the fallopian tubes and predispose to infertility and ectopic tubal pregnancy. A tubal factory must be considered in young women with infertility of undetermined significance.[40]
- A pudendal nerve block is an option to alleviate the pain of labor. However, these blocks are rarely used since the popularity of epidurals has increased.
- The pubococcygeus muscle bears the largest tissue strain compared to other pelvic floor muscles and is at significant risk of being compromised during vaginal delivery.
- Study results have shown that the round ligament's embolization may help control recurrent or persistent postpartum hemorrhage.[41] Interventional radiologists should consider this as an alternative for hard-to-control cases.[42]
- There have been rare cases of femoral herniation containing the ovary, fallopian tube, and uterus.[36][43][44]
- Pelvic organ prolapse is prevalent among women.[45] Many treatment strategies exist, including conservative management, mechanical support, or surgical repair.[46] Laparoscopic promontofixation is a surgical approach that generally delivers improved long-term outcomes.
Media
(Click Image to Enlarge)
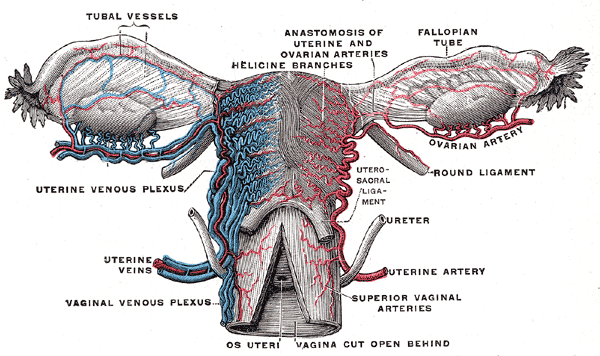
Female Reproductive System Blood Supply. Anterior-view illustration showing the fallopian tube vessels, anastomosis of the uterine and ovarian arteries, helicine branches, ovarian artery, uterine venous plexus, uterine artery, and veins, superior vaginal arteries, and vaginal venous plexus. Other structures shown are the ovaries, fallopian tubes, uterus, os uteri, uterosacral ligament, and ureter.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
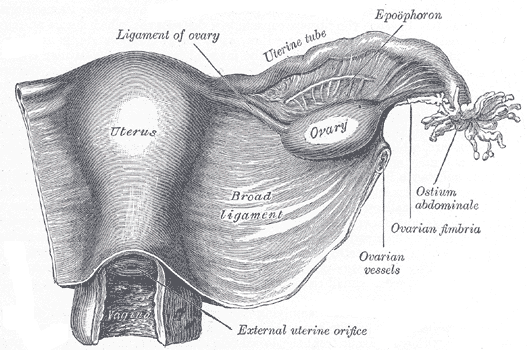
The Female Genital Organs. The uterus and right broad ligament are seen from behind, along with the uterine tube, ovary, epoophoron, and ovarian fimbria.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
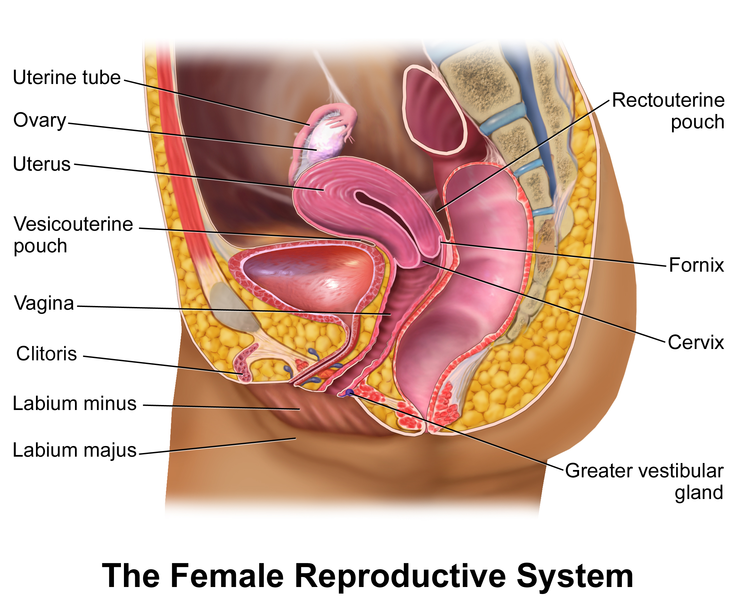
The Female Reproductive System. In females, pelvic organs include the reproductive organs (the uterus, paired fallopian tubes, and ovaries) and other organs, such as the bladder and rectum, surrounded by ligaments, nerves, lymph nodes, and vasculature.
Blausen.com staff. Medical Gallery of Blausen Medical 2014. WikiJournal of Medicine. doi: 10.15347/wjm/2014.010.
ISSN 2002-4436. [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)] via Wikimedia Commons.
References
Nizić D, Pervan M, Kos I, Šimunović Marko. [Flexion and version of the uterus on pelvic ultrasound examination]. Acta medica Croatica : casopis Hravatske akademije medicinskih znanosti. 2014 Jun:68(3):311-5 [PubMed PMID: 26016224]
Cagnacci A, Grandi G, Cannoletta M, Xholli A, Piacenti I, Volpe A. Intensity of menstrual pain and estimated angle of uterine flexion. Acta obstetricia et gynecologica Scandinavica. 2014 Jan:93(1):58-63. doi: 10.1111/aogs.12266. Epub 2013 Dec 3 [PubMed PMID: 24116846]
Mihu D, Mihu CM. Ultrasonography of the uterus and ovaries. Medical ultrasonography. 2011 Sep:13(3):249-52 [PubMed PMID: 21894299]
Roach MK, Andreotti RF. The Normal Female Pelvis. Clinical obstetrics and gynecology. 2017 Mar:60(1):3-10. doi: 10.1097/GRF.0000000000000259. Epub [PubMed PMID: 28005593]
Park HJ, Kim YS, Yoon TK, Lee WS. Chronic endometritis and infertility. Clinical and experimental reproductive medicine. 2016 Dec:43(4):185-192. doi: 10.5653/cerm.2016.43.4.185. Epub 2016 Dec 26 [PubMed PMID: 28090456]
Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, Brölmann HA, Mol BW, Huirne JA. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Human reproduction (Oxford, England). 2015 Dec:30(12):2695-702. doi: 10.1093/humrep/dev240. Epub 2015 Sep 25 [PubMed PMID: 26409016]
Catenaccio E, Mu W, Lipton ML. Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain structure & function. 2016 Nov:221(8):3845-3867 [PubMed PMID: 26897178]
DeMayo FJ, Zhao B, Takamoto N, Tsai SY. Mechanisms of action of estrogen and progesterone. Annals of the New York Academy of Sciences. 2002 Mar:955():48-59; discussion 86-8, 396-406 [PubMed PMID: 11949965]
Level 3 (low-level) evidenceKhan YS, Fakoya AO, Sajjad H. Anatomy, Thorax, Mammary Gland. StatPearls. 2025 Jan:(): [PubMed PMID: 31613446]
Briceag I, Costache A, Purcarea VL, Cergan R, Dumitru M, Briceag I, Sajin M, Ispas AT. Fallopian tubes--literature review of anatomy and etiology in female infertility. Journal of medicine and life. 2015 Apr-Jun:8(2):129-31 [PubMed PMID: 25866566]
Coy P, García-Vázquez FA, Visconti PE, Avilés M. Roles of the oviduct in mammalian fertilization. Reproduction (Cambridge, England). 2012 Dec:144(6):649-60. doi: 10.1530/REP-12-0279. Epub 2012 Oct 1 [PubMed PMID: 23028122]
Level 3 (low-level) evidenceZulfiqar M, Shetty A, Yano M, McGettigan M, Itani M, Naeem M, Ratts VS, Siegel CL. Imaging of the Vagina: Spectrum of Disease with Emphasis on MRI Appearance. Radiographics : a review publication of the Radiological Society of North America, Inc. 2021 Sep-Oct:41(5):1549-1568. doi: 10.1148/rg.2021210018. Epub 2021 Jul 23 [PubMed PMID: 34297630]
Migda MS, Migda M, Słapa R, Mlosek RK, Migda B. The use of high-frequency ultrasonography in the assessment of selected female reproductive structures: the vulva, vagina and cervix. Journal of ultrasonography. 2019 Dec:19(79):261-268. doi: 10.15557/JoU.2019.0039. Epub 2019 Dec 31 [PubMed PMID: 32021707]
Ernest A, Emmanuel M, Gregory K. Post-coital posterior fornix perforation with vaginal evisceration. BMC women's health. 2014 Nov 25:14():141. doi: 10.1186/s12905-014-0141-6. Epub 2014 Nov 25 [PubMed PMID: 25420670]
Level 3 (low-level) evidenceCiebiera M, Słabuszewska-Jóźwiak A, Ledowicz W, Jakiel G. Vaginal foreign body mimicking cervical cancer in postmenopausal woman - case study. Przeglad menopauzalny = Menopause review. 2015 Sep:14(3):208-10. doi: 10.5114/pm.2015.54348. Epub 2015 Sep 30 [PubMed PMID: 26528112]
Level 3 (low-level) evidenceLee IO, Lee JY, Kim S, Kim SW, Kim YT, Nam EJ. Sentinel lymph node mapping with indocyanine green in vaginal cancer. Journal of gynecologic oncology. 2017 Jul:28(4):e29. doi: 10.3802/jgo.2017.28.e29. Epub [PubMed PMID: 28541627]
Hasanzadeh M, Jafarian AH, Mousavi Seresht L. Primary Clear Cell Carcinoma with no Diethylstilbestrol Exposure; Case Series. Iranian journal of medical sciences. 2019 Mar:44(2):163-167 [PubMed PMID: 30936603]
Level 2 (mid-level) evidenceYao HH. The pathway to femaleness: current knowledge on embryonic development of the ovary. Molecular and cellular endocrinology. 2005 Jan 31:230(1-2):87-93 [PubMed PMID: 15664455]
Level 3 (low-level) evidenceSingh A, Rappolee DA, Ruden DM. Epigenetic Reprogramming in Mice and Humans: From Fertilization to Primordial Germ Cell Development. Cells. 2023 Jul 17:12(14):. doi: 10.3390/cells12141874. Epub 2023 Jul 17 [PubMed PMID: 37508536]
Kleppe M, Kraima AC, Kruitwagen RF, Van Gorp T, Smit NN, van Munsteren JC, DeRuiter MC. Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015 Oct:25(8):1405-14. doi: 10.1097/IGC.0000000000000514. Epub [PubMed PMID: 26397066]
Level 2 (mid-level) evidenceMigale R, Neumann M, Mitter R, Rafiee MR, Wood S, Olsen J, Lovell-Badge R. FOXL2 interaction with different binding partners regulates the dynamics of ovarian development. Science advances. 2024 Mar 22:10(12):eadl0788. doi: 10.1126/sciadv.adl0788. Epub 2024 Mar 22 [PubMed PMID: 38517962]
Level 3 (low-level) evidenceP A A, Arbor TC, Krishan K. Embryology, Sexual Development. StatPearls. 2025 Jan:(): [PubMed PMID: 32491533]
Şimşek E, Doğan Durdağ G, Alkaş Yağınç D, Aydın Ş, Yüksel Şimşek S, Çağlar Aytaç P. The effect of unicornuate uterus on reproductive outcomes in infertile patients. European journal of obstetrics, gynecology, and reproductive biology. 2024 Nov:302():38-42. doi: 10.1016/j.ejogrb.2024.08.040. Epub 2024 Aug 27 [PubMed PMID: 39216408]
Boyd CA, Riall TS. Unexpected gynecologic findings during abdominal surgery. Current problems in surgery. 2012 Apr:49(4):195-251. doi: 10.1067/j.cpsurg.2011.12.002. Epub [PubMed PMID: 22424211]
Fernandes R, Naik SM, Naik S. Surgical Management of a Rare Case of a Large Gartner's Cyst: A Case Report. Journal of obstetrics and gynaecology of India. 2023 Dec:73(Suppl 2):301-303. doi: 10.1007/s13224-023-01820-5. Epub 2023 Nov 4 [PubMed PMID: 38143967]
Level 3 (low-level) evidenceGarge S, Paliwal G, Mittal S, Kakani N. Mesonephric Remnant (Paroophoron) Presenting as a Congenital Lumbar Sinus in a Child. Journal of Indian Association of Pediatric Surgeons. 2020 Jan-Feb:25(1):58-59. doi: 10.4103/jiaps.JIAPS_242_18. Epub 2019 Nov 27 [PubMed PMID: 31896904]
Peters A, Stuparich MA, Mansuria SM, Lee TT. Anatomic vascular considerations in uterine artery ligation at its origin during laparoscopic hysterectomies. American journal of obstetrics and gynecology. 2016 Sep:215(3):393.e1-3. doi: 10.1016/j.ajog.2016.06.004. Epub 2016 Jun 8 [PubMed PMID: 27287682]
Mikhael M, Khan YS. Anatomy, Abdomen and Pelvis: Lymphatic Drainage. StatPearls. 2025 Jan:(): [PubMed PMID: 32491652]
Anderson D. Pudendal nerve block for vaginal birth. Journal of midwifery & women's health. 2014 Nov-Dec:59(6):651-659. doi: 10.1111/jmwh.12222. Epub 2014 Oct 7 [PubMed PMID: 25294258]
Level 3 (low-level) evidenceAshton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Annals of the New York Academy of Sciences. 2007 Apr:1101():266-96 [PubMed PMID: 17416924]
de Tayrac R, Panel L, Masson G, Mares P. [Episiotomy and prevention of perineal and pelvic floor injuries]. Journal de gynecologie, obstetrique et biologie de la reproduction. 2006 Feb:35(1 Suppl):1S24-1S31 [PubMed PMID: 16495824]
Level 1 (high-level) evidenceKalis V, Chaloupka P, Turek J, Rokyta Z. [The perineal body length and injury at delivery]. Ceska gynekologie. 2005 Sep:70(5):355-61 [PubMed PMID: 16180795]
Sabbagh R, Mandron E, Piussan J, Brychaert PE, Tu le M. Long-term anatomical and functional results of laparoscopic promontofixation for pelvic organ prolapse. BJU international. 2010 Sep:106(6):861-6. doi: 10.1111/j.1464-410X.2009.09173.x. Epub 2010 Jan 19 [PubMed PMID: 20089111]
Level 2 (mid-level) evidenceWeekes AR, Atlay RD, Brown VA, Jordan EC, Murray SM. The retroverted gravid uterus and its effect on the outcome of pregnancy. British medical journal. 1976 Mar 13:1(6010):622-4 [PubMed PMID: 1252851]
Dabrowiecki A, Newsome J, Bercu ZL, Martin JG. Postpartum haemorrhage requiring embolisation of a hypertrophied round ligament artery. BMJ case reports. 2019 Aug 30:12(8):. doi: 10.1136/bcr-2019-230071. Epub 2019 Aug 30 [PubMed PMID: 31473635]
Level 3 (low-level) evidenceAmbedkar V, Singh A, Bain J, Singh LM. A rare case of femoral herniation of female internal genitalia. Journal of natural science, biology, and medicine. 2015 Jul-Dec:6(2):454-6. doi: 10.4103/0976-9668.160038. Epub [PubMed PMID: 26283851]
Level 3 (low-level) evidenceSanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR. American journal of roentgenology. 2014 Jul:203(1):W117-24. doi: 10.2214/AJR.12.10403. Epub [PubMed PMID: 24951223]
Albulescu D, Constantin C, Constantin C. Uterine artery emerging variants - angiographic aspects. Current health sciences journal. 2014 Jul-Sep:40(3):214-6. doi: 10.12865/CHSJ.40.03.11. Epub 2014 Aug 4 [PubMed PMID: 25729609]
Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, Schlessinger D, Ottolenghi C. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC developmental biology. 2009 Jun 18:9():36. doi: 10.1186/1471-213X-9-36. Epub 2009 Jun 18 [PubMed PMID: 19538736]
Level 3 (low-level) evidencePatil M. Assessing tubal damage. Journal of human reproductive sciences. 2009 Jan:2(1):2-11. doi: 10.4103/0974-1208.51335. Epub [PubMed PMID: 19562067]
Lindquist JD, Vogelzang RL. Pelvic Artery Embolization for Treatment of Postpartum Hemorrhage. Seminars in interventional radiology. 2018 Mar:35(1):41-47. doi: 10.1055/s-0038-1636520. Epub 2018 Apr 5 [PubMed PMID: 29628615]
Chen C, Lee SM, Kim JW, Shin JH. Recent Update of Embolization of Postpartum Hemorrhage. Korean journal of radiology. 2018 Jul-Aug:19(4):585-596. doi: 10.3348/kjr.2018.19.4.585. Epub 2018 Jun 14 [PubMed PMID: 29962865]
Soeta N, Saito T, Nemoto T, Oshibe I, Gotoh M. Laparoscopic repair of irreducible femoral hernia containing the fallopian tube alone: a case report. Surgical case reports. 2016 Dec:2(1):57. doi: 10.1186/s40792-016-0185-y. Epub 2016 Jun 6 [PubMed PMID: 27271469]
Level 3 (low-level) evidenceCoyle D, Kavanagh N, Mahmoud A, Lowery AJ, Khan W, Barry K. Incarcerated femoral hernia containing ovary and fallopian tube in a 54-year-old. BMJ case reports. 2011 Aug 4:2011():. doi: 10.1136/bcr.05.2011.4263. Epub 2011 Aug 4 [PubMed PMID: 22687676]
Level 3 (low-level) evidenceGiarenis I, Robinson D. Prevention and management of pelvic organ prolapse. F1000prime reports. 2014:6():77. doi: 10.12703/P6-77. Epub 2014 Sep 4 [PubMed PMID: 25343034]
Chung SH, Kim WB. Various Approaches and Treatments for Pelvic Organ Prolapse in Women. Journal of menopausal medicine. 2018 Dec:24(3):155-162. doi: 10.6118/jmm.2018.24.3.155. Epub 2018 Dec 31 [PubMed PMID: 30671407]