Introduction
Proper palate formation in the developing fetus is essential for functional phonation and feeding after birth. The palate forms the roof of the mouth and separates the oral cavity from the nasal cavity. The palate undergoes complex morphological changes during embryogenesis to achieve its final form and divides into an anterior immobile hard bony segment and a posterior mobile soft palate that does not contain bone. The superior aspect of the palate forms the floor of the nasal cavities and has a lining of ciliated pseudostratified columnar epithelium (respiratory epithelium). The inferior aspect of the palate forms the roof of the oral cavity and is lined with stratified squamous epithelium (oral mucosa) that contains secretory salivary glands. The hard palate comprises about two-thirds of the total palate surface area, and its underlying bony structure consists of the palatine processes of the maxilla and the horizontal plates of the palatine bones. The soft palate is comprised of muscle fibers covered by a mucus membrane, specifically five muscles which have a functional role in breathing and swallowing:
- Levator veli palatini muscle: elevates the soft palate and is involved in swallowing.
- Musculus uvulae muscle: functions to shorten the uvula.
- Palatoglossus muscle: pulls the soft palate towards the tongue and is involved in swallowing.
- Palatopharyngeus muscle: tenses the soft palate and draws the pharynx anteriorly, involved in breathing.
- Tensor veli palatini muscle: tenses the soft palate and is involved in swallowing.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
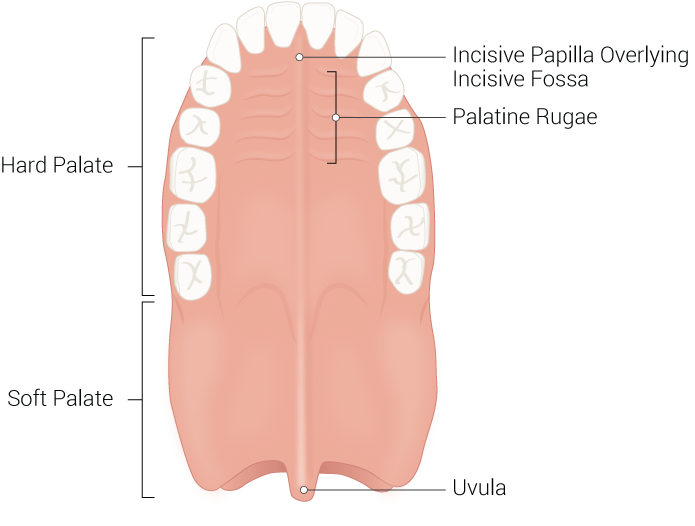
Hard Palate
The hard palate comprises the anterior two-thirds of the palate and is an immobile hard bony segment whose underlying bony structure consists of the palatine processes of the maxilla and the horizontal plates of the palatine bones. Situated anteriorly, the palatine processes of the maxilla comprise most of the hard palate and cover the area between the two sides of the maxillary dental arch. The palatine processes of the maxilla meet the two horizontal plates of the palatine bones posteriorly, which fuse down the midline. The hard palate formally separates the oral cavity from the nasal cavities, forming the floor of the nasal cavity and the roof of the oral cavity. The hard palate is covered superiorly by ciliated pseudostratified columnar epithelium (respiratory mucosa) and inferiorly by stratified squamous epithelium (oral mucosa). Anteriorly, the hard palate has irregular ridges or rugae, called rugae palatinae, on the mucous membrane that facilitates the passage of food posteriorly towards the pharynx. The hard palate contains three foramina/canals that serve as passageways for critical neurovascular structures that supply the oral cavity. These important landmarks include the incisive canal, the greater palatine foramen, and the lesser palatine foramen. The incisive canal is located at the anterior midline of the maxilla, directly posterior to the central maxillary incisor teeth, and contains the nasopalatine nerve and the descending palatine artery. The greater palatine foramen is located in the posterior hard palate medial to the third molar tooth and is traversed by the greater palatine vessels and nerve. The lesser palatine foramen is located posterior to the greater palatine foramen on the base of the pyramidal process and is traversed by the lesser palatine nerve. Structurally, the hard palate provides a rigid floor to the nasal cavity that prevents changes in pressure within the mouth from closing off nasal passages. Historically, infants with a cleft palate could not suckle and often died from malnutrition; this is because infants cannot effectively create negative sucking pressure without a properly formed hard palate. The hard palate is also essential for phonation and contains rugae that aid in mastication and digestion.[1][2]
Soft Palate
The soft palate makes up the posterior third of the palate and is a posterior continuation of the hard palate. The soft palate consists of muscle fibers and connective tissue covered by a mucus membrane consisting of a stratified squamous epithelium with secretory salivary glands. Unlike the hard palate, the soft palate is very flexible and does not contain any bony structures. The soft palate serves to elevate the nasopharynx, effectively closing the communication from the oropharynx to the nasopharynx. The soft palate is comprised of five muscles that play essential roles in breathing, phonation, and swallowing.
Anteriorly, the soft palate is continuous with the hard palate and the palatine aponeurosis. Posteriorly, the soft palate ends as a midline projection called the uvula that projects into the oral cavity. The soft palate forms the roof of the oropharyngeal isthmus, an area connecting the pharynx and oral cavity. Two arches bind the soft palate to the tongue and pharynx, the palatoglossal arches, and palatopharyngeal arches, respectively. The palatine tonsils appear between these arches in the tonsillar fossa of the oropharynx.
The soft palate has five muscles that are innervated by the pharyngeal plexus of CN X, except for the tensor veli palatini muscle, which receives innervation from a branch of the trigeminal nerve called the medial pterygoid nerve. The muscles that make up the soft palate include the palatoglossus, palatopharyngeus, tensor veli palatini, musculus uvulae, and levator veli palatini muscles. The tensor veli palatini muscle attaches to the palatine aponeurosis from its origin at the medial pterygoid plate of the sphenoid. The tensor veli palatini muscle functions to tense the soft palate during swallowing, preventing the entry of food into the nasopharynx. The levator veli palatini muscle emerges from the eustachian tube and the petrous temporal bone before attaching to the palatine aponeurosis, this muscle functions to elevate the soft palate during swallowing to prevent the entry of food into the nasopharynx. The palatoglossus muscle arises from the palatine aponeurosis and travels inferiorly, anteriorly, and laterally to attach into the side of the tongue—the palatoglossus muscle functions to pull the soft palate towards the tongue and initiates the act of swallowing. The palatopharyngeus muscle emerges from the hard palate and the palatine aponeurosis and attaches to the superior border of the thyroid cartilage. The palatopharyngeus muscle tenses the soft palate and draws the pharynx superiorly and anteriorly during the act of swallowing. The palatopharyngeus muscle closes the laryngeal airway during swallowing to prevent the aspiration of food. The musculus uvulae muscle derives from the palatine aponeurosis and the posterior nasal spine and attaches to the mucous membrane of the uvula. The musculus uvulae muscle functions to shorten the uvula. Ipsilateral contraction of the musculus uvulae muscle draws up the uvula on the same side.
During the act of swallowing, the levator veli palatini muscle elevates the soft palate, approximating it with the posterior pharyngeal wall and completely blocking off the airway and nasal passages. As a result, the soft palate forms a vacuum in the oral cavity, keeping food from entering the respiratory tract. Breathing ceases briefly during swallowing; this is because of the physical closure of the airway by elevation of the soft palate. During a sneeze, the soft palate protects the nasal passage by diverting some secretions to the oral cavity. The soft palate plays a role in the gag reflex, touching the end of the soft palate or uvula evokes the gag reflex in the majority of people.[3][4][5]
Embryology
Early in embryonic development, the nasal cavity runs continuously with the oral cavity, as there is no anatomic structure separating these two cavities. As embryonic development progresses, palate formation ensues, formally separating the oral and nasal cavities from one another. Proper palate development requires the formation of a primary palate in the sixth week of development, as well as the formation of a secondary palate between the sixth and eighth weeks of development. The primary palate includes the alveolar arch. The secondary palate consists of the hard and soft palate. The external human face develops between the fourth and sixth weeks of embryonic development. The primary palate develops around the same time as the external face, specifically the sixth week of development, as a result of the fusion of the medial nasal and maxillary processes. Subsequently, between the sixth and twelfth weeks of embryonic development, the secondary palate forms as the result of the fusion between palatal processes growing from the medial walls of the maxillary processes. During secondary palate formation, the palatal shelves extend medially and fuse superior to the tongue. As the palatal shelves expand medially, the developing mandible continues to grow, allowing for the tongue to get out of the way of the growing palatal shelves. By the ninth week of development, the palatal shelves fuse as well as with the primary palate anteriorly to form the definitive palate.[6][7][8]
Blood Supply and Lymphatics
The greater palatine arteries run anteriorly, from the greater palatine foramen to supply the palate. The greater palatine arteries serve as the primary arterial supply to the palate. Anastomosis between the ascending palatine artery and the lesser palatine artery provides collateral supply to the palate. The greater palatine and lesser palatine arteries are branches of the maxillary artery, which arises from the external carotid artery. The ascending palatine artery is a branch of the facial artery, which also arises from the external carotid artery. The palate’s venous drainage directs towards the pterygoid venous plexus. The lymph vessels of the soft palate drain into the sub-digastric and/or lateral pharyngeal nodes.[9][10][11]
Nerves
Excluding the tensor veli palatini muscle, all of the palatal muscles are innervated by fibers from the pharyngeal plexus derived from the vagus nerve. The tensor veli palatini muscle receives its innervation from the medial pterygoid nerve, a branch of the trigeminal nerve. The sensory innervation of the palate originates from the maxillary branch of the trigeminal nerve. The maxillary division of the trigeminal nerve gives rise to many sensory branches that innervate the middle portion of the face, including the nasopalatine, greater palatine, lesser palatine, superior alveolar, middle meningeal, infraorbital, zygomatic, inferior palpebral, superior labial, and pharyngeal nerves. The greater palatine nerve arises from its canal through the greater palatine foramen and courses anteriorly in the roof of the palate, innervating the gingivae and mucosa of the hard palate while communicating with the nasopalatine nerve anteriorly. The lesser palatine nerve runs alongside, the greater palatine nerve, arising from the lesser palatine foramen to provide sensory innervation to the tonsils, uvula, and soft palate.[12]
Muscles
The soft palate has five muscles that receive innervation by the pharyngeal plexus of the vagus nerve, except for the tensor veli palatini muscle, which is innervated by a branch of the trigeminal nerve called the medial pterygoid nerve. The muscles that make up the soft palate include the palatoglossus, palatopharyngeus, tensor veli palatini, musculus uvulae, and levator veli palatini muscles.
The tensor veli palatini muscle attaches to the palatine aponeurosis from its origin at the medial pterygoid plate of the sphenoid. The tensor veli palatini muscle functions to tense the soft palate during swallowing, preventing the entry of food into the nasopharynx.
The levator veli palatini muscle emerges from the eustachian tube and the petrous temporal bone before attaching to the palatine aponeurosis, this muscle functions to elevate the soft palate during swallowing to prevent the entry of food into the nasopharynx.
The palatoglossus muscle arises from the palatine aponeurosis. It travels inferiorly, anteriorly, and laterally to attach into the side of the tongue—the palatoglossus muscle functions to pull the soft palate towards the tongue and initiates the act of swallowing.
The palatopharyngeus muscle emerges from the hard palate and the palatine aponeurosis and attaches to the superior border of the thyroid cartilage. The palatopharyngeus muscle tenses the soft palate and draws the pharynx superiorly and anteriorly during the act of swallowing.
The palatopharyngeus muscle closes the laryngeal airway during swallowing to prevent the aspiration of food.
The musculus uvulae muscle derives from the palatine aponeurosis and the posterior nasal spine and attaches to the mucous membrane of the uvula. The musculus uvulae muscle functions to shorten the uvula. Ipsilateral contraction of the musculus uvulae muscle draws up the uvula on the same side.
Physiologic Variants
Studies have demonstrated that the structural and obstructive patterns of the pharynx and palate vary among the general population. It is essential to recognize these phenotypic variations and understand how they generate the shape and collapsibility of the retropalatal airway. Understanding phenotypic variations in palatal anatomy are imperative in the diagnosis and treatment of sleep apnea.
The soft palate has both distal and proximal segments separated by a structure called the palatal genu. The palatal genu approximates the border between the aponeurotic and muscular components of the soft palate. The length of both the distal and proximal soft palate may vary among individuals, potentially predisposing certain individuals to sleep apnea. A distal palatal segment length greater than fifteen millimeters has been shown to increase the risk of sleep apnea dramatically and correlates with poorer palatopharyngoplasty outcomes.
The configuration of the soft palate and the position of the palatal genu is strongly correlated to the length of the levator veli palatini muscle. The levator veli palatini muscle comprises forty percent of the soft palate length between the hard palate and the base of the uvula. The proximal soft palatal segment maintains a variable angle from the hard palate, termed the alpha angle, which is determined by the length and position of the levator veli palatini muscle.
Studies have described three palatal airway phenotypes based on the measurement of the alpha angle; oblique, intermediate, and vertical. In individuals with intermediate and oblique palatal phenotypes, the angle between the proximal soft palate and hard palate is less acutely downwards, allowing for the soft palate to maintain a position more parallel to the hard palate. The airways of patients with oblique and intermediate phenotypes typically collapse circularly. In individuals who display a vertical palatal phenotype, the angle between the proximal soft palate and hard palate is more acutely downward, allowing for the soft palate to maintain a position more parallel to the posterior pharyngeal wall, rather than the hard palate. Airway collapse occurs in an anterior-posterior fashion in these patients.[13]
Surgical Considerations
Surgical techniques involving the palate are rapidly evolving to improve the quality of life for patients further. Palate surgery is typically indicated for patients with severe obstructive sleep apnea, cleft palate repair, and cancer resection.
Cleft palate repair, termed palatoplasty, should be performed between six to twelve months of age to optimize feeding and speech development without interrupting normal maxillofacial growth. There are three types of palatoplasty techniques: one type is for soft palate repair, the second type is for hard palate repair, and the third involves the surgical schedule. Soft palate repair techniques are radical muscle dissection, intravelar veloplasty, primary pharyngeal flap, and double opposing Z-plasty techniques. The hard palate repair techniques consist of the von Langenbeck, Veau-Wardill-Kilner V-Y, vomer flap, and alveolar extension palatoplasty techniques. The surgical schedule/protocol based techniques are whole in one, Malek’s, and modified schedule with palatoplasty before lip repair. The goal of surgery is to reapproximate or realign the palatal muscles. Upon completion of the surgery, this can help expedite the development of normal speech and sound. Further, cleft palate repair with repositioning of muscles can also improve the functioning of the eustachian tube and hearing. When a surgeon repositions the palatal muscles correctly, this maneuver can improve middle ear ventilation and decrease episodes of otitis media and hearing defects.
The most common surgical procedure used to treat obstructive sleep apnea is called uvulopalatopharyngoplasty (UPPP) and involves the repositioning and/or removal of tissue at the soft palate, uvula, and throat walls to increase airway size and decrease tissue collapse.
Soft palate implants, or the pillar procedure, is a minimally invasive procedure that has demonstrated to help with mild to moderate cases of sleep apnea. The pillar procedure requires the placement of three rods made of polyester into the soft palate. These polyester rods incite an inflammatory reaction within the surrounding soft tissue, which results in a stiffening of the soft palate. This inflammatory reaction results in a stiffer soft palate that is less apt to make contact with the posterior pharyngeal wall as the muscles relax during the deep stages of sleep.
Surgical resection is the treatment for cancer of the hard palate. Extension into surrounding bone is common, and frequently warrants removal of adjacent bone. If the tumor is small, the excision may be closed after surgery. If the tumor is large, a prosthetic device can serve to cover the opening in the palate as the excised area cannot be closed.[1][3][13][14]
Clinical Significance
Tumors of the palate, both benign and malignant, most often present asymptomatically or are associated with a low level of discomfort. The appearance of benign and malignant lesions of the palate is also quite similar, making it of utmost importance that the clinician be able to differentiate between them. Malignant squamous epithelial neoplasms such as verrucous carcinoma, carcinoma of the maxillary sinus, and squamous cell carcinoma may appear in the hard palate. Carcinoma of the maxillary sinus is often asymptomatic for a long time and usually presents at an advanced stage. This tumor is associated with the elderly and is most commonly diagnosed once the tumor expands to fill the sinus and produces a bulge of the alveolar ridge or palatal area on physical examination. Squamous cell carcinomas constitute more than ninety percent of all oral cancers. Non-squamous cell carcinomas of the oral cavity are rare. Risk factors for squamous cell carcinoma of the palate include alcohol, tobacco, and HPV infection. Primary squamous cell carcinoma of the soft palate is painful, causes dysphagia, and portends a much worse prognosis than tumors located more anteriorly. Verrucous carcinoma is a form of squamous cell carcinoma that rarely metastasizes, behaves indolently, and demonstrates a white papillary clinical appearance. The most common locations for verrucous carcinoma are the alveolar ridge and hard palate, and it is associated with elderly patients who wear complete denture prosthesis. Tumors of the palate can follow the palatine nerves through the greater and lesser palatine foramen and extend into surrounding bone, nerves, and soft tissue. Up to seventy percent of squamous cell carcinomas of the hard palate extends beyond the hard palate into neighboring structures. Radiographically, malignant tumors of the palate may show evidence of bone destruction and sometimes reveal a radioopacity produced by the neoplastic mass.[15]
Minor salivary gland carcinomas represent less than five percent of oral cavity cancers, and around sixty percent of them arise on the hard palate. Both malignant and benign salivary gland tumors of the palate appear as well-circumscribed, smooth, dome-shaped, non-moveable swellings that demonstrate a slow growth pattern.[16]
Pleomorphic adenoma is the most common benign salivary gland neoplasm found within the palate. The most frequently discovered malignant salivary gland tumors of the palate in descending order are adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and mucoepidermoid carcinoma.[17][18]
Melanoma is a malignant tumor of melanocytes most often associated with cancer of the skin. Melanoma may develop anywhere melanocytes exist and have the potential to form from melanocytes located within the palate. Oral melanoma is extremely rare, accounting for less than one percent of all oral malignancies. The most common site for oral melanoma is the hard palate, which accounts for approximately forty percent of cases. Oral melanoma most commonly appears on the maxillary alveolus or hard palate and tends to have a much worse prognosis than cutaneous melanoma. Initially, melanoma lesions appear flat and later become fixed and nodular in appearance. Melanomas often appear as light/dark brown lesions.[15]
The most common locations of extranodal lymphoma in the head and neck are the soft palate and posterior hard palate. On examination, lymphomas typically appear as a non-ulcerated, non-tender diffuse mass.
Sarcomas are malignant tumors of non-epithelial-tissue origin that may arise anywhere in the human body, including the palate. Sarcoma of the palate appears as ulcerated masses in the middle-aged or young patient.[15]
Cleft palate and lip are the most common congenital orofacial deformities in the world. Cleft palate represents the third most frequently occurring congenital deformity after clubfoot and cleft lip. Cleft lip and palate demonstrate a multifactorial inheritance pattern. They are the result of a combination of genetic predispositions and in-utero exposure to teratogens like nicotine, alcohol, antiepileptics, and folate antagonists. Cleft palate results in severe facial deformities, feeding difficulties, nasal regurgitation of milk, coughing/choking during feeds, difficulties with phonation, dentition defects, and predisposes patients to recurrent episodes of otitis media with effusion. Cleft palate occurs in a variety of chromosomal abnormalities like the Pierre-Robin sequence, Patau syndrome (trisomy 13), and Edward syndrome (trisomy 18). Embryologically, a cleft palate occurs due to a partial or total failure of fusion of the palatine prominences by the ninth week of development. Cleft palate may be unilateral or bilateral and complete or incomplete. There are three types of cleft palate: submucosal cleft palate, incomplete cleft palate, and a complete cleft palate. In the submucosal cleft palate, the palatine mucosa remains intact, but an underlying bony palatal or muscular defect is present. Incomplete cleft palate refers to clefting of the secondary palate exclusively, while complete cleft palate refers to clefting of the entire soft palate, hard palate, and uvula. Cleft palate repair, termed palatoplasty, should be performed between six to twelve months of age to optimize feeding and speech development without interrupting normal maxillofacial growth.[14][19]
Repeated bouts of upper airway obstruction cause obstructive sleep apnea during the deep stages of sleep as a result of the narrowing/collapse of the respiratory passages. Patients with obstructive sleep apnea tend to be overweight with associated peripharyngeal fat infiltration and/or increased size of the tongue and soft palate. These anatomic abnormalities predispose patients to obstructive sleep apnea because they decrease the cross-sectional area of the upper airway. An increased neck circumference corresponds to increased peripharyngeal fat infiltration, specifically lining the airway and at the base of the tongue. Neck circumference should be measured when obstructive sleep apnea is suspected. A neck circumference greater than seventeen inches in men and greater than sixteen inches in women increases the risk for obstructive sleep apnea. One local airway factor thought to be a causative agent of obstructive sleep apnea is an elongated soft palate. A distal palatal segment length greater than fifteen millimeters has shown to increase the risk of sleep apnea dramatically. Initial treatment of sleep apnea is primarily nonsurgical and consists of positive airway pressure therapy, most commonly in the form of CPAP. Historically, many of the surgical techniques used to correct sleep apnea in adults were sub-optimal, and palatopharyngoplasty techniques have evolved from primarily excisional methods to those that reposition and reconstruct the palate. Examples of these more advanced surgical techniques include the expansion sphincter-pharyngoplasty, lateral pharyngoplasty, relocation pharyngoplasty, and uvulopalatopharyngoplasty (UPPP).[1][5]
Media
(Click Image to Enlarge)
References
Olszewska E, Woodson BT. Palatal anatomy for sleep apnea surgery. Laryngoscope investigative otolaryngology. 2019 Feb:4(1):181-187. doi: 10.1002/lio2.238. Epub 2019 Jan 10 [PubMed PMID: 30828637]
Hourfar J, Kanavakis G, Bister D, Schätzle M, Awad L, Nienkemper M, Goldbecher C, Ludwig B. Three dimensional anatomical exploration of the anterior hard palate at the level of the third ruga for the placement of mini-implants--a cone-beam CT study. European journal of orthodontics. 2015 Dec:37(6):589-95. doi: 10.1093/ejo/cju093. Epub 2015 Jan 6 [PubMed PMID: 25564503]
Level 2 (mid-level) evidenceCho JH, Kim JK, Lee HY, Yoon JH. Surgical anatomy of human soft palate. The Laryngoscope. 2013 Nov:123(11):2900-4. doi: 10.1002/lary.24067. Epub 2013 May 17 [PubMed PMID: 23686451]
Grimaldi A, Parada C, Chai Y. A Comprehensive Study of Soft Palate Development in Mice. PloS one. 2015:10(12):e0145018. doi: 10.1371/journal.pone.0145018. Epub 2015 Dec 15 [PubMed PMID: 26671681]
Shigeta Y, Ogawa T, Tomoko I, Clark GT, Enciso R. Soft palate length and upper airway relationship in OSA and non-OSA subjects. Sleep & breathing = Schlaf & Atmung. 2010 Dec:14(4):353-8. doi: 10.1007/s11325-009-0318-7. Epub 2009 Dec 8 [PubMed PMID: 19997779]
Dursun A, Öztürk K, Albay S. Development of Hard and Soft Palate During the Fetal Period and Hard Palate Asymmetry. The Journal of craniofacial surgery. 2018 Nov:29(8):2358-2362. doi: 10.1097/SCS.0000000000005016. Epub [PubMed PMID: 30320695]
Greene RM, Pisano MM. Palate morphogenesis: current understanding and future directions. Birth defects research. Part C, Embryo today : reviews. 2010 Jun:90(2):133-54. doi: 10.1002/bdrc.20180. Epub [PubMed PMID: 20544696]
Level 3 (low-level) evidenceMatsumoto R, Evans SE. The palatal dentition of tetrapods and its functional significance. Journal of anatomy. 2017 Jan:230(1):47-65. doi: 10.1111/joa.12534. Epub 2016 Aug 19 [PubMed PMID: 27542892]
Cho JH, Kim JW, Park HW, Suh JD, Kim JK, Yoon JH. Arterial supply of the human soft palate. Surgical and radiologic anatomy : SRA. 2017 Jul:39(7):731-734. doi: 10.1007/s00276-016-1798-3. Epub 2017 Jan 30 [PubMed PMID: 28138793]
Mercer NS, MacCarthy P. The arterial supply of the palate: implications for closure of cleft palates. Plastic and reconstructive surgery. 1995 Oct:96(5):1038-44 [PubMed PMID: 7568477]
Zhong W, Zhang K, Wang F. [Applied anatomical study of blood supply in human palate]. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chinese journal of stomatology. 2001 Mar:36(2):136-8 [PubMed PMID: 11812326]
Level 2 (mid-level) evidenceMu L, Chen J, Li J, Arnold M, Sobotka S, Nyirenda T, Fowkes M, Christopherson M, Sanders I. Sensory Innervation of the Human Soft Palate. Anatomical record (Hoboken, N.J. : 2007). 2018 Nov:301(11):1861-1870. doi: 10.1002/ar.23864. Epub 2018 Aug 6 [PubMed PMID: 30079585]
Mendonca DA, Patel KB, Skolnick GB, Woo AS. Anatomical study of the effects of five surgical maneuvers on palate movement. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2014 Jun:67(6):764-9. doi: 10.1016/j.bjps.2014.02.014. Epub 2014 Feb 21 [PubMed PMID: 24721126]
Kosowski TR, Weathers WM, Wolfswinkel EM, Ridgway EB. Cleft palate. Seminars in plastic surgery. 2012 Nov:26(4):164-9. doi: 10.1055/s-0033-1333883. Epub [PubMed PMID: 24179449]
Montero PH, Patel SG. Cancer of the oral cavity. Surgical oncology clinics of North America. 2015 Jul:24(3):491-508. doi: 10.1016/j.soc.2015.03.006. Epub 2015 Apr 15 [PubMed PMID: 25979396]
Ginsberg LE, DeMonte F. Imaging of perineural tumor spread from palatal carcinoma. AJNR. American journal of neuroradiology. 1998 Sep:19(8):1417-22 [PubMed PMID: 9763370]
Level 3 (low-level) evidenceUral A, Livaoğlu M, Bektaş D, Bahadır O, Hesapçıoğlu A, Imamoğlu M, Işık AU. Approach to benign tumors of the palate: analysis of 28 cases. Ear, nose, & throat journal. 2011 Aug:90(8):382-5 [PubMed PMID: 21853443]
Level 3 (low-level) evidenceJu WT, Zhao TC, Liu Y, Tan YR, Dong MJ, Sun Q, Wang LZ, Li J, Zhong LP. Computed tomographic features of adenoid cystic carcinoma in the palate. Cancer imaging : the official publication of the International Cancer Imaging Society. 2019 Jan 31:19(1):3. doi: 10.1186/s40644-019-0190-z. Epub 2019 Jan 31 [PubMed PMID: 30704527]
Worley ML, Patel KG, Kilpatrick LA. Cleft Lip and Palate. Clinics in perinatology. 2018 Dec:45(4):661-678. doi: 10.1016/j.clp.2018.07.006. Epub 2018 Sep 18 [PubMed PMID: 30396411]