Introduction
The spinal cord is a collection of nerve tissue extending from the caudal aspect of the brainstem to the lumbar region of the spinal canal. It terminates at approximately the level of the L2 vertebral body as the conus medullaris. Together with the brain, it comprises the central nervous system (CNS). The spinal cord has various functions, which include: conveying afferent, sensory information from the peripheral nervous system to the brain; transmitting signals from the motor cortex of the brain to the periphery; and serving as the center of reflex responses.
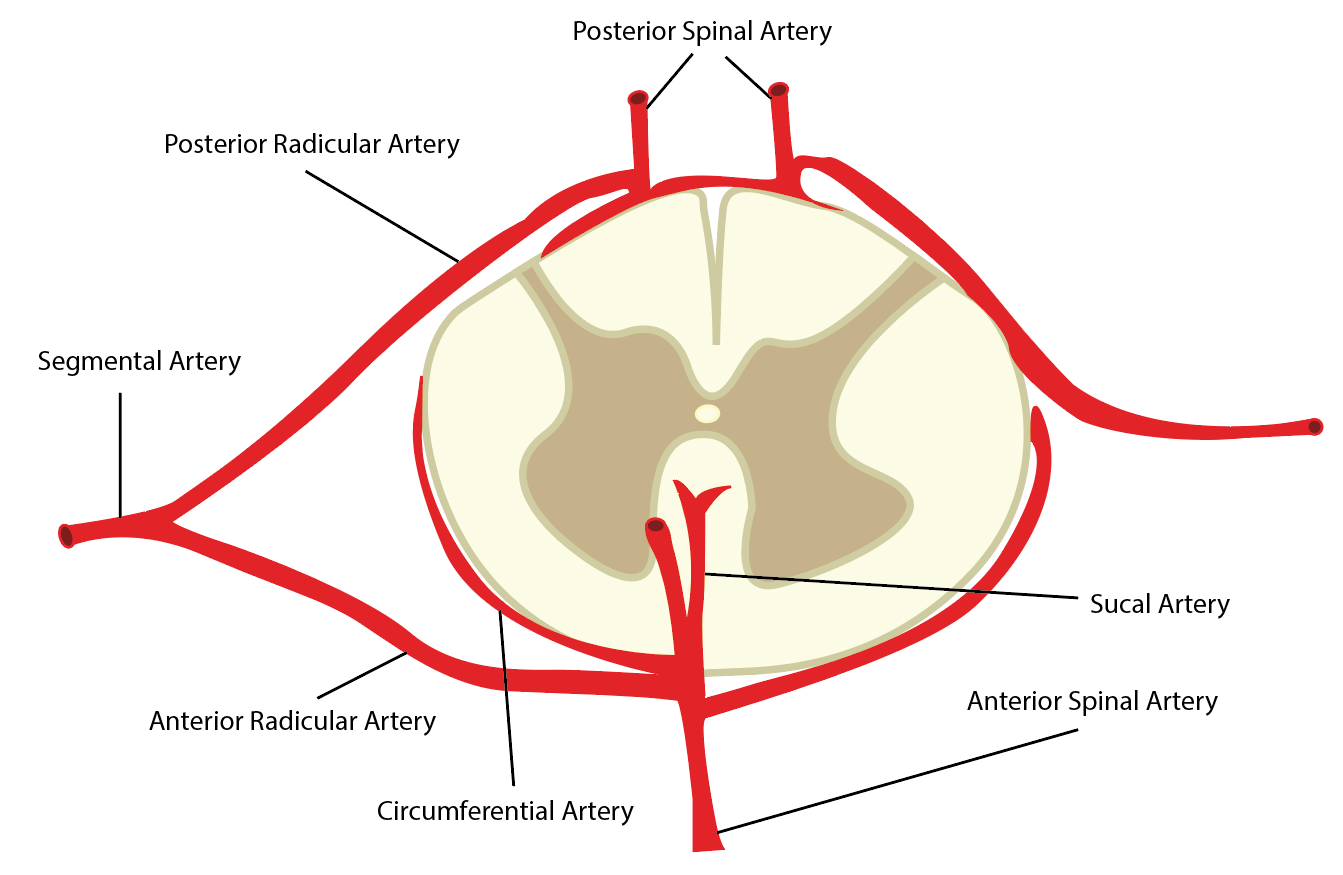
Arterial supply to the spinal cord is vital to supplement the high metabolic requirements of this nervous tissue. The vasculature has a certain degree of variability, although common features and structures exist. It includes vasculature lying directly superficial to the cord that courses longitudinally along the structure and radicular arteries that supply the cord transversely in a segmental fashion.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Blood supply to the spinal cord is highly complex and anastomotic to ensure adequate supply to the entire structure. Variability in the vasculature is not uncommon. Additionally, blood flows preferentially to certain regions of the spinal cord, particularly the cervical and lumbar enlargements.[1]
Longitudinally, the spinal cord receives vascular supply by the anterior spinal artery (ASA) and a pair of posterior spinal arteries (PSA). These three arteries typically originate from the distal vertebral arteries at the superior aspect of the spinal cord. However, some literature has described the PSAs as branches of the posterior inferior cerebellar artery. The ASA is the predominant supply of the spinal cord. It supplies the anterior two-thirds of the cross-sectional area through its terminal branches, the central arteries. The paired PSAs provide blood to the spinal posterior one-third cross-sectional area. The three vessels also connect circumferentially around the structure via the pial plexus, often referred to as the vasocorona. It supplies the periphery of the cord via small perforators, known as rami perforantes.[2][3]
Segmentally, numerous anterior and posterior radicular arteries (see below) provide collateral blood flow to the entire vertebral column, including the vertebral bodies and intervertebral discs, in addition to the spinal cord.[1][3]
Embryology
The cardiovascular system is the initial organ system formed in neonatal development. Its primary functions include gas exchange, nutritional supplementation, and metabolic waste removal to maintain the viability and growth of the cells and tissues. Additionally, the vascular system is a significant provider of hormonal regulation and guidance, specifically the vascular endothelial growth factor (VGEF).[4][5]
During development, the peri-neural vascular plexus (PNVP) supplies blood to the spinal cord. Some of the PNVP eventually integrates into the CNS to become the intra-neural vascular plexus (INVP). The INVP is susceptible to environmental and hormonal cues that lead to preferential patterning of blood supply.[5]
Present throughout the CNS vascular system, the blood-brain barrier (BBB) plays an important role in protecting the neural tissue from variations in blood composition in addition to blood-borne pathogens. The BBB derives from the development of widespread tight junctions formed by endothelial cells that prevent cellular diffusion into the neural tissues.[6]
The artery of Adamkiewicz is a vital vessel that supplies the lumbar spinal cord. It is often identified by a trademark “hairpin” loop that arises due to differential growth of the spinal column and the spinal cord.
Blood Supply and Lymphatics
Cervical Spinal Cord
Arterial supply to the cervical spinal cord displays a marked variation between individuals because of differences in embryonic development—the ASA and two PSAs branch off the distal vertebral arteries at the level of the foramen magnum. The ASA courses midline on the ventral sulcus of the spinal cord, while the PSAs travel parallel to each other in a paramedian location on the dorsal aspect. In the cervical spine, two or three supportive anterior and posterior radiculomedullary arteries branch from the vertebral arteries. The largest of these radiculomedullary vessels is the artery of cervical enlargement, usually identified between the C4-C8 vertebral levels, most often coursing along the C6 nerve root. The radiculomedullary arteries join with the ASA and PSAs to carry supplemental blood flow to these nervous tissues. The provision of supplemental flow to the cord is from branches of the vertebral, ascending cervical, and deep cervical arteries, and potentially the ascending pharyngeal and occipital arteries.[1][3]
Thoracic Spinal Cord
The diameter of the ASA through the thoracic spinal cord is notably narrower compared to its size in the cervical and lumbar regions. Thus, much of the blood flow to the thoracic spine derives from segmental arteries branching from the dorsal aorta. Most often, nine pairs of posterior intercostal arteries in addition to one pair of subcostal arteries supply the spinal cord in the thorax; however, variation in the quantity of segmental vasculature is not uncommon. Rostral to the T3 level, the segmental arteries arise from the supreme intercostal artery, also known as the superior intercostal artery. Caudal to this level, minor segmental arteries branch directly from the dorsal aorta and derive their name according to the rib under which they track. Anatomically, the minor segmental arteries branch from the aorta at one to two vertebral levels caudal to their perfusion site and are seen traveling in an upward course from their site of origin to the spinal column.[3]
Lumbar Spinal Cord
The largest and most important radiculomedullary artery supplying the lumbar spinal cord is the artery of Adamkiewicz (AKA). Like most other vasculature structures supplying the spinal cord, its level of origin is variable based on embryonic development. It usually originates between T8-L2, most commonly at T10/T11. The artery lies to the left of the spinal cord and forms a hairpin loop as it bifurcates into ascending and larger descending branches to meet the ASA. There is little, if any, collateral blood supply inferior to the AKA and ASA junction on the cord’s anterior surface. However, the largest posterior radiculomedullary artery often enters the spinal cord below the junction of the AKA and ASA, serving as the most distal collateral blood supply to the cord.[1][3]
The ASA and the two PSAs course caudally past the conus medullaris. At this level, the ASA is at its thickest. After coursing inferiorly past the conus, with the filum terminale, the ASA ends as a collection of rami cruciantes that anastomose with each distal PSA.[3]
Physiologic Variants
The vasculature of the spinal cord is highly variable in size and structure. This is attributable to differences in embryological development and proliferation as well as metabolic needs. Some variants are described in the text above, such as the development of the artery of Adamkiewicz, the branching origin of the PSAs, and the number of segmental radicular arteries supplying the thoracic spinal cord. However, these are not the only identified instances of variation in spinal cord vasculature.
Rather than existing as a single continuous vessel, duplication of the ASA until the C5/C6 level is common. Radicular arteries supplying the ASA also differ in both number and origin. Literature has identified the average number of cervical radicular arteries to be two to three, arising from all branches of the subclavian artery in the neck. Specifics are less defined in the thoracic and lumbar regions due to an even more irregular and unpredictable pattern display.
The PSAs display an equal amount of irregularity. The posterior spinal arteries are more numerous and richer in collateralization than their anterior counterpart. They often present as discontinuous in a cruciate configuration and provide blood supply to the spinal cord in a contralateral fashion.[1][2][3]
Surgical Considerations
Many vascular complications can arise during surgical procedures of the spine. Besides the complex vasculature providing blood flow to the nervous structures in the spinal cord, the vertebral column is near vital vascular structures, including the aorta, iliac vessels, and carotid and vertebral arteries. Anterior cervical exposures come close to the carotid sheath, and injuries to the carotid artery—especially during revision surgery—have been documented.[7] Posterior cervical procedures, mainly those involving lateral mass screw placement, risk injuring the vertebral arteries that course in the transverse foramina, entering the C6 and exiting the C2 vertebrae.[8]
Potential vascular complications in the thoracic and thoracolumbar spine are injuries to the major vessels and their branches and the sequelae of compromising the blood supply to the thoracic and/or lumbar spinal cord. Paraplegia due to a unilateral vascular injury of a thoracic segmental vessel is uncommon. However, bilateral disruption of the segmental blood supply, such as in the case of aortic surgery or aortic dissection, presents a higher risk of paraplegia.[9] Thoracic and vascular literature has extensively documented the consequences of bilateral disruption of the blood flow to the thoracic cord during complex thoracic and thoracolumbar aneurysm reconstructive surgery.[10]
The transthoracic approach to the anterior thoracic spine typically requires the mobilization of segmental arteries over several vertebral levels. A right-sided approach is recommended to avoid the pericardial structures since the artery of Adamkiewicz has been reported to originate on the left side of the aorta between T8 and L1 in 75% of patients.[11]
Clinical Significance
Adequate vascular supply to the spinal cord is vital in maintaining the integrated function of the central nervous system. Supply to the cord is highly complex and interconnected to maintain the tissue’s metabolic needs.
Acute central cord syndrome is the most common type of spinal cord injury, accounting for over two-thirds of all incomplete cervical spinal cord injuries. Its predominant etiology is from cervical spine hyperextension trauma that compresses the spinal cord. Trauma to the cord often results in centro-medullary hemorrhage and edema. Characteristic symptoms are upper extremity weakness, sensory deficits, and bowel, and bladder dysfunction. Magnetic resonance imaging (MRI) is vital for diagnosing central cord syndrome and other complications such as hemorrhage and spinal column instability. Treatment specifics vary based on severity, but ensuring spinal column stability is always paramount. Surgical approaches are necessary in severe cases with marked instability and neurological impairment.[12]
Spinal cord infarction (SCI) results from an interruption in spinal cord blood supply and can lead to acute spinal syndrome. Though the development of SCI is rare (comprises only 1% of stroke), infarct of the anterior spinal artery and/or the artery of Adamkiewicz is overwhelmingly more common than infarct of a posterior spinal artery. Lesions of the anterior supply lead to clinical presentation characterized as an anterior spinal syndrome. Symptoms will depend on the location and severity of the infarct but include sudden back pain progressing to sensation deficits, extremity weakness or paresis, and bladder or bowel dysfunction. Onset is typically sudden, with peak symptoms arising within 30 to 45 minutes. In the typical ASA infarct, the anterior two-thirds of the cord are affected, leading to bilateral weakness, progressive sensory loss, and potential autonomic symptoms. An infarct of a PSA will typically cause unilateral symptoms. Diagnosis is made via detection on MRI, often after visualization of the trademark “owl’s eye” hyperintensity appearance on T2 axial images. Underlying etiology is wide-ranging; thus, a range of treatment methods exist.[13][14]
Similarly, subarachnoid hemorrhage (SAH) of spinal origin is uncommon but has received coverage in the literature. Spinal SAH is attributable to either arteriovenous fistula or malformation, as well as a spinal aneurysm. Symptoms usually include sudden onset neck or back pain and extremity weakness with signs indicating spinal cord compression. Again, computed tomogram (CT)/computed tomogram angiogram (CTA) and MRI are important for urgent diagnosis. Treatment varies depending on severity, location, and size, but both conservative treatment and endovascular or surgical management have proven effective.[15][16]
Media
References
Prince EA, Ahn SH. Basic vascular neuroanatomy of the brain and spine: what the general interventional radiologist needs to know. Seminars in interventional radiology. 2013 Sep:30(3):234-9. doi: 10.1055/s-0033-1353475. Epub [PubMed PMID: 24436544]
Chakravorty BG. Arterial supply of the cervical spinal cord and its relation to the cervical myelopathy in spondylosis. Annals of the Royal College of Surgeons of England. 1969 Oct:45(4):232-51 [PubMed PMID: 4980920]
Level 3 (low-level) evidenceSantillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A. Vascular anatomy of the spinal cord. Journal of neurointerventional surgery. 2012 Jan 1:4(1):67-74. doi: 10.1136/neurintsurg-2011-010018. Epub 2011 May 2 [PubMed PMID: 21990489]
Level 3 (low-level) evidenceUdan RS, Culver JC, Dickinson ME. Understanding vascular development. Wiley interdisciplinary reviews. Developmental biology. 2013 May-Jun:2(3):327-46. doi: 10.1002/wdev.91. Epub 2012 Oct 5 [PubMed PMID: 23799579]
Level 3 (low-level) evidenceTakahashi T, Takase Y, Yoshino T, Saito D, Tadokoro R, Takahashi Y. Angiogenesis in the developing spinal cord: blood vessel exclusion from neural progenitor region is mediated by VEGF and its antagonists. PloS one. 2015:10(1):e0116119. doi: 10.1371/journal.pone.0116119. Epub 2015 Jan 13 [PubMed PMID: 25585380]
Level 3 (low-level) evidenceTata M, Ruhrberg C, Fantin A. Vascularisation of the central nervous system. Mechanisms of development. 2015 Nov:138 Pt 1():26-36. doi: 10.1016/j.mod.2015.07.001. Epub 2015 Jul 26 [PubMed PMID: 26222953]
Chozick BS, Watson P, Greenblatt SH. Internal carotid artery thrombosis after cervical corpectomy. Spine. 1994 Oct 1:19(19):2230-2 [PubMed PMID: 7809760]
Level 3 (low-level) evidenceSchweighofer F, Passler JM, Wildburger R, Hofer HP. Interbody fusion of the lower cervical spine: a dangerous surgical method? Langenbecks Archiv fur Chirurgie. 1992:377(5):295-9 [PubMed PMID: 1405955]
Estrera AL, Rubenstein FS, Miller CC 3rd, Huynh TT, Letsou GV, Safi HJ. Descending thoracic aortic aneurysm: surgical approach and treatment using the adjuncts cerebrospinal fluid drainage and distal aortic perfusion. The Annals of thoracic surgery. 2001 Aug:72(2):481-6 [PubMed PMID: 11515886]
Kari FA, Saravi B, Krause S, Puttfarcken L, Scheumann J, Förster K, Rylski B, Maier S, Göbel U, Siepe M, Czerny M, Beyersdorf F. New insights into spinal cord ischaemia after thoracic aortic procedures: the importance of the number of anterior radiculomedullary arteries for surgical outcome. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2018 Jul 1:54(1):149-156. doi: 10.1093/ejcts/ezy058. Epub [PubMed PMID: 29917121]
Lazorthes G, Gouaze A, Zadeh JO, Santini JJ, Lazorthes Y, Burdin P. Arterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathways. Journal of neurosurgery. 1971 Sep:35(3):253-62 [PubMed PMID: 22046635]
Molliqaj G, Payer M, Schaller K, Tessitore E. Acute traumatic central cord syndrome: a comprehensive review. Neuro-Chirurgie. 2014 Feb-Apr:60(1-2):5-11. doi: 10.1016/j.neuchi.2013.12.002. Epub 2014 Mar 7 [PubMed PMID: 24613283]
Level 3 (low-level) evidenceYadav N, Pendharkar H, Kulkarni GB. Spinal Cord Infarction: Clinical and Radiological Features. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2018 Oct:27(10):2810-2821. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.008. Epub 2018 Aug 6 [PubMed PMID: 30093205]
Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. 2015 Mar:57(3):241-57. doi: 10.1007/s00234-014-1464-6. Epub 2014 Nov 16 [PubMed PMID: 25398656]
Aljuboori Z, Sharma M, Simpson J, Altstadt T. Surgical Management of Ruptured Isolated Aneurysm of Artery of Adamkiewicz: Interesting Report and Overview of Literature. World neurosurgery. 2018 Mar:111():36-40. doi: 10.1016/j.wneu.2017.11.179. Epub 2017 Dec 9 [PubMed PMID: 29229349]
Level 3 (low-level) evidenceMaiti TK, Bir SC, Nanda A. Spinal subarachnoid hemorrhage and aneurysms. Handbook of clinical neurology. 2017:143():215-223. doi: 10.1016/B978-0-444-63640-9.00020-5. Epub [PubMed PMID: 28552143]