 Radiation Therapy for Heterotopic Ossification Prophylaxis
Radiation Therapy for Heterotopic Ossification Prophylaxis
Introduction
Heterotopic ossification (HO) is the formation of mature lamellar bone in soft tissues where bone does not normally exist. HO is commonly seen following trauma or surgical intervention in periarticular soft tissue and is commonly associated with hip injury. The 3 primary causes can be grouped into traumatic, neurogenic, and genetic etiologies.
HO from trauma includes fractures, dislocations, and operative procedures such as open reduction-internal fixation (ORIF) or total hip arthroplasties (THA). The hip's most involved compartment is the abductor compartment.[1] The elbow is the next most commonly involved joint following burns or elbow replacements.
Neurogenic causes include spinal trauma and head injuries and again occur around major joints. The relationship between neurohumoral factors and the development of HO is poorly understood but is centered around the stimulation of osteoblasts to lay down ectopic bone.[2]
The last main etiology includes genetic disorders, such as fibrodysplasia ossificans progressiva, progressive osseous heteroplasia, and Albright hereditary osteodystrophy. These rare conditions lead to the development of HO early in life, resulting in debilitating morbidity.[3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The sensitivity of osteogenic progenitors to radiation therapy was known starting in the 1950s during experiments with bone repair.[4] These early studies harnessed the HO process; primitive mesenchymal cells in the surrounding soft tissues are transformed into osteoblastic tissue. This tissue then forms mature lamellar bone from dysfunctional bone formation/remodeling. It is thought that Craven et al found osteogenic progenitor cells to be radiosensitive because of a high mitotic rate as they proliferate and differentiate into osteoblasts and chondrocytes.[5] Thus, low-dose radiation is used as a prophylactic treatment to decrease the chance of HO formation, as discussed below.
Epidemiology
The incidence of heterotrophic ossification is more than 50% and as high as 90% in patients with moderate or severe osteophytes at the femoral head and socket in the setting of a hip replacement.[6][7] The hip is the most common site, followed by the elbow. Other major joints are sometimes affected. Soft tissue locations that are not associated with joints are rarely involved.
Several risk factors have been identified. High-risk patients include those with bilateral hypertrophic osteoarthritis, a prior history of HO, and arthritis caused by trauma characterized by hypertrophic osteophytosis.[8] Moderate-risk patients include those with unilateral hypertrophic osteoarthritis, ankylosing spondylitis, Paget disease, or diffuse idiopathic skeletal hyperostosis (DISH). Furthermore, HO is twice as common in males compared to females over 65 at the time of surgery and lateral surgical approach of the hip.[8][9]
History and Physical
Most commonly, patients present with hip stiffness after surgery or trauma that can lead to decreased range of motion and functional impairment. Symptoms associated with severe HO may include fever, warmth, erythema, tenderness, and swelling. Pain alone within several days after surgery could be a presenting symptom of HO, but this presentation is less common.
HO could also be an incidental radiograph finding, where calcified structures with blurred contours are seen as early as 2 to 6 weeks postoperatively. Bone scans usually show increased uptake in the soft tissues adjacent to the hip. Scans can detect HO several days before it becomes apparent on plain film, but these findings are not specific to HO.[10] Maturation of the HO may take up to 1 or 2 years. Rarely have correlations been reported with a sedimentation rate greater than 35 mm/hr and a serum alkaline phosphatase greater than 250 international units/L in cases with severe HO.[11][12]
Evaluation
HO is typically evaluated radiographically. The Brooker Staging system is the most widely accepted classification system and includes 4 grades based on an anteroposterior (AP) radiograph of the pelvis and hip.[13] Grades 3 and 4 are clinically relevant, without pain or impaired mobility.
Brooker Staging System
- Grade 1: Bone islands in the soft tissue around the hip
- Grade 2: Exophytes in the pelvis or proximal end of the femur with at least 1 cm between opposing bone surfaces
- Grade 3: Exophytes in the pelvis or proximal end of the femur with less than 1 cm between opposing bone surfaces
- Grade 4: Bony ankylosis between the proximal femur and pelvis [3]
Treatment / Management
Treatment depends on the severity and symptoms of HO or the patient's risk of developing HO. The orthopedic surgeon often determines the type of HO prophylaxis the patient obtains. Early-grade cases that are asymptomatic may be observed. Advanced HO may require surgical resection followed by prophylactic modalities, such as medical therapy and radiation therapy (RT), to help minimize the high risk for re-development of HO. When a patient does not have a history of HO, the decision to offer prophylactic treatment is often weighed with patient-related, clinical, and surgical risk factors against the potential risks of preventative treatment.
Medical Therapy
The main medical therapy used for HO prophylaxis is nonsteroidal anti-inflammatory drugs (NSAIDs). While indomethacin is the most commonly used NSAID, other NSAIDs, such as ibuprofen, have been effective with the added benefit of ease of administration and low cost. The recommended dose of indomethacin is 75 to 100 mg/day for 7 to 14 days postoperatively, with care for gastrointestinal side effects such as gastritis and ulcer formation.[14](A1)
Differential Diagnosis
The differential diagnoses for radiation therapy for heterotopic ossification prophylaxis include the following:
- Cellulitis
- Fracture
- Hematoma
- Local trauma
- Septic arthritis
- Thrombophlebitis
Radiation Oncology
Radiation therapy is more effective than NSAIDs in multiple trials. The effectiveness of indomethacin versus radiation therapy for HO prevention was compared in a prospective randomized trial comparing post-open reduction/internal fixation surgery for patients with an acetabular fracture. RT was given as 8 Gy in 1 fraction within 72 hours of surgery, and indomethacin was given for 6 weeks. There was a greater risk of nonunion of long bone fractures among those who received indomethacin over RT (26% versus 7%, p = 0.004).[15] A subsequent meta-analysis by Pakos et al analyzed 7 randomized trials for 1,143 patients and found that RT was almost twice as effective as NSAIDs. Still, the absolute benefit was less than 2%, with efficacy being dose-dependent.[16]
Radiation Dosing and Fractionation
External beam radiation therapy for HO prophylaxis is often prescribed at a 7 to 8 Gy single fraction dose. Still, various studies have shown that several fractionation and dosing regimens are acceptable. Historically, 20 Gy in 10 fractions was administered based on pediatric observational studies of bony growth inhibition. Sylvester et al retrospectively compared this regimen to 10 Gy in 5 fractions in 27 hips after THA and found the 2 groups similarly effective.[17] Around this time, Lo et al retrospectively examined outcomes after a single fraction RT with a 7 Gy dose and found that none of the patients developed grade 3 or 4 HO.[18] Another single fraction regimen with 8 Gy was compared in a randomized trial with 10 Gy in 5 fractions in 62 hips. With a median follow-up of 6 months, the failure rate in both groups was equivalent to 21%.[19] As the single fraction was shown to be effective, Healy et al explored the efficacy of lowering the dose. In this retrospective analysis, 107 hips were given either 7 Gy or 5.5 Gy postoperatively. The radiographic failure rate was 10% versus 63% in the lower-dose arm, which was significantly different.[20] A recent study compared lower doses in a randomized prospective trial in 59 high-risk patients for HO. The 2 arms were 5 Gy in 2 fractions or 10 Gy in 5 fractions, and all patients were treated within 4 days of surgery. There was a trend toward increased HO of any grade in the 5 Gy arm (69% versus 43%, p = 0.09).[21]
Radiation Timing
RT is typically given either preoperatively within 24 hours or postoperatively within 72 hours. If surgery is planned, it is ideal that the patient be consulted and consented to before surgery since the anesthesia may render the patient unable to give informed consent. Also, the patient could be in more pain postoperatively and may not be able to lie still during treatment. However, in trauma or emergencies, the radiation would be given postoperatively.
A randomized, multi-institutional trial by Gregoritch et al compared patients treated with pre-op versus post-op 7-8 Gy in 1 fraction and found that the radiographic and clinical failure rates between the 2 groups were not significantly different.[22] This was examined in another randomized study of 161 patients treated preoperatively less than 4 hours before surgery or postoperatively less than 72 hours after surgery. There was no significant difference amongst patients with Brooker grades 0 to 2; however, treatment failures in the overall cohort were significantly lower in the postoperative group. This was likely due to a higher biological equivalent dose as the preoperative group received 7 Gy in 1 fraction, while the postoperative group received 17.5 Gy in 5 fractions. Despite these findings, the current standard is to use the same dose of single fraction 7 to 8 Gy within 24 hours pre-operatively or within 72 hours postoperatively.
Radiation Treatment Planning
The general principle of treatment planning should include standard techniques with immobilization devices if necessary and placement of the patient in a comfortable position that allows reproducibility for daily treatments. The target volumes should include the surfaces of the bone that are most often involved with HO or the periarticular HO. HO often forms between the femoral head and pelvis, either from the greater trochanter to the ilium or the lesser trochanter to the ischium, so these surfaces must be included in the radiation field.
The radiation techniques to treat a hip with HO have been well described for the hip as this is the most commonly treated site. The patient is treated supine in a comfortable and reproducible position. Each field is customized to the patient’s anatomy. Still, generally, the cranial border is about 3 cm above the acetabulum, the inferior border about 3 cm below the lesser trochanter of the femoral head, or could include the upper 1/3 of the implant shaft (if used) with a field size of about 14 x 10 cm. Soft tissue can be blocked by collimating the field to use the field edges or using the multi-leaf collimators to shape the field to allow about a 1.5 to 2 cm margin on the bone and prior areas of HO. Three-dimensional conformal radiotherapy (3DCRT) using anterior-posterior/posterior-anterior (AP/PA) fields with 6 to 15MV photons are often used to create a homogeneous plan to cover the target to at least the 95% isodose line while minimizing dose to the surrounding soft tissue and skin (See Image. Heterotopic Ossification of the Hip).
Other Sites
The hip is the most common site of HO development and treatment. However, other joints are also susceptible to HO. RT has also been effective in these areas, particularly with the elbow. A study of 36 patients treated at the Cleveland Clinic with 7 Gy in 1 fraction following elbow surgery found that RT was associated with favorable functional and radiographic outcomes.[23] Furthermore, a retrospective study by Strauss et al of 44 patients found radiographic evidence of HO in 48% of the group with no complications after high-risk elbow surgery.[24] Favorable outcomes were reported from a case series of 9 patients with clinically significant HO at the elbow. Fractionation included 5 Gy in 2 fractions and 6-7 Gy in 1 fraction with a median follow-up of 7.7 months; there were no failures, with the majority showing clinical improvement.[25]
Toxicity from Radiation
HO is not a malignant condition, yet it can cause pain and limited mobility and negatively affect the quality of life. Thus, the benefits of treatment must be weighed against the risks. Patients are often counseled that the low RT dose administered for HO prophylaxis decreases the risk of HO significantly with minimal side effects and is considered a safe treatment overall. Potential side effects are rare but include fatigue, wound healing delays, joint swelling, and an extremely low chance of secondary cancer from the RT.
Secondary malignancies from radiation can develop in the bone or tissues included in a previous RT field. Since the total dose given for HO prophylaxis is very low, the changes of secondary malignancies are also extremely low, but not zero. At least 2 case reports have described secondary malignancies after a single fraction. The first case described a 51-year-old patient who received 7 Gy x 1 for HO 15 years earlier. He underwent a second course of 7 Gy x 1 for debilitating HO but developed high-grade undifferentiated sarcoma of the proximal thigh 16 months after his second treatment.[26] Another case described a 26-year-old male who underwent surgery for an acetabular fraction and received 7 Gy in 1 fraction postoperatively for HO prophylaxis. He presented 11 years later with osteosarcoma of the treated area, which was treated definitively with neoadjuvant cisplatin and adriamycin, with plans for surgical resection at the time of the report.[27] Otherwise, there are no other reported cases of secondary malignancies from RT given for HO prophylaxis treatment, as it is a rare complication.
Trochanteric nonunion and wound healing complications are also potential toxicities from RT. One study found that the rate of nonunion ranged from 12% to 30% after RT, while the rate of nonunion was diminished by 2% to 15% of the time for those who did not get RT.[3] Since modern hardware for total hip arthroplasties is cementless and porous, they may rely on bony ingrowth at the acetabulum or the proximal femur region. There is concern that RT may inhibit the necessary bony ingrowth and lead to prosthesis failure, which has prompted some providers to utilize shielding of the acetabular cup to prevent this complication. However, a clinical study of patients receiving preoperative and postoperative radiation found no evidence of prosthesis failure, even without shielding.[28]
For male patients, the RT dose to the testicles is of concern as it can reduce sperm count, even from doses as low as 20 Gy. Testicular shielding is recommended as it can reduce the dose to the testicles by approximately 50%.
Pearls and Other Issues
Key facts to keep in mind about radiation therapy for heterotopic ossification prophylaxis are as follows:
- Heterotopic ossification (HO) is the abnormal formation of bone in soft tissues where bone does not normally exist.
- HO is most commonly seen following trauma or surgical intervention in periarticular soft tissue and is commonly associated with injury to the hip.
- Radiation to the peri-articular joint prescribed to 7 to 8 Gy in 1 fraction given less than 24 hours preoperatively or less than 72 hours postoperatively can decrease the risk of HO development.
- Prophylactic RT for HO is safe and is more efficacious compared to NSAIDs.
Enhancing Healthcare Team Outcomes
HO is best managed by an interprofessional team. Today, radiation is being recommended in select patients, and while effective, one has to weigh the benefits versus harm of this therapy in asymptomatic patients. The patient should be involved in decision-making because RT is not entirely harmless.
Media
(Click Image to Enlarge)
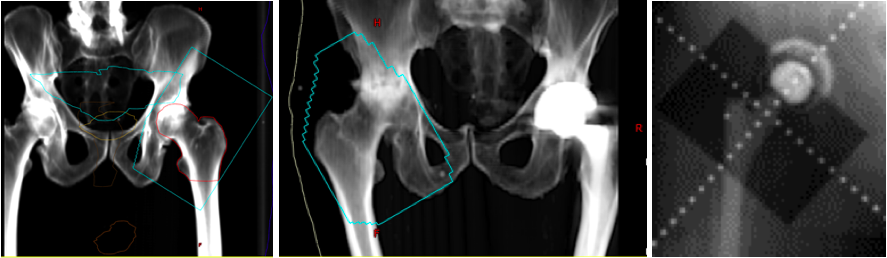
Heterotopic Ossification of the Hip. Digitally reconstructed radiographs (DRR) based on original portal images showing fields of three patients receiving radiation for heterotopic ossification of the hip. The blue line represents the field edge in the first two images. Take note that the surfaces of the greater trochanter to the ilium and the lesser trochanter to the ischium are not blocked out since this is the most common location for HO formation. The third image shows the shielding of the acetabular cup to prevent bony ingrowth and prosthesis failure.
Contributed by NP Amin
References
Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clinical orthopaedics and related research. 1991 Feb:(263):13-29 [PubMed PMID: 1899635]
Level 3 (low-level) evidenceSullivan MP, Torres SJ, Mehta S, Ahn J. Heterotopic ossification after central nervous system trauma: A current review. Bone & joint research. 2013 Mar:2(3):51-7. doi: 10.1302/2046-3758.23.2000152. Epub 2013 Mar 1 [PubMed PMID: 23610702]
Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. International journal of radiation oncology, biology, physics. 2006 Aug 1:65(5):1289-99 [PubMed PMID: 16863921]
COOLEY LM, GOSS RJ. The effects of transplantation and x-irradiation on the repair of fractured bones. The American journal of anatomy. 1958 Mar:102(2):167-81 [PubMed PMID: 13571154]
Craven PL, Urist MR. Osteogenesis by radioisotope labelled cell populations in implants of bone matrix under the influence of ionizing radiation. Clinical orthopaedics and related research. 1971 May:76():231-43 [PubMed PMID: 4931060]
Level 3 (low-level) evidenceDeLee J, Ferrari A, Charnley J. Ectopic bone formation following low friction arthroplasty of the hip. Clinical orthopaedics and related research. 1976 Nov-Dec:(121):53-9 [PubMed PMID: 825341]
Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. The Journal of bone and joint surgery. American volume. 1977 Apr:59(3):345-51 [PubMed PMID: 403193]
Ahrengart L, Lindgren U. Heterotopic bone after hip arthroplasty. Defining the patient at risk. Clinical orthopaedics and related research. 1993 Aug:(293):153-9 [PubMed PMID: 8339476]
Horwitz BR, Rockowitz NL, Goll SR, Booth RE Jr, Balderston RA, Rothman RH, Cohn JC. A prospective randomized comparison of two surgical approaches to total hip arthroplasty. Clinical orthopaedics and related research. 1993 Jun:(291):154-63 [PubMed PMID: 8504594]
Level 1 (high-level) evidenceOrzel JA, Rudd TG. Heterotopic bone formation: clinical, laboratory, and imaging correlation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1985 Feb:26(2):125-32 [PubMed PMID: 3918147]
Kjaersgaard-Andersen P, Schmidt SA, Pedersen NW, Kristensen SS, Pedersen P. Erythrocyte sedimentation rate and heterotopic bone formation after cemented total hip arthroplasty. Clinical orthopaedics and related research. 1989 Nov:(248):189-94 [PubMed PMID: 2509120]
Level 1 (high-level) evidenceKjaersgaard-Andersen P, Pedersen P, Kristensen SS, Schmidt SA, Pedersen NW. Serum alkaline phosphatase as an indicator of heterotopic bone formation following total hip arthroplasty. Clinical orthopaedics and related research. 1988 Sep:(234):102-9 [PubMed PMID: 3136963]
Level 1 (high-level) evidenceBrooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. The Journal of bone and joint surgery. American volume. 1973 Dec:55(8):1629-32 [PubMed PMID: 4217797]
Fransen M, Anderson C, Douglas J, MacMahon S, Neal B, Norton R, Woodward M, Cameron ID, Crawford R, Lo SK, Tregonning G, Windolf M, HIPAID Collaborative Group. Safety and efficacy of routine postoperative ibuprofen for pain and disability related to ectopic bone formation after hip replacement surgery (HIPAID): randomised controlled trial. BMJ (Clinical research ed.). 2006 Sep 9:333(7567):519 [PubMed PMID: 16885182]
Level 1 (high-level) evidenceBurd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. The Journal of bone and joint surgery. British volume. 2003 Jul:85(5):700-5 [PubMed PMID: 12892193]
Level 1 (high-level) evidencePakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. International journal of radiation oncology, biology, physics. 2004 Nov 1:60(3):888-95 [PubMed PMID: 15465207]
Level 1 (high-level) evidenceSylvester JE, Greenberg P, Selch MT, Thomas BJ, Amstutz H. The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. International journal of radiation oncology, biology, physics. 1988 Mar:14(3):471-6 [PubMed PMID: 3343154]
Lo TC, Healy WL, Covall DJ, Dotter WE, Pfeifer BA, Torgerson WR, Wasilewski SA. Heterotopic bone formation after hip surgery: prevention with single-dose postoperative hip irradiation. Radiology. 1988 Sep:168(3):851-4 [PubMed PMID: 3136510]
Pellegrini VD Jr, Konski AA, Gastel JA, Rubin P, Evarts CM. Prevention of heterotopic ossification with irradiation after total hip arthroplasty. Radiation therapy with a single dose of eight hundred centigray administered to a limited field. The Journal of bone and joint surgery. American volume. 1992 Feb:74(2):186-200 [PubMed PMID: 1541613]
Level 1 (high-level) evidenceHealy WL, Lo TC, DeSimone AA, Rask B, Pfeifer BA. Single-dose irradiation for the prevention of heterotopic ossification after total hip arthroplasty. A comparison of doses of five hundred and fifty and seven hundred centigray. The Journal of bone and joint surgery. American volume. 1995 Apr:77(4):590-5 [PubMed PMID: 7713977]
Padgett DE, Holley KG, Cummings M, Rosenberg AG, Sumner DR, Conterato D, Galante JO. The efficacy of 500 CentiGray radiation in the prevention of heterotopic ossification after total hip arthroplasty: a prospective, randomized, pilot study. The Journal of arthroplasty. 2003 Sep:18(6):677-86 [PubMed PMID: 14513439]
Level 3 (low-level) evidenceGregoritch SJ, Chadha M, Pelligrini VD, Rubin P, Kantorowitz DA. Randomized trial comparing preoperative versus postoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement: preliminary results. International journal of radiation oncology, biology, physics. 1994 Aug 30:30(1):55-62 [PubMed PMID: 8083129]
Level 3 (low-level) evidenceRobinson CG, Polster JM, Reddy CA, Lyons JA, Evans PJ, Lawton JN, Graham TJ, Suh JH. Postoperative single-fraction radiation for prevention of heterotopic ossification of the elbow. International journal of radiation oncology, biology, physics. 2010 Aug 1:77(5):1493-9. doi: 10.1016/j.ijrobp.2009.06.072. Epub [PubMed PMID: 20637977]
Level 2 (mid-level) evidenceStrauss JB, Wysocki RW, Shah A, Chen SS, Shah AP, Abrams RA, Cohen MS. Radiation therapy for heterotopic ossification prophylaxis afer high-risk elbow surgery. American journal of orthopedics (Belle Mead, N.J.). 2011 Aug:40(8):400-5 [PubMed PMID: 22016869]
Level 2 (mid-level) evidenceHeyd R, Strassmann G, Schopohl B, Zamboglou N. Radiation therapy for the prevention of heterotopic ossification at the elbow. The Journal of bone and joint surgery. British volume. 2001 Apr:83(3):332-4 [PubMed PMID: 11341414]
Mourad WF, Packianathan S, Shourbaji RA, Russell G, Khan MA, Vijayakumar S. Radiation-induced sarcoma following radiation prophylaxis of heterotopic ossification. Practical radiation oncology. 2012 Apr-Jun:2(2):151-4. doi: 10.1016/j.prro.2011.06.005. Epub 2011 Jul 22 [PubMed PMID: 24674090]
Farris MK, Chowdhry VK, Lemke S, Kilpatrick M, Lacombe M. Osteosarcoma following single fraction radiation prophylaxis for heterotopic ossification. Radiation oncology (London, England). 2012 Aug 21:7():140. doi: 10.1186/1748-717X-7-140. Epub 2012 Aug 21 [PubMed PMID: 22908888]
Level 3 (low-level) evidenceSeegenschmiedt MH, Keilholz L, Martus P, Goldmann A, Wölfel R, Henning F, Sauer R. Prevention of heterotopic ossification about the hip: final results of two randomized trials in 410 patients using either preoperative or postoperative radiation therapy. International journal of radiation oncology, biology, physics. 1997 Aug 1:39(1):161-71 [PubMed PMID: 9300751]
Level 1 (high-level) evidence