Introduction
Benedikt syndrome is an extremely rare eponymously named cluster of symptoms relating to anatomically specific damage of varying etiology to the midbrain.[1]
It is characterized by:
- Ipsilateral oculomotor nerve palsy
- Contralateral hemiparesis
- Contralateral cerebellar ataxia and/or Holmes tremor and/or choreoathetosis.[1][2]
A more precise description remains elusive for Benedikt syndrome owing to historical legacy with significant variance in the description in textbooks and clinical case studies. Moiz Benedikt, an Austrian neurologist, first described this constellation of symptoms in a lecture delivered in 1889. He described three similar cases involving oculomotor palsy, contralateral hemiparesis, and contralateral tremors.[1] One of these cases was a 4-year-old with a right oculomotor palsy, contralateral hand jerking/tremor, contralateral involuntary leg movements, and contralateral upper motor neuron facial weakness. An autopsy revealed a tuberculoma a "pigeon's egg" in size amongst other smaller tuberculomas in the cerebral hemispheres and cerebellum.[1]
In 1893 Charcot was the first to use the term Benedikt syndrome to describe a similar presentation of a 37-year-old male with left-sided ptosis, adduction weakness, mydriasis, right arm choreoathetosis and right leg weakness with resting tremor. 2 more cases were presented in 1900: the first with right hemiplegia, involuntary movements, and bilateral oculomotor palsy; the second case with hemiplegia on the left side, tremor of the left arm, right exotropia and right mydriasis. These first and subsequent descriptions over the next 40 years describe patients with Benedikt syndrome with slightly varying symptoms, etiology, and lesion location.[1]
This historical legacy has subsequently led to patients with pupil sparing, associated "plus-minus lid" syndrome, and varied associated tremors and choreoathetosis' being included in the umbrella nomenclature of "Benedikt syndrome."[3][4][5][1] Current consensus requires the above three symptoms to be considered to be Benedikt syndrome, but as mentioned, additional symptoms do not exclude the diagnosis.[1][2][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Benedikt syndrome is best understood as a collection of symptoms, as described above, arising from mesencephalic damage to the ipsilateral red nucleus, substantia nigra, third cranial nerve, and cerebral peduncle.[2][6] There is, therefore, a multitude of disease processes that can cause focal neurological damage and, consequently, Benedikt syndrome. Varied etiology of this syndrome includes, as described in case report literature: meningioma, prostatic carcinoma, posterior cerebral artery stenosis, trauma, cyst formation, iatrogenic injury following a stereotactic procedure, iatrogenic injury following aneurysm clipping, cavernous hemangioma, and unruptured aneurysm.[7][8][5][2][9][10][11][6][4]
Although often associated with thrombo-ischemic events as in Weber and Claude syndromes, there are scant case reports in the literature describing specifically ischaemic thromboembolism causing Benedikt syndrome, a study of 308 brainstem infarcts failed to detect a single case of Benedikt syndrome.[12] This, however, may be due to the late development of movement disorders in subsequent weeks not seen in the acute setting.[13]
Epidemiology
Due to the extremely low frequency of reported cases, there remain no epidemiological studies outlying the frequency of this condition. However, the condition has been reported in both sexes and from around the world.[1][9][4]
Pathophysiology
Understanding the underlying anatomy and function of mesencephalic structures is key to understanding why specific damage in the tegmentum of the midbrain can, in some cases, cause the aforementioned symptom cluster.[2] Furthermore, it may explain why some patients present with Benedikt syndrome symptoms with additional neurological symptoms and why associated contralateral movement disorders vary considerably.
The midbrain is classically divided cross-sectionally into the: tegmentum (or a ventral portion, excluding the cerebral crus/peduncle) and tectum (dorsal portion) and longitudinally into the: superior colliculus and inferior colliculus. The following major structures are encountered if one travels ventrally from the periaqueductal grey matter and stays in the medial half of the tegmentum at the level of the superior colliculus: 1) Edinger-Westphal nucleus, 2) oculomotor nerve, 3) medial longitudinal fasciculus, 4) red nucleus, 5) substantia nigra and finally 6) cerebral peduncle.[14] Other major structures more laterally located in the tegmentum are the medial lemniscus and medial geniculate nucleus, amongst others [14]. Benedikt syndrome has also been referred to as paramedian midbrain syndrome, so understanding the function of those above medially located tegmentum structures can help elucidate the syndrome’s symptom cluster:
- Edinger-Westphal nucleus: the origin of the parasympathetic supply to iris sphincter and ciliary muscle travels with the oculomotor nerve. Damage causes mydriasis and difficulty with visual accommodation.[14]
- Oculomotor nucleus: innervates 4/6 muscles controlling eye movement and levator palpebrae superioris. Damage causes a classic ‘down and out’ pupil and ptosis.[14]
- Medial longitudinal fasciculus: integrates eye movements from cranial nerves III, IV, and VI. Isolated damage causes internuclear ophthalmoplegia not seen in Benedikt syndrome due to oculomotor palsy.[15]
- Red nucleus: receives input via the dentatorubrothalamic tract from the cerebellum and cerebral cortex before subsequently decussating to form the rubrospinal tract, which is a descending motor tract that plays an important role in flexor muscle tone, coordinated movement, and motor reflexes. Damage can, therefore, cause problems on the contralateral part of the body in forming a coordinated movement.[16][13]
- Substantia nigra: part of the basal ganglia and has a complex but critical role in regulating movement as part of the nigrostriatal pathway. Damage or loss of function (as is the case seen in Parkinson disease) causes resting tremor, postural instability, and difficulty in initiating movement.[17]
- Cerebral peduncle: contain the main descending motor pathways, including the corticospinal tract from the cerebrum that controls voluntary movement, these decussate in the medulla oblongata. Damage to this structure causes contralateral muscle weakness with loss of voluntary control of muscles, increased tone, and hyperreflexia.[14][2]
Putting the above together illuminates both the syndrome and its variation. The red nucleus, substantia nigra, and cerebral peduncle all control movement (in the case of the red nucleus and substantia nigra in complicated relaying pathways), therefore, slight variations in how damaged each of these structures is in different patients could give heterogenous presentations despite having very similar localized damage. Thus in this light, Holmes tremor, choreoathetosis, cerebellar ataxia all become plausibly part of the same condition across different case reports with varying clinical presentations.[5][1][16] Considering the proximity of the medial lemniscus pathway (carrying ascending sensory information) and the trochlear cranial nerve nucleus, associated deficits have been described in patients with this syndrome, for example, plus-minus lid syndrome.[3][7]
The structures mentioned above are supplied by numerous peduncular arterial branches derived from the proximal posterior cerebral artery. These branches penetrate the cerebral peduncle and go on to supply the rest of the structures in turn involved with this syndrome.[5][14] Whether thrombotic, stenotic, or aneurysmal, vascular pathologies in these peduncular branches could cause Benedikt syndrome.
History and Physical
A detailed history is vital in determining the symptoms and their time course. For example, a Holmes tremor classically takes a few weeks after the original insult to present itself.[13] It is equally important to uncover any lifestyle factors, past medical history, family history, medication history, and social history. A detailed neurological examination is also essential as with any patient presenting with neurological symptoms.
Evaluation
If the patient presents with symptoms in the acute setting and a stroke is considered a possibility, a CT scan as per routine management of an acute stroke is necessary.[18] If symptoms present more insidiously, an MRI with contrast is frequently done to assess location and size, which in turn helps determine the likely etiology of the lesion when considered alongside the clinical context along with the patient's history and examination findings.[6][7][2]
Treatment / Management
Owing to the varied etiology of this syndrome, treatment is dependent on the underlying pathology. For example, mesencephalic cavernoma removed via surgical excision or superior cerebellar artery aneurysm treated via aneurysmal clipping.[7][11] Thromboembolism is treated as per any thromboembolic stroke and can be treated via thrombolysis, thrombectomy, and secondary prevention depending on the clinical presentation and imaging findings.[18] A Holmes tremor has been reported in the literature successfully managed with deep brain stimulation to the thalamus or the contralateral lenticular fasciculus.[19][20][19] Neurorehabilitation has been reported to be efficacious in this syndrome, for example, in a Benedikt syndrome secondary to trauma.[2](B3)
Differential Diagnosis
Closely related syndromes: Weber, Nothnagel, and Claude syndromes. These are all eponymously named syndromes relating to the midbrain's damage and are classically considered caused by cerebrovascular-related ischemia.[12][6]
Weber syndrome is characterized by oculomotor palsy and contralateral hemiparesis. Underlying structures involved are thought to be the cerebral peduncle and oculomotor fascicles.[6][21]
Claude syndrome shows oculomotor palsy and contralateral ataxia. Underlying structures involved are thought to be the dentatorubrothalamic tract and oculomotor fascicles.[6][21]
Nothnagel syndrome has oculomotor palsy and ipsilateral limb ataxia. Underlying structures involved are thought to be the superior cerebellar peduncle and tectum of the midbrain.[22][21]
Prognosis
Prognosis varies widely according to the underlying etiology. Significant regression of symptoms has been reported in patients who have had successful removal of lesions causing symptoms when combined with rehabilitation.[7] However, as with the first-ever patient described with this condition, patients can succumb to the underlying disease pathology or be left with significant morbidity and decreased quality of life. Movement disorders have a significantly negative impact on activities of daily living and quality of life.[11][19][16]
Complications
Complications are dependent on the underlying etiology, and regression of symptoms can sometimes be seen.[2][7] However, as previously mentioned movement disorders and hemiparesis can have a significant impact on an individual's quality of life and activities of daily living.[23]
Deterrence and Patient Education
Owing to the diverse etiology and extremely low incidence of Benedikt syndrome, deterrence, and patient education have a minor role. Doctors should advise their patients to engage with healthy lifestyles to prevent cerebrovascular accidents.[18]
Pearls and Other Issues
Damage from any etiology to the red nucleus, oculomotor fascicles, cerebral peduncle, and substantia nigra causes Benedikt syndrome and explains the clinical variation seen with movement disorders.
Enhancing Healthcare Team Outcomes
As with any patient presenting with neurological symptoms, multidisciplinary teamwork provides the best outcomes for patients with regards to diagnosis, management, treatment, and rehabilitation. A patient with Benedikt syndrome will often require the involvement of neurology, neurosurgery, radiology, nursing care, physiotherapy, occupational therapy, to name a few.[24][25] [Level 5]
Media
(Click Image to Enlarge)
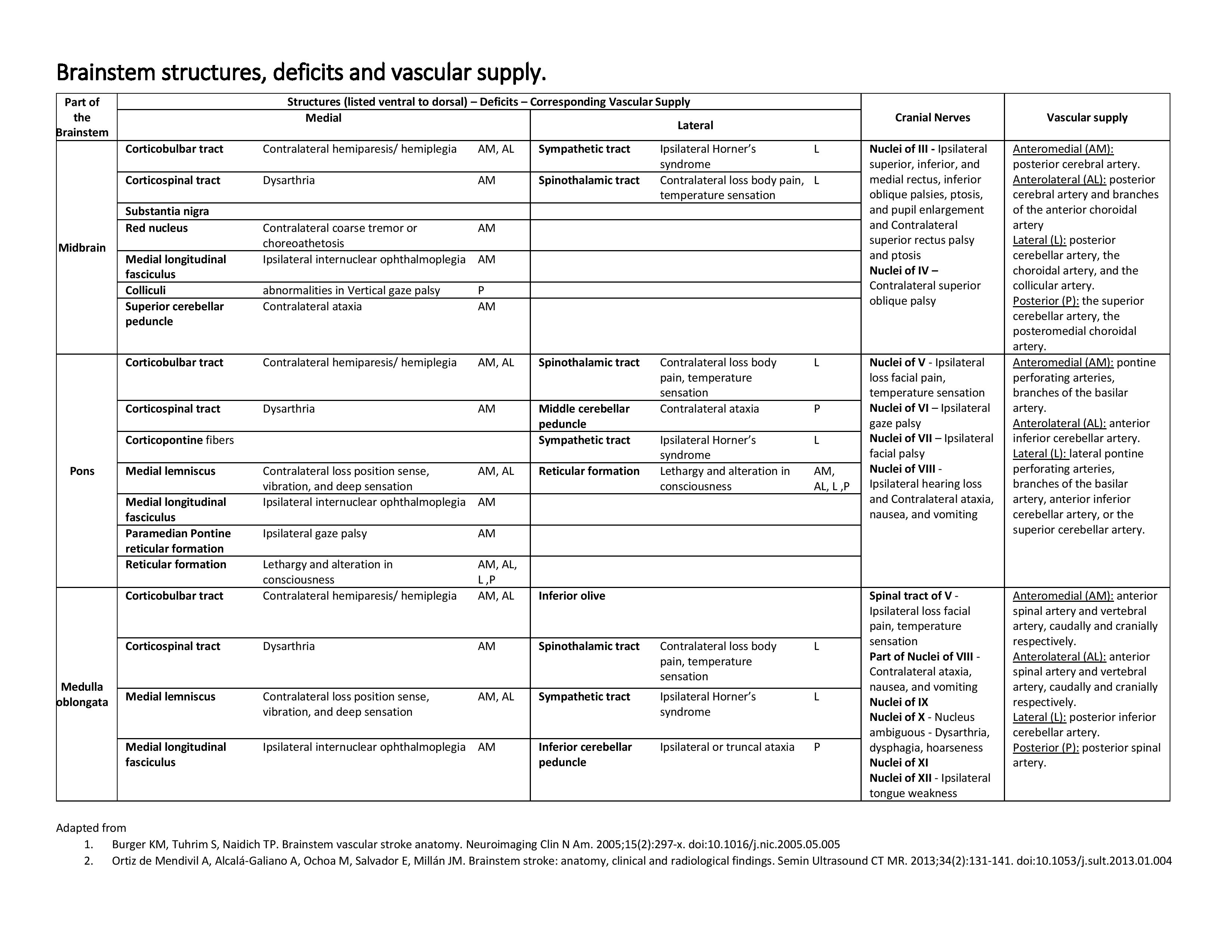
Brainstem Structures, Deficits, and Vascular Supply Table. Brainstem infarction is an area of tissue death resulting from a lack of oxygen supply to any part of the brainstem. The knowledge of anatomy, vascular supply, and physical examination can be life-saving in the setting of an acute infarct and provide precise diagnosis and management. Time becomes an essential factor in management. Early intervention has shown dramatically reduced morbidity and mortality.
Contributed by SN Gowda, MBBS
References
Liu GT, Crenner CW, Logigian EL, Charness ME, Samuels MA. Midbrain syndromes of Benedikt, Claude, and Nothnagel: setting the record straight. Neurology. 1992 Sep:42(9):1820-2 [PubMed PMID: 1513475]
Paidakakos NA, Rokas E, Theodoropoulos S, Dimogerontas G, Konstantinidis E. Posttraumatic Benedikt's syndrome: a rare entity with unclear anatomopathological correlations. World neurosurgery. 2012 Dec:78(6):715.e13-5. doi: 10.1016/j.wneu.2012.03.028. Epub 2012 Apr 3 [PubMed PMID: 22484069]
Level 3 (low-level) evidenceAkdal G, Kutluk K, Men S, Yaka E. Benedikt and "plus-minus lid" syndromes arising from posterior cerebral artery branch occlusion. Journal of the neurological sciences. 2005 Jan 15:228(1):105-7 [PubMed PMID: 15607218]
Level 3 (low-level) evidenceKoskela E, Setälä K, Kivisaari R, Hernesniemi J, Laakso A. Neuro-ophthalmic presentation and surgical results of unruptured intracranial aneurysms—prospective Helsinki experience of 142 patients. World neurosurgery. 2015 Apr:83(4):614-9. doi: 10.1016/j.wneu.2014.12.017. Epub 2014 Dec 18 [PubMed PMID: 25527884]
Duncan GW, Weindling SM. Posterior cerebral artery stenosis with midbrain infarction. Stroke. 1995 May:26(5):900-2 [PubMed PMID: 7740587]
Level 3 (low-level) evidenceRuchalski K, Hathout GM. A medley of midbrain maladies: a brief review of midbrain anatomy and syndromology for radiologists. Radiology research and practice. 2012:2012():258524. doi: 10.1155/2012/258524. Epub 2012 May 22 [PubMed PMID: 22693668]
Maduri R, Barbagallo G, Iofrida G, Signorelli M, Signorelli F. Regression of Benedikt's syndrome after single-stage removal of mesencephalic cavernoma and temporal meningioma: a case report. Clinical neurology and neurosurgery. 2013 Jun:115(6):748-50. doi: 10.1016/j.clineuro.2012.06.033. Epub 2012 Jul 21 [PubMed PMID: 22824723]
Level 3 (low-level) evidenceLoseke N, Retif J, Noterman J, Flament-Durand J. Inferior red nucleus syndrome (Benedikt's syndrome) due to a single intramesencephalic metastasis from a prostatic carcinoma. Case report. Acta neurochirurgica. 1981:56(1-2):59-64 [PubMed PMID: 7246282]
Level 3 (low-level) evidenceOno Y, Suzuki M, Kayama T, Yoshimoto T. Multilobulated cystic formation in the brain stem with Benedikt's syndrome: case report. Neurosurgery. 1994 Apr:34(4):726-9; discussion 729 [PubMed PMID: 8008173]
Level 3 (low-level) evidenceBorrás JM, Salazar FG, Grandas F. Oculomotor palsy and contralateral tremor (Benedikt's syndrome) following a stereotactic procedure. Journal of neurology. 1997 Apr:244(4):272-4 [PubMed PMID: 9112599]
Level 3 (low-level) evidenceYamanaka Y, Shinohara T, Kozano I, Yoshizumi T, Kawasaki T. [Benedikt Syndrome Associated with Neck Clipping of Ruptured Basilar-Superior Cerebellar Artery Aneurysm:A Case Report]. No shinkei geka. Neurological surgery. 2018 Nov:46(11):1007-1012. doi: 10.11477/mf.1436203855. Epub [PubMed PMID: 30458438]
Level 3 (low-level) evidenceMarx JJ, Thömke F. Classical crossed brain stem syndromes: myth or reality? Journal of neurology. 2009 Jun:256(6):898-903. doi: 10.1007/s00415-009-5037-2. Epub 2009 Feb 28 [PubMed PMID: 19252797]
Level 2 (mid-level) evidenceRaina GB, Cersosimo MG, Folgar SS, Giugni JC, Calandra C, Paviolo JP, Tkachuk VA, Zuñiga Ramirez C, Tschopp AL, Calvo DS, Pellene LA, Uribe Roca MC, Velez M, Giannaula RJ, Fernandez Pardal MM, Micheli FE. Holmes tremor: Clinical description, lesion localization, and treatment in a series of 29 cases. Neurology. 2016 Mar 8:86(10):931-8. doi: 10.1212/WNL.0000000000002440. Epub 2016 Feb 10 [PubMed PMID: 26865524]
Level 3 (low-level) evidenceCaminero F, Cascella M. Neuroanatomy, Mesencephalon Midbrain. StatPearls. 2023 Jan:(): [PubMed PMID: 31855353]
Feroze KB, Wang J. Internuclear Ophthalmoplegia. StatPearls. 2023 Jan:(): [PubMed PMID: 28722999]
Choi SM. Movement Disorders Following Cerebrovascular Lesions in Cerebellar Circuits. Journal of movement disorders. 2016 May:9(2):80-8. doi: 10.14802/jmd.16004. Epub 2016 May 25 [PubMed PMID: 27240809]
Sonne J, Reddy V, Beato MR. Neuroanatomy, Substantia Nigra. StatPearls. 2023 Jan:(): [PubMed PMID: 30725680]
Khaku AS, Tadi P. Cerebrovascular Disease. StatPearls. 2023 Jan:(): [PubMed PMID: 28613677]
Cheng G, Yang Y, Wang Y, Tan H, Zhang S. Deep brain stimulation of the thalamic ventral intermediate nucleus for Benedikt's syndrome mainly present as tremor: a long-term case observation. Acta neurochirurgica. 2018 Jul:160(7):1349-1353. doi: 10.1007/s00701-018-3526-8. Epub 2018 Mar 30 [PubMed PMID: 29600395]
Level 3 (low-level) evidenceBandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. Journal of neurosurgery. 2008 Oct:109(4):635-9. doi: 10.3171/JNS/2008/109/10/0635. Epub [PubMed PMID: 18826349]
Level 3 (low-level) evidenceSciacca S, Lynch J, Davagnanam I, Barker R. Midbrain, Pons, and Medulla: Anatomy and Syndromes. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Jul-Aug:39(4):1110-1125. doi: 10.1148/rg.2019180126. Epub [PubMed PMID: 31283463]
Moncayo J. Midbrain infarcts and hemorrhages. Frontiers of neurology and neuroscience. 2012:30():158-61. doi: 10.1159/000333630. Epub 2012 Feb 14 [PubMed PMID: 22377886]
Ramos-Lima MJM, Brasileiro IC, Lima TL, Braga-Neto P. Quality of life after stroke: impact of clinical and sociodemographic factors. Clinics (Sao Paulo, Brazil). 2018 Oct 8:73():e418. doi: 10.6061/clinics/2017/e418. Epub 2018 Oct 8 [PubMed PMID: 30304300]
Level 2 (mid-level) evidenceEpstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surgical neurology international. 2014:5(Suppl 7):S295-303. doi: 10.4103/2152-7806.139612. Epub 2014 Aug 28 [PubMed PMID: 25289149]
Moodley KK, Nitkunan A, Pereira AC. Acute neurology: a suggested approach. Clinical medicine (London, England). 2018 Oct:18(5):418-421. doi: 10.7861/clinmedicine.18-5-418. Epub [PubMed PMID: 30287440]