Introduction
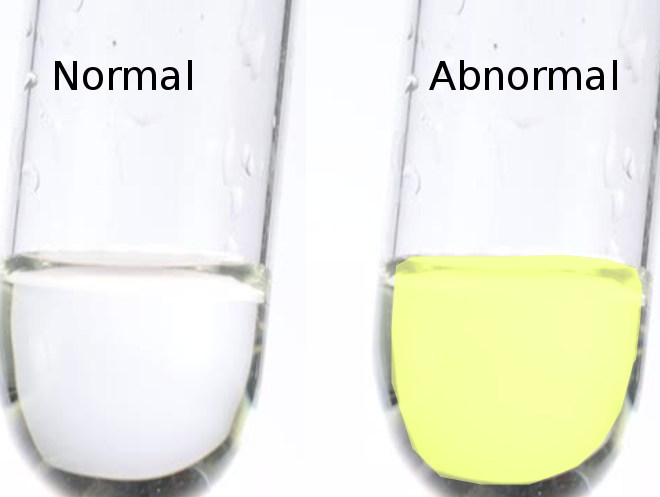
Xanthochromia is derived from the Greek word "xanthos," meaning yellow. The term was first used to describe the pink or yellow pigmentation of cerebrospinal fluid (CSF).[1] This color change is attributed to varying concentrations of pigmented compounds such as oxyhemoglobin, bilirubin, and methemoglobin, which are typically the byproducts of red blood cell degradation.[2] The term is now more widely accepted to represent the yellow color created by the presence of bilirubin in the CSF. The presence of bilirubin resulting in yellow discoloration of the CSF is the contemporary definition of xanthochromia.[3][4][5] It can be diagnosed by 2 methods: the traditional visualization or eye test and the more sensitive and specific spectrophotometry.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The yellowish hue seen with xanthochromia is caused by the presence of bilirubin, which is the byproduct of the degradation of oxyhemoglobin by the enzyme oxygenase. In the CSF, this occurs by the macrophages that line the arachnoid space. There are many causes of xanthochromia, including acute intracerebral hemorrhage, brain tumors, infection, conditions that cause an increase of CSF protein (≥150 mg/dL), and severe systemic jaundice (serum bilirubin levels >10 g/dL). The finding of xanthochromia in the CSF is most commonly used in diagnosing subarachnoid hemorrhage (SAH) in the presence of a normal head computed tomography (CT).[6][7]
Epidemiology
The true prevalence and incidence of xanthochromia are not known, as various pathologies can cause it; however, it is more commonly discussed surrounding the diagnosis of a ruptured intracerebral aneurysm, which most commonly causes SAH. The incidence of SAH is anywhere from 9 to 15 people per 100,000 in the United States (US), with rates that vary by geography. Risk factors for SAH include hypertension, cigarette smoking, alcohol use, some sympathomimetic drugs, and some genetic causes that increase the risk of a cerebral aneurysm.[8]
It is known that xanthochromia can be seen very soon after an SAH (up to 2 hours); within 12 hours of a ruptured SAH, more than 90% of patients will show xanthochromia in their CSF that can persist for up to 4 weeks.[9] With newer imaging options and better-quality CT scanners available, the diagnosis of SAH is most commonly made with either a noncontrasted CT of the brain or a CT-angiogram (CTA) of the brain. However, SAH can be missed on imaging, and analysis of CSF for xanthochromia can help with the diagnosis of SAH (which occurs in <1% of cases), as it has a 100% sensitivity and 99% specificity when using the United Kingdom National External Quality Assessment Service (UK-NEQAS) method of spectrophotometry is used.[6][10]
Pathophysiology
No matter the etiology, xanthochromia represents the presence of degraded red blood cells. This process takes place over time as blood is degraded by macrophages and broken down into its byproducts containing bilirubin. Conversion from heme to bilirubin in the CSF takes roughly 6 to 12 hours. Therefore, xanthochromia is best identified 6 to 12 hours after the onset of a bleed, and its presence suggests that blood has been present in the spinal fluid for at least 2 hours, with 100% of patients having xanthochromia present by visual inspection by 12 hours.[11]
Xanthochromia is seen in over 90% of patients very soon after an SAH and can be present for up to 3 to 4 weeks, with some studies showing that more than 75% of patients can have xanthochromia present at 21 days following SAH. Even though a noncontrasted CT scan of the brain is the initial imaging modality of choice for a suspected SAH, nearly 5% of cases will have no CT evidence of hemorrhage within the first 24 hours.[12] This percentage increases to 50% by the first week and is about 30% at 2 weeks.
Histopathology
There are currently 2 different methods to identify xanthochromia in the CSF.
Visual Detection
In the US, visual detection is still one of the most commonly used methods to evaluate patients for xanthochromia. During this process, a CSF sample is spun in a centrifuge, and the supernatant is visually inspected with the naked eye for a yellow or pink tint. This is accomplished by holding a vial of water next to a vial of supernatant against a white backdrop. A yellow or pink tint suggests that blood has been in the spinal fluid for at least 2 hours, with 100% of patients having xanthochromia present by visual inspection by 12 hours.[11]
False positives can result from several causes, including patients taking rifampin, those eating excess carotenoids (carrots and spinach), the presence of clinical jaundice, and elevated protein levels in the CSF (>150 mg/dL). Some common causes of elevated CSF protein include carcinomatosis, intracranial neoplasms, Froin's syndrome, tuberculosis, cryptococcal meningitis, and radicular demyelination.
Spectrophotometry
The second and perhaps more reliable way to test for xanthochromia is by using spectrophotometry.[13] A spectrophotometer measures the absorption of light in a material and identifies that material based on the wavelength of light it absorbs. Bilirubin itself has a narrow window of detection with a wavelength of 440 to 460 nm.
Multiple studies show the superiority of spectrophotometry when compared with visual inspection.[14] There are several factors that can interfere with the appearance of xanthochromia on visual inspection alone. For instance, the presence of proteins or pigments such as carotenoids can obscure the color change associated with the presence of xanthochromia. The presence of oxyhemoglobin with an acute bleed or traumatic lumbar puncture appears pink or orange and can hide the yellow discoloration of xanthochromia, leading to a false-negative result. Furthermore, unlike spectrophotometry, a visual inspection cannot detect low concentrations of bilirubin and cannot distinguish bilirubin from oxyhemoglobin.[3]
History and Physical
Patients who have xanthochromia can have a variety of conditions, with the most severe being an SAH. Although rare, SAH carries significant morbidity and mortality risk, as it can lead to sudden death and disability if not diagnosed and treated properly. Patients often present complaining of the worst headache of their life.[5] A sudden onset at a maximal intensity or within the first hour of onset often characterizes headaches. They can be associated with meningismus, nausea, vomiting, photophobia, or phonophobia but rarely with acute neurologic deficits.[15] It can be difficult to distinguish between a nontraumatic headache associated with SAH or another lessseveres cause.
Evaluation
Noncontrast CT of the head (NCHCT) is still the initial test of choice in the diagnosis of SAH.[16] The sensitivity of this test is greatest within the first 6 hours of the onset of symptoms.[5] The current standard of care is to obtain an NCHCT followed by a lumbar puncture to evaluate for xanthochromia if the NCHCT is negative. The finding of xanthochromia in the CSF is helpful in that it is 93% sensitive and 95% specific, with a positive predictive value of 72% and a negative predictive value of 99%.[17]
Xanthochromia is typically present in the CSF within 6 to 12 hours after the onset of symptoms. Unlike CT, xanthochromia is present in the CSF in all patients up to 2 weeks post ictus and is still present in 70% of patients up to 3 weeks later.[11] Furthermore, sensitivity for detecting a bleed by CT decreases from up to 95% on day 1 to less than 10% in 3 weeks, with the sensitivity of CSF analysis remaining constant near 100% over this time.[14] Because the production of bilirubin is a process that happens only in vivo, the presence of xanthochromia in the CSF is the only way to differentiate between a true hemorrhage and a traumatic tap, as both can contain large amounts of red blood cells.[18]
Treatment / Management
Once the patient is diagnosed with an SAH based on the findings of xanthochromia in the CSF, they may be sent for magnetic resonance imaging (MRI) or CTA or taken directly for treatment. The next step in management is to find the location of an aneurysm and, thus, the cause of the SAH.[19]
Differential Diagnosis
The differential diagnoses for xanthochromia include:
- Aneurysmal leaks
- Severe hyperbilirubinemia
- SAH
- Sentinel hemorrhage
- Excess carotenoid intake (carrots and spinach)
- Conditions that cause elevated protein levels in the CSF (eg, carcinomatosis, intracranial neoplasms, Froin's syndrome, tuberculosis, cryptococcal meningitis, and radicular demyelination)
Complications
It is challenging to determine the pretest probability of SAH based on a single presentation alone, as no one factor could determine the need for further testing for an acute nontraumatic headache. Lumbar puncture is an invasive procedure, and often the results are misleading and nondiagnostic.[20][21] However, there is a particular subset of patients in whom a lumbar puncture can identify xanthochromia and help correctly diagnose those with SAH.
Most patients with negative head CT will not be diagnosed with SAH. However, the overuse of CTA can expose patients to unnecessary radiation.[22] Furthermore, the use of CTA leads to the finding of incidental aneurysms, which may or may not be clinically significant. This, in turn, may expose the patient to even more unnecessary testing and intervention. In these cases, A lumbar puncture has a high diagnostic yield, potentially eliminating the need for neurosurgical consultation or intervention in most cases with a low risk of infection, bleeding, and postdural headache.[23] The test is cost-effective, time-efficient, and can potentially reduce the number of patients exposed to the radiation and contrast associated with angiography.[23]
Pearls and Other Issues
- Xanthochromia describes the presence of yellowish supernatant in the CSF that is caused by the presence of bilirubin.
- The presence of xanthochromia on visual inspection can also be seen in other conditions; increased vitamin A intake, certain medications like rifampin, and other infectious conditions can lead to false positives.
- Visual inspection of the CSF is the most common method of detection in the US
- Spectrophotometry is proven to be a superior method of detection to that of visual inspection.[3][14][24]
- It is essential to know the screening pitfalls of those with possible SAH, as there are false positive and false negative scenarios.
Enhancing Healthcare Team Outcomes
Observing xanthochromia in CSF is an important diagnostic finding, given the potential morbidity of an underlying illness such as SAH. Because of the limitations of CT imaging for detecting SAH, the presence of xanthochromia might be the only evidence pointing to its diagnosis.
Twenty-four hours after symptom onset, CT scans give an increasing false negative rate for SAH. Therefore, there must be good communication between the ordering physician and the radiologist regarding the clinical history and appropriate imaging modality.
Standard practice in diagnosing SAH with a negative head CT still includes using a lumbar puncture to detect the presence of xanthochromia. This test is more cost-effective and may reduce the need for CTA with contrast in many patients.[23][25][26]
Additionally, collaboration with the hospital's lab technicians is important, as understanding which method they use for evaluating xanthochromia can help with differential diagnoses. Traditionally, xanthochromia is detected in the lab by visualizing the supernatant CSF under incandescent light for the presence of a yellow tint. Multiple studies have shown the superiority of using spectrophotometry instead of visualization; despite this finding, visual inspection remains the most commonly used modality in the US. Spectrophotometry can detect the presence of xanthochromia at smaller concentrations than the naked eye. It is more specific, as it relies on detecting a certain wavelength of emitted light corresponding to xanthochromia.[24]
Media
References
Shah KH, Edlow JA. Distinguishing traumatic lumbar puncture from true subarachnoid hemorrhage. The Journal of emergency medicine. 2002 Jul:23(1):67-74 [PubMed PMID: 12217474]
Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. American family physician. 2003 Sep 15:68(6):1103-8 [PubMed PMID: 14524396]
Chu KH, Bishop RO, Brown AF. Spectrophotometry, not visual inspection for the detection of xanthochromia in suspected subarachnoid haemorrhage: A debate. Emergency medicine Australasia : EMA. 2015 Jun:27(3):267-72. doi: 10.1111/1742-6723.12398. Epub 2015 Apr 28 [PubMed PMID: 25919441]
Gill HS, Marcolini EG, Barber D, Wira CR. The Utility of Lumbar Puncture After a Negative Head CT in the Emergency Department Evaluation of Subarachnoid Hemorrhage. The Yale journal of biology and medicine. 2018 Mar:91(1):3-11 [PubMed PMID: 29599652]
Long B, Koyfman A, Runyon MS. Subarachnoid Hemorrhage: Updates in Diagnosis and Management. Emergency medicine clinics of North America. 2017 Nov:35(4):803-824. doi: 10.1016/j.emc.2017.07.001. Epub 2017 Aug 24 [PubMed PMID: 28987430]
Rankin S, McGuire J, Chekroud M, Alakandy L, Mukhopadhyay B. Evaluating xanthochromia in the diagnosis of subarachnoid haemorrhage in Scotland in the Era of modern computed tomography. Scottish medical journal. 2022 May:67(2):71-77. doi: 10.1177/00369330211072264. Epub 2022 Feb 1 [PubMed PMID: 35105220]
Ichiba T, Hara M, Nishikawa K, Tanabe T, Urashima M, Naitou H. Comprehensive Evaluation of Diagnostic and Treatment Strategies for Idiopathic Spinal Subarachnoid Hemorrhage. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2017 Dec:26(12):2840-2848. doi: 10.1016/j.jstrokecerebrovasdis.2017.07.003. Epub 2017 Aug 9 [PubMed PMID: 28802522]
Yu SD, Chen MY, Johnson AJ. Factors associated with traumatic fluoroscopy-guided lumbar punctures: a retrospective review. AJNR. American journal of neuroradiology. 2009 Mar:30(3):512-5. doi: 10.3174/ajnr.A1420. Epub 2009 Jan 15 [PubMed PMID: 19147709]
Level 2 (mid-level) evidenceUK National External Quality Assessment Scheme for Immunochemistry Working Group. National guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Annals of clinical biochemistry. 2003 Sep:40(Pt 5):481-8 [PubMed PMID: 14503985]
Level 2 (mid-level) evidenceBazer DA, Koroneos N, Orwitz M, Amar J, Corn R, Wirkowski E. The Diagnostic Dilemma in Delayed Subarachnoid Hemorrhage: A Case Report. Clinical practice and cases in emergency medicine. 2023 Aug:7(3):175-177. doi: 10.5811/cpcem.1586. Epub [PubMed PMID: 37595310]
Level 3 (low-level) evidenceVermeulen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. Journal of neurology, neurosurgery, and psychiatry. 1989 Jul:52(7):826-8 [PubMed PMID: 2769274]
Chakraborty T, Daneshmand A, Lanzino G, Hocker S. CT-Negative Subarachnoid Hemorrhage in the First Six Hours. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2020 Dec:29(12):105300. doi: 10.1016/j.jstrokecerebrovasdis.2020.105300. Epub 2020 Oct 10 [PubMed PMID: 33051138]
Petzold A, Keir G, Sharpe LT. Spectrophotometry for xanthochromia. The New England journal of medicine. 2004 Oct 14:351(16):1695-6 [PubMed PMID: 15483297]
Level 3 (low-level) evidencePetzold A, Keir G, Sharpe TL. Why human color vision cannot reliably detect cerebrospinal fluid xanthochromia. Stroke. 2005 Jun:36(6):1295-7 [PubMed PMID: 15879320]
Moore SA, Rabinstein AA, Stewart MW, David Freeman W. Recognizing the signs and symptoms of aneurysmal subarachnoid hemorrhage. Expert review of neurotherapeutics. 2014 Jul:14(7):757-68. doi: 10.1586/14737175.2014.922414. Epub [PubMed PMID: 24949896]
Abraham MK, Chang WW. Subarachnoid Hemorrhage. Emergency medicine clinics of North America. 2016 Nov:34(4):901-916. doi: 10.1016/j.emc.2016.06.011. Epub [PubMed PMID: 27741994]
Dupont SA, Wijdicks EF, Manno EM, Rabinstein AA. Thunderclap headache and normal computed tomographic results: value of cerebrospinal fluid analysis. Mayo Clinic proceedings. 2008 Dec:83(12):1326-31. doi: 10.1016/S0025-6196(11)60780-5. Epub [PubMed PMID: 19046551]
Level 2 (mid-level) evidenceMark DG, Kene MV, Offerman SR, Vinson DR, Ballard DW, Kaiser Permanente CREST Network. Validation of cerebrospinal fluid findings in aneurysmal subarachnoid hemorrhage. The American journal of emergency medicine. 2015 Sep:33(9):1249-52. doi: 10.1016/j.ajem.2015.05.012. Epub 2015 May 15 [PubMed PMID: 26022754]
Level 1 (high-level) evidencede Oliveira Manoel AL, Goffi A, Marotta TR, Schweizer TA, Abrahamson S, Macdonald RL. The critical care management of poor-grade subarachnoid haemorrhage. Critical care (London, England). 2016 Jan 23:20():21. doi: 10.1186/s13054-016-1193-9. Epub 2016 Jan 23 [PubMed PMID: 26801901]
Doherty CM, Forbes RB. Diagnostic Lumbar Puncture. The Ulster medical journal. 2014 May:83(2):93-102 [PubMed PMID: 25075138]
Wisborg T. [Overuse of CT at the trauma centre?]. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2019 Mar 12:139(5):. doi: 10.4045/tidsskr.19.0038. Epub 2019 Mar 11 [PubMed PMID: 30872827]
Ohana O, Soffer S, Zimlichman E, Klang E. Overuse of CT and MRI in paediatric emergency departments. The British journal of radiology. 2018 May:91(1085):20170434. doi: 10.1259/bjr.20170434. Epub 2018 Feb 5 [PubMed PMID: 29271231]
Martin SC, Teo MK, Young AM, Godber IM, Mandalia SS, St George EJ, McGregor C. Defending a traditional practice in the modern era: The use of lumbar puncture in the investigation of subarachnoid haemorrhage. British journal of neurosurgery. 2015:29(6):799-803. doi: 10.3109/02688697.2015.1084998. Epub 2015 Sep 16 [PubMed PMID: 26373397]
Chu K, Hann A, Greenslade J, Williams J, Brown A. Spectrophotometry or visual inspection to most reliably detect xanthochromia in subarachnoid hemorrhage: systematic review. Annals of emergency medicine. 2014 Sep:64(3):256-264.e5. doi: 10.1016/j.annemergmed.2014.01.023. Epub 2014 Mar 11 [PubMed PMID: 24635988]
Level 1 (high-level) evidenceGoyale A, O'Shea J, Marsden J, Keep J, Vincent RP. Analysis of cerebrospinal fluid for xanthochromia versus modern computed tomography scanners in the diagnosis of subarachnoid haemorrhage: experience at a tertiary trauma referral centre. Annals of clinical biochemistry. 2016 Jan:53(Pt 1):150-4. doi: 10.1177/0004563215579454. Epub 2015 Mar 12 [PubMed PMID: 25766384]
Level 2 (mid-level) evidenceNagy K, Skagervik I, Tumani H, Petzold A, Wick M, Kühn HJ, Uhr M, Regeniter A, Brettschneider J, Otto M, Kraus J, Deisenhammer F, Lautner R, Blennow K, Shaw L, Zetterberg H, Mattsson N. Cerebrospinal fluid analyses for the diagnosis of subarachnoid haemorrhage and experience from a Swedish study. What method is preferable when diagnosing a subarachnoid haemorrhage? Clinical chemistry and laboratory medicine. 2013 Nov:51(11):2073-86. doi: 10.1515/cclm-2012-0783. Epub [PubMed PMID: 23729569]