Introduction
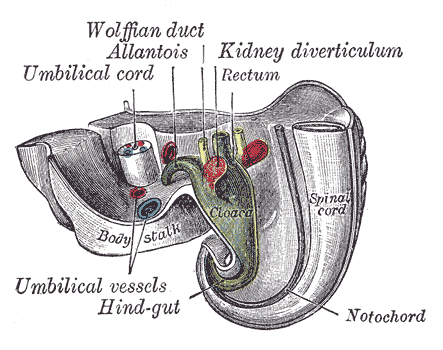
The Wolffian ducts, or mesonephric ducts, are paired embryonic structures that serve as progenitors of the male internal genitalia and are involved in the incipient development of the renal system in both sexes. The Wolffian ducts (WDs) develop in male and female embryos but are only maintained in males by testosterone, and they give rise to the epididymis, vas deferens, and seminal vesicles.[1] Although the development of the WDs into the male internal genitalia is dependent on testosterone, there is a dynamic and complex interplay of androgens, growth factors, gene expression in the duct epithelium, and mesenchyme for the proper formation of the male reproductive tract.[1]
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
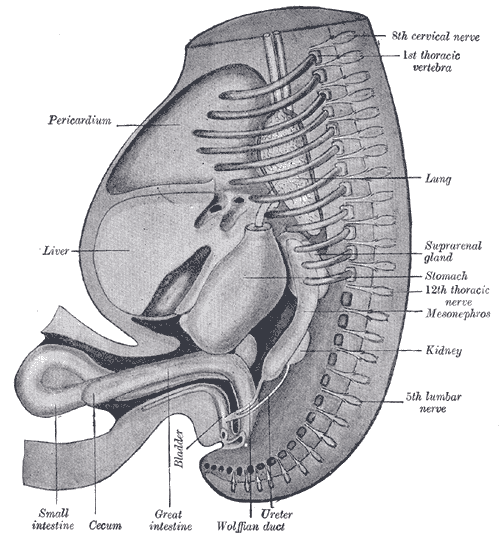
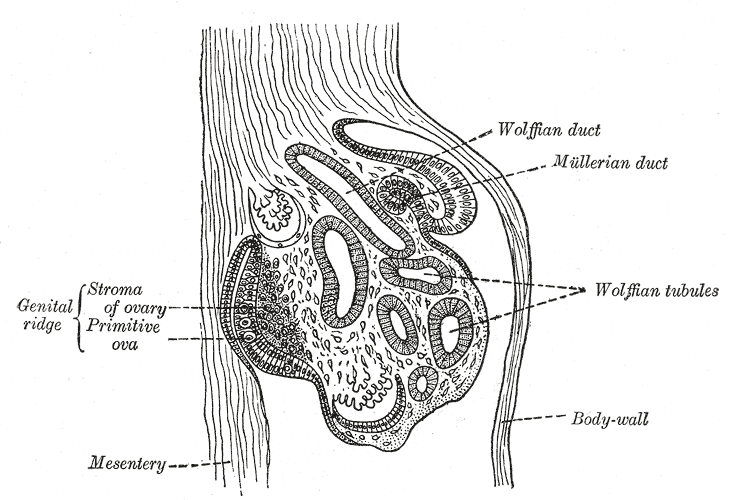
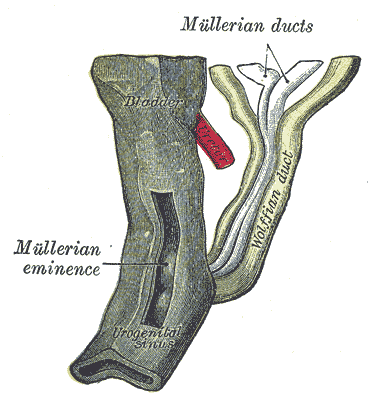
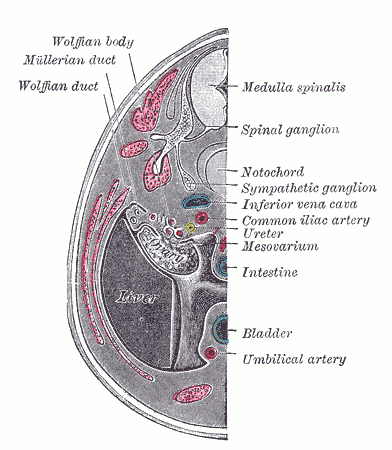
The Wolffian ducts (WDs) develop from the intermediate mesoderm in craniocaudal succession. They extend from the pronephric duct near the future forelimb buds caudally to the cloaca.[1] The WDs stimulate the growth of mesonephric tubules as mesonephric mesenchymal proliferations, which extend to the epithelial cells of the gonads in males and females. In mouse and rat studies, four to six pairs of cranial mesonephric tubules form as outgrowths of the WDs, but most of the tubules are caudal, never fusing with the WDs, and instead differentiate similar to the metanephric nephrons.[2] The ureteric bud stems from the WDs posteriorly and gives rise to the pronephros, mesonephros, and metanephros of the kidney primordia by interacting with the metanephric mesenchyme. The pronephros and mesonephros degenerate after their development in females, but in males, the mesonephric tubules become the precursor to the epididymal ducts and efferent ducts.[2]
Many genes and growth factors appear to be crucial for the proper formation of the WDs, based largely on rodent studies. Pax2 and Pax8 are known for inducing WD formation, and Lim1 is necessary for WD extension.[3][4] Pax2, Pax8, Emx2, and Lim1 are expressed in the condensed nephrite cord and are necessary for tubulogenesis and WD development.[5] WT-1 and Six1 are also expressed in the nephrogenic mesenchymal condensation, and rodents without the WT-1 or Six-1 expression have been shown to lack caudal MTs.[6][7] Mice with a null Emx2 have regular WD formation until the ducts degenerate, resulting in the failure of the kidney and reproductive tract to form.[8] Mice with a null Gata3 mutation also have defects with WD initiation.[9] Fgf8, which is signaled by the intermediate mesoderm, is also essential, and not expressing it results in the nonexistence of the cranial mesonephros and mesonephric tubules (MTs) in mice. During mesonephric development, the mesenchyme expresses Fgfr1 while the epithelium expresses Fgfr2, thus maintaining the WD and mesonephric mesenchyme.[10] Fgfr2 appears to sustain the caudal portion of the WD by managing cell proliferation.[11] The absence of forkhead transcription factors, Foxc1 and Foxc2, and Shh results in supernumerary MT formation in mouse models, which suggests these genes have suppressive effects on MT development.[12][13] Retinoic acid signaling is also critical in WD development, as compound null mutations in retinoid acid receptors α and γ can cause agenesis or dysplasia of the epididymis, vas deferens, and seminal vesicles.[14]
The epithelium of the WDs also expresses Wnt9b, and Wnt7b is signaled, starting from E9.5. The lack of Wnt9b expression correlates with the absence of MTs and epididymis at birth, and β-catenin-dependent canonical Wnt signaling induces MT formation in Wnt9b null mice.[15] During the formation of the metanephric kidney, Wnt9b attenuation influences the epithelial cell polarity and increases the diameter of the tubules.[15]
Stabilization and Elongation of the WDs
In males, the mesonephros serves as the precursor to the male reproductive tract; whereas, in females, the mesonephros regresses. After gonadal sex differentiation, the Leydig cells of the testes produce testosterone, and it is locally generated androgens that are required for WD stabilization.[16] However, some studies suggest androgens transported through the systemic circulation are enough to prevent WD regression.[5] Androgens act on the androgen receptor (AR) and growth factors, such as FGF and epidermal growth factor (EGF), to mediate androgen functions in the prostate and WDs.[17]
After the WDs have stabilized, their elongation is dependent on the expression of androgens and growth factor signaling. Inhba is a paracrine factor that controls the coiling of the epithelium in the anterior WD, and Pkd1 appears to be involved in signaling the transduction of growth factors and cytoskeleton dynamics.[18][19]
Differentiation of the Regions of the WDs
Research suggests that the expression of some homeobox genes in specific regions is critical for WD differentiation into the epididymis, vas deferens, and seminal vesicles.[1] The Drosophila Abdominal B-related homeobox genes are important in distinguishing tissue boundaries between these anatomic structures in mice. In male mice, the epididymis expresses Hoxa9, Hoxa10, Hoxd10, and Hoxd9; while the vas deferens expresses Hoxa9, Hoxd9, Hoxa10, Hoxd10, and Hoxa11. Hoxa13 and Hoxd13 are also expressed via the caudal part of the WD and seminal vesicles. Hoxa10 mutations can cause WD anterior homeotic transformation, whereby the distal epididymis and proximal vas deferens display morphological qualities of more anterior segments. Research has demonstrated Hoxa11 mutations to cause a homeotic transformation of the vas deferens to an epididymis-like phenotype. Hoxd13 mutations could lead to decreased size and cleating of the seminal vesicles.[1]
Clinical Significance
WDs develop in male and female embryos and contribute to their renal development. In males, however, exposure to adequate amounts of androgens is critical for WD development of the male internal genitalia. The differentiated male gonad must function in utero to support typical WD development. Thus, in females, the gonads differentiate into ovaries, and the lack of Leydig cell-secreted testosterone results in the gradual regression of the WDs. However, small WD inclusions or remnants may persist in females. For instance, the paraurethral (or Skene glands) have long been suggested to be developmentally homologous with the WD-derived male prostate. In addition, a visible remnant of the WD-derived mesonephric tubules called the epoophoron may be present in females. An elongate remnant of the WD-derived mesonephric tubules may also persist as a Gartner duct or cyst within the lateral vaginal wall. These lesions are benign and not life-threatening.
Some disorders may arise as a result of improper development of WDs. For instance, congenital bilateral absence of the vas deferens, which is distinguished by the nonexistence of the body and tail of the epididymis, vas deferens, and seminal vesicles, accounts for 1 to 2% of male infertility cases.[20] However, individuals with this disorder retain an apparent head of the epididymis with normal function. Mutations in the cystic fibrosis transmembrane conductance regulator gene account for 80% of cases and early obstruction of the WDs by dehydrated secretions seem to cause the nonexistence of the more distal WD derivatives. The head of the epididymis expresses high CFTR levels, and CFTR malfunction at the head of the epididymis could cause flow obstruction in more distal areas of the male internal genitalia.[21][22]
Severe mutations in the androgen receptor (AD) could also cause complete androgen insensitivity syndrome, where patients typically present with female external genitalia.[23] However, some WD derivatives are sometimes present in these patients, and these mutant AR receptors respond to high concentrations of testosterone in the WDs in vivo but not to reduced androgen concentrations in the external genitalia.[24]
17beta-hydroxysteroid dehydrogenase deficiency is another WD-related disorder. Patients with this condition have impaired conversion of androstenedione to testosterone and usually have female external genitalia at birth[1]. However, individuals with this condition have epididymides and vasa deferentia that are typically developed. [1] Previous research demonstrated that patients with this condition often have testosterone levels 15 to 70-fold lower and androstenedione levels 15 to 20-fold higher than those in normal patients, which may be enough for WD stabilization since the AR is capable of binding to androstenedione with a lower affinity than testosterone.[25]
Patients with 5alpha-reductase deficiency also have mild to severe undervirilization of the external genitalia at birth, since testosterone is not converted effectively into dihydrotestosterone in utero. However, the development of WDs is normal given that WD development of the male reproductive organs depends on testosterone, which is present at normal or high concentrations in these patients.[1]
LH receptor mutations could also result in Leydig cell hypoplasia and a variety of disorders of sex development.[26] Patients with these mutations often have low testosterone concentrations and female external genitalia with normal epididymides and vasa deferentia.[27]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hannema SE, Hughes IA. Regulation of Wolffian duct development. Hormone research. 2007:67(3):142-51 [PubMed PMID: 17077643]
Level 3 (low-level) evidenceSainio K, Hellstedt P, Kreidberg JA, Saxén L, Sariola H. Differential regulation of two sets of mesonephric tubules by WT-1. Development (Cambridge, England). 1997 Apr:124(7):1293-9 [PubMed PMID: 9118800]
Level 3 (low-level) evidenceTorres M, Gómez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development (Cambridge, England). 1995 Dec:121(12):4057-65 [PubMed PMID: 8575306]
Level 3 (low-level) evidencePedersen A, Skjong C, Shawlot W. Lim 1 is required for nephric duct extension and ureteric bud morphogenesis. Developmental biology. 2005 Dec 15:288(2):571-81 [PubMed PMID: 16216236]
Level 3 (low-level) evidenceMurashima A, Xu B, Hinton BT. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian journal of andrology. 2015 Sep-Oct:17(5):749-55. doi: 10.4103/1008-682X.155540. Epub [PubMed PMID: 26112482]
Level 3 (low-level) evidenceKreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27:74(4):679-91 [PubMed PMID: 8395349]
Level 3 (low-level) evidenceKobayashi H, Kawakami K, Asashima M, Nishinakamura R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mechanisms of development. 2007 Apr:124(4):290-303 [PubMed PMID: 17300925]
Level 3 (low-level) evidenceMiyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development (Cambridge, England). 1997 May:124(9):1653-64 [PubMed PMID: 9165114]
Level 3 (low-level) evidenceGrote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development (Cambridge, England). 2006 Jan:133(1):53-61 [PubMed PMID: 16319112]
Level 3 (low-level) evidencePoladia DP,Kish K,Kutay B,Hains D,Kegg H,Zhao H,Bates CM, Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Developmental biology. 2006 Mar 15; [PubMed PMID: 16442091]
Level 3 (low-level) evidenceOkazawa M, Murashima A, Harada M, Nakagata N, Noguchi M, Morimoto M, Kimura T, Ornitz DM, Yamada G. Region-specific regulation of cell proliferation by FGF receptor signaling during the Wolffian duct development. Developmental biology. 2015 Apr 1:400(1):139-47. doi: 10.1016/j.ydbio.2015.01.023. Epub 2015 Feb 9 [PubMed PMID: 25678108]
Level 3 (low-level) evidenceKume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development (Cambridge, England). 2000 Apr:127(7):1387-95 [PubMed PMID: 10704385]
Level 3 (low-level) evidenceMurashima A, Akita H, Okazawa M, Kishigami S, Nakagata N, Nishinakamura R, Yamada G. Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm. Developmental biology. 2014 Feb 1:386(1):216-26. doi: 10.1016/j.ydbio.2013.12.026. Epub 2013 Dec 24 [PubMed PMID: 24370450]
Level 3 (low-level) evidenceMendelsohn C, Lohnes D, Décimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development (Cambridge, England). 1994 Oct:120(10):2749-71 [PubMed PMID: 7607068]
Level 3 (low-level) evidenceKarner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nature genetics. 2009 Jul:41(7):793-9. doi: 10.1038/ng.400. Epub 2009 Jun 21 [PubMed PMID: 19543268]
Level 3 (low-level) evidenceShima Y, Miyabayashi K, Haraguchi S, Arakawa T, Otake H, Baba T, Matsuzaki S, Shishido Y, Akiyama H, Tachibana T, Tsutsui K, Morohashi K. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Molecular endocrinology (Baltimore, Md.). 2013 Jan:27(1):63-73. doi: 10.1210/me.2012-1256. Epub 2012 Nov 2 [PubMed PMID: 23125070]
Level 3 (low-level) evidenceDonjacour AA, Thomson AA, Cunha GR. FGF-10 plays an essential role in the growth of the fetal prostate. Developmental biology. 2003 Sep 1:261(1):39-54 [PubMed PMID: 12941620]
Level 3 (low-level) evidenceTomaszewski J, Joseph A, Archambeault D, Yao HH. Essential roles of inhibin beta A in mouse epididymal coiling. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 3:104(27):11322-7 [PubMed PMID: 17592132]
Level 3 (low-level) evidenceNie X, Arend LJ. Pkd1 is required for male reproductive tract development. Mechanisms of development. 2013 Nov-Dec:130(11-12):567-76. doi: 10.1016/j.mod.2013.07.006. Epub 2013 Aug 9 [PubMed PMID: 23933588]
Level 3 (low-level) evidenceJequier AM, Ansell ID, Bullimore NJ. Congenital absence of the vasa deferentia presenting with infertility. Journal of andrology. 1985 Jan-Feb:6(1):15-9 [PubMed PMID: 3918979]
McCallum TJ, Milunsky JM, Cunningham DL, Harris DH, Maher TA, Oates RD. Fertility in men with cystic fibrosis: an update on current surgical practices and outcomes. Chest. 2000 Oct:118(4):1059-62 [PubMed PMID: 11035677]
Level 2 (mid-level) evidenceTizzano EF, Silver MM, Chitayat D, Benichou JC, Buchwald M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. The American journal of pathology. 1994 May:144(5):906-14 [PubMed PMID: 7513949]
MORRIS JM. The syndrome of testicular feminization in male pseudohermaphrodites. American journal of obstetrics and gynecology. 1953 Jun:65(6):1192-1211 [PubMed PMID: 13057950]
Hannema SE, Scott IS, Hodapp J, Martin H, Coleman N, Schwabe JW, Hughes IA. Residual activity of mutant androgen receptors explains wolffian duct development in the complete androgen insensitivity syndrome. The Journal of clinical endocrinology and metabolism. 2004 Nov:89(11):5815-22 [PubMed PMID: 15531547]
Level 3 (low-level) evidenceChen F, Knecht K, Leu C, Rutledge SJ, Scafonas A, Gambone C, Vogel R, Zhang H, Kasparcova V, Bai C, Harada S, Schmidt A, Reszka A, Freedman L. Partial agonist/antagonist properties of androstenedione and 4-androsten-3beta,17beta-diol. The Journal of steroid biochemistry and molecular biology. 2004 Aug:91(4-5):247-57 [PubMed PMID: 15336702]
Level 3 (low-level) evidenceBerthezène F, Forest MG, Grimaud JA, Claustrat B, Mornex R. Leydig-cell agenesis: a cause of male pseudohermaphroditism. The New England journal of medicine. 1976 Oct 28:295(18):969-72 [PubMed PMID: 184390]
Level 3 (low-level) evidenceChu L, Li J, Liu Y, Hu W, Cheng CH. Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Molecular endocrinology (Baltimore, Md.). 2014 Nov:28(11):1785-95. doi: 10.1210/me.2014-1061. Epub 2014 Sep 19 [PubMed PMID: 25238195]
Level 3 (low-level) evidence