Introduction
According to the Surveillance, Epidemiology, and End Results (SEER) Program, vulvar cancer represents 0.3% of all new cancer cases annually at a rate of 2.6 per 100,000 women per year in the United States. Diagnosis is usually made in the sixth through eighth decades of life and is commonly identified early in the disease. Squamous cell carcinoma (SCC) accounts for most vulvar cancers, while basal cell carcinoma (BCC), extramammary Paget disease, and vulvar melanoma comprise the less common subtypes. Surgery remains the mainstay of vulvar cancer treatment, with medical and radiation oncology playing an increasingly important role in preventing recurrence and improving outcomes.[1][2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Risk factors for the development of vulvar cancer include increasing age, infection with human papillomavirus (HPV), smoking, inflammatory conditions of the vulva, prior pelvic radiation, and immunodeficiency.[3]
Epidemiology
Squamous cell carcinoma is the most common type of vulvar cancer.[1] SEER data supports that vulvar cancer is most commonly diagnosed at ages 65 to 74, with the median age at diagnosis of 69 years. The data notes that sixty percent of diagnoses are localized and exhibit an 85% five-year survival. Vulvar melanoma is the second most common vulvar malignancy, representing 5% of vulvar cancers.[4] It more commonly affects White race women ages 50 to 70. The median age at diagnosis for vulvar melanoma is similar to SCC (68 years), but approximately 8.4% present with advanced disease and have a lower survival rate.[5]
Pathophysiology
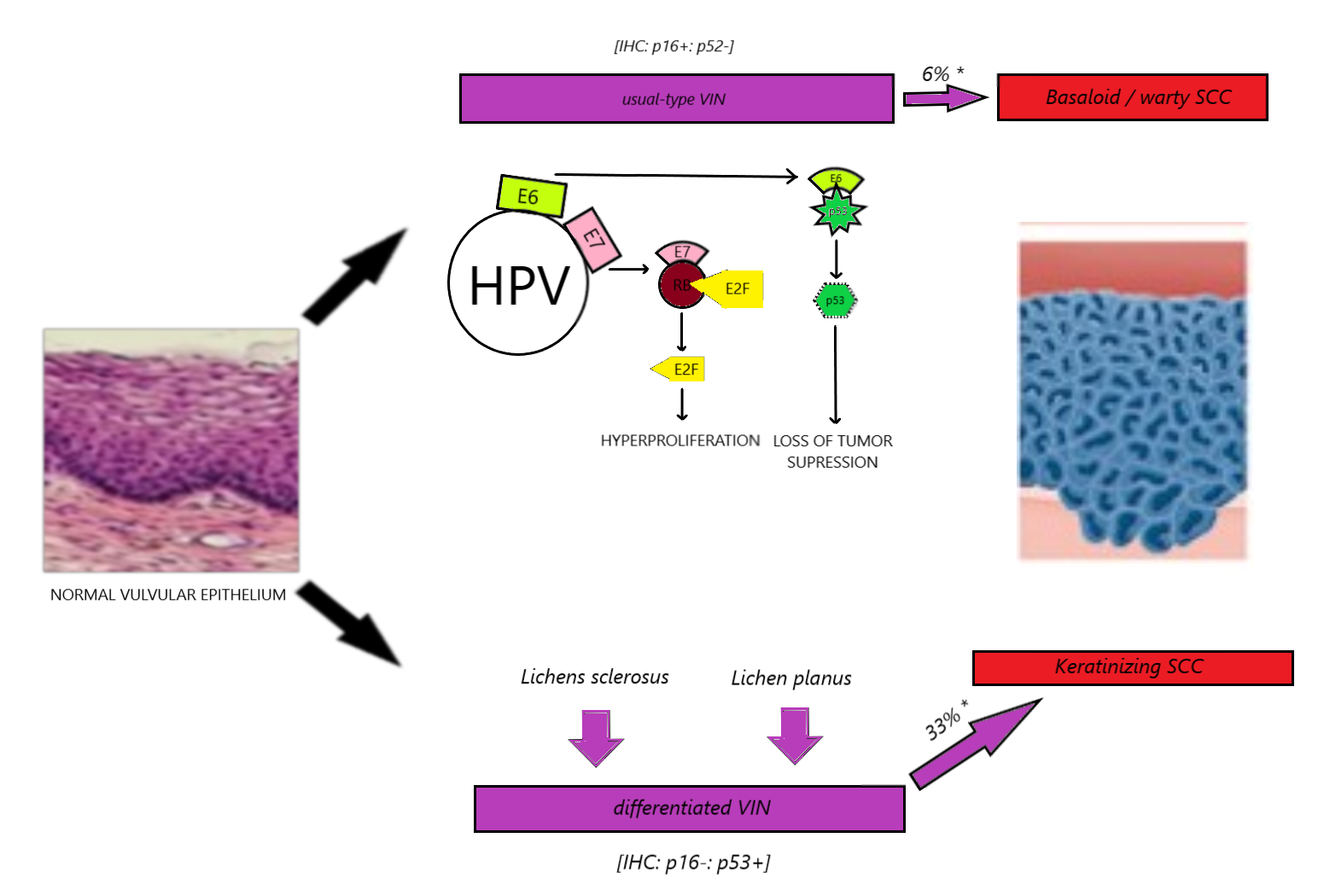
Vulvar SCC represents 90% of all vulvar cancers and typically develops by one of two pathways (see Image. Vulvar Cancer Development).[6] Thirty to forty percent of vulvar cancer cases are associated with high-risk human papillomavirus (HR-HPV), resulting from the classic “two-hit hypothesis” for cancer development.[6][7] HPV is known to have E6 and E7 oncoproteins, which inactivate the p53 and RB tumor suppressor proteins, respectively. The loss of these tumor suppressor genes leads to unregulated hyperproliferation. Another pathway involves inflammatory changes that result in cells with intact p53 status but the loss of cyclin-dependent kinase inhibitor 2A (p16), also resulting in unregulated cell cycle proliferation and eventually cancer (see Image. Human Papillomavirus [HPV 16]).[2][7][8] Pathophysiology of usual-type and differentiated VIN and its progression to SCC. Suggested progression of usual-type (uVIN) and differentiated vulvar intraepithelial neoplasia (dVIN) to SCC.[2]
Histopathology
Squamous Cell Carcinoma: Squamous cell carcinoma is the most common histologic subtype of vulvar cancer (see Image. Vulvar Squamous Cell Carcinoma). The precursor lesion for SCC is vulvar intraepithelial neoplasia (VIN) and can be subdivided into two categories: HPV-dependent usual type (uVIN) and HPV-independent differentiated type (VIN).[2] As of 2012, the Lower Anogenital Squamous Terminology unified terminology for all HPV-associated squamous lesions and recommended the use of Low-Grade Squamous Intraepithelial Lesions (LSIL) and High-Grade Squamous Intraepithelial Lesions (HSIL).[9] In 2014, the WHO classification of tumors subdivided squamous intraepithelial lesions of the vulva into LSIL, HSIL, and dVIN. In 2015, the International Society for the Study of Vulvovaginal Disease (ISSVD) accepted and approved a similar terminology classification.[10]
uVIN: (LSIL, HSIL). This is the HPV-dependent usual type that typically affects younger patients and is less likely to progress to SCC than dVIN.[10][11] There is a strong association between this subtype and a history of smoking, and it is more commonly seen in basaloid or warty SCC. This subtype is usually p16 positive and p53 negative on immunohistochemistry, whereas dVIN is usually p16 negative and p53 positive.[12] The usual type of VIN progresses to invasive SCC in only 5% of cases but is responsible for 40% of all vulvar SCC.[6]
dVIN: HPV-independent differentiated type typically progresses to keratinized SCC (see Image. Vulvar Squamous Cell Carcinoma, Histopathology). This subtype arises mostly from chronic dermatoses, with lichen sclerosis and lichen planus being the most common.[2] It is characterized by cellular atypia of the basal layers of the vulvar epithelium. dVIN makes up only 5% of preinvasive vulvar lesions but has a higher rate of malignant transformation than uVIN and is identified as a precursor in approximately 35% of vulvar SCC.[9] Patients with lichen sclerosus need lifelong monitoring due to the risk of eventually developing vulvar SCC, increasing the duration of the disease (1% at 2 years with lichen sclerosus and 37% at 25 years with the disease).[13] See Image. Kraurosis Vulvae, Lichen Sclerosus, Pathology.
Vulvar SCC can be categorized into three histological subtypes: warty, basaloid, and keratinizing. A warty and basaloid subtype is found mostly in patients ages 40 to 44 years and is associated with HPV. The keratinizing subtype is associated with older patients and is HPV-independent. This type accounts for 60-80% of all SCC subtypes. It may occur anywhere on the vulva but is most commonly found on the labia majora and perineum.
Basal Cell Carcinoma: Basal cell carcinoma is a relatively rare vulvar malignancy. Approximately 2% of BCC affects the vulva, and vulvar BCC is diagnosed in only 8% of all vulvar malignancies.[14] Diagnosis of vulvar BCC is usually made in a seventh or eighth decade, most commonly with a labia majora and vulvar pruritis lesion.[9] Most BCC is of the nodular subtype, with the superficial subtype being the second most common. Dermoscopy diagnostic of BCC reveals arborizing vessels, telangiectasias, blue ovoid nests, blue globules, and shiny white structures. Imaging studies are only needed for extensive local disease suspicious for underlying structural destruction and invasion. Analogous to BCC in other body areas, treatment is the primary complete excision with negative margins. BCC in the genital area is at high risk for recurrence; however, the prognosis of vulvar BCC is favorable, and overall survival is unaffected by the lesion's size. The Mohs surgical technique has been used successfully with a 3-year recurrence-free survival rate of 100%.[15]
Paget Disease: Extramammary Paget disease is a rare skin malignancy affecting the apocrine gland-bearing skin, with 65% of all cases occurring in the vulva[16], comprising only 1-2% of all vulvar malignancies.[17] This disease occurs primarily in Caucasian women in their sixth to seventh decade of life. Pruritis is the presenting symptom in 54%[18] to 72%[19]. Vulvar Paget disease can be broadly categorized as a primary or secondary disease. Primary vulvar Paget disease is an intraepithelial adenocarcinoma with Paget cells that arise from within the epidermis and extend into the epithelium of adjacent skin appendages.[20] This disease can become locally invasive when Paget cells break through the basement membrane and infiltrate deeper tissue layers. Secondary vulvar Paget disease occurs less frequently but is associated with epidermotropic metastases or direct invasion of an occult adenocarcinoma. Noninvasive vulvar Paget disease is associated with underlying adenocarcinoma in 4-17% of patients.[18][19][21]Histologically, Paget cells are epithelial tumor cells with clear cytoplasm. These can either heterogeneously invade the epidermis or spread in a nest-like fashion. Immunohistochemical (IHC) markers of vulvar Paget disease include Cytokeratin 7, CEA, pan-CK, and EMA. IHC markers CK20 and CDX2 are more prevalent in secondary vulvar Paget disease and can help differentiate primary from secondary disease. Vulvar Paget lesions can be slow-growing for many years, but after invasion through the dermis, the spread can be rapid and aggressive via lymphatic or hematogenous routes. The adnexal extension is frequent, occurring in up to 90% of recurrent cases, most commonly involving hair follicles and eccrine ducts.[22]
Vulvar Melanoma: Three types of vulvar melanomas exist. The mucosal lentigous subtype is most common, followed by the nodular and superficial spreading subtypes. This differs from cutaneous melanoma, where the superficial spreading subtype is most common.[23] Vulvar and cutaneous melanomas share similar IHC markers (S100B, HMB45, and Melan-A) that differ significantly by mutational analyses. Vulvar melanomas more rarely exhibit V600-BRAF mutations (7%[24] to 26%[25]) than mucosal melanomas, while mutations in cell regulatory proteins such as c-KIT and PD-L1 are significantly more common in vulvar melanoma, approximating 25-31%.[24][25] In the fifth to seventh decades, white women are most commonly affected with lesions most often found on the clitoris and labia minora. The American Joint Committee on Cancer (AJCC) staging provides the best predictor for survival, with Breslow depth of invasion and lymphovascular space invasion predictive of nodal metastases.[26] Surgical resection with adjuvant-targeted medical therapy is currently recommended.[27]
Verrucous Carcinoma: The etiology of vulvar verrucous carcinoma (VC) is unknown, and no precursor lesions to this disease have been described. However, an association between lichen simplex chronicus and lichen sclerosus has been reported.[28] Studies evaluating an association with HPV status are mixed. Histologically, VC is a well-differentiated tumor with marked acanthotic epithelial proliferation and minimal nuclear atypia. The tumor expands with elongating rete ridges that have become characteristic of this type of lesion. These elongating ridges advance into the dermis, causing a pushing rather than infiltrating pattern.[2] Proliferation occurs primarily at basal and parabasal layers. The increased expression of cellular proteins Ki67, MCM2, and TOP2A demonstrates this.[29] In contrast with SCC, VC does not have overexpression of p53.[30] The appearance of these lesions is typically warty and can become quite large without the risk of metastasis. The recommended treatment is local excision.[30]
Sarcoma: Although rare, cancers of mesenchymal origin have been described in the setting of vulvar cancer. The most common sarcoma of the vulva is leiomyosarcoma (LMS), followed by dermatofibrosarcoma protuberans (DFSP), epithelioid sarcoma, malignant fibrohistiocytomas, and synovial sarcomas. Synovial sarcomas are divided into monophasic, biphasic, and undifferentiated histologic types. Monophasic contains only spindle cells, biphasic type contains both epithelial and spindle cell types, while undifferentiated share characteristics of both. Vulvar sarcomas are slow-growing tumors, most commonly of the clitoris and labia minora, have a younger age at diagnosis (median age 41 years) and rare lymph node invasion.[31] DFSP median age at diagnosis is 45 years old and is related to a translocation leading to tyrosine kinase disinhibition. This presents a possible use for tyrosine kinase inhibitors in managing this subtype of vulvar sarcoma.[32] Vulvar epithelioid sarcoma is diagnosed at the youngest mean age (31 years) and tends to exhibit the lowest survival of all vulvar cancers.[33]
Bartholin Gland and Other Adenocarcinomas: The Bartholin gland's primary cancer is exceedingly rare and arises from either the gland or duct. Presentation is usually the growth of a painless mass in the labia majora during the 5 to 6 decades. Cancer of the Bartholin’s gland most commonly exhibits either SCC or adenocarcinoma histologic subtypes.[34][35]
History and Physical
The anatomy of the vulva includes the mons pubis, labia majora, labia minora, clitoris, vestibule, vestibular bulb, and the greater vestibular glands. The internal and external pudendal arteries are responsible for most of the vulvar blood supply. The ilioinguinal, genitofemoral, and pudendal nerves are responsible for the innervation of the vulvar tissue. Vulvar lymphatic drainage is via the inguinal lymph nodes. The first lymphatic chain encountered is the superficial inguinal nodes. Then, after crossing through the cribriform fascia, lymphatic drainage is to the deep inguinal nodes, followed by the external iliac nodes and, finally, the paraaortic nodes. History of vulvar illness may include pruritis, irritation, or pain, but the patient can also be asymptomatic. Most patients with vulvar melanoma present with advanced symptoms, including bleeding, mass, and ulceration.[9]
Approximately 25% of vulvar melanomas are amelanotic, making diagnosis difficult in many patients.[36] Vulvar Paget disease can have a very nonspecific presentation, which has often led to the diagnosis being delayed by a median of two years, typically after topical steroids or antifungals have failed. Similarly, Bartholin’s gland carcinoma presents nonspecifically as a painless visible tumor and is often misdiagnosed and incorrectly treated as an abscess or cyst before a definitive diagnosis.[34] A physical exam may show an erythematous lesion, a scaly patch, plaques, an ulcer, or an ill-defined mass. Lesions of verrucous carcinoma commonly have a cauliflower-like appearance. Any suspicious lesions warrant further investigation, including a pelvic exam, speculum exam, colposcopy of the vulva and vagina, and biopsy.[37] In cases of vulvar melanoma, attention to the ABCDE rule (a dermatologic acronym for asymmetry, border irregularity, color, diameter, and evolving) can aid in clinical diagnosis.[38]
Evaluation
The gold standard for diagnosing vulvar cancer remains histologic diagnosis, although clinical correlation does have significant value. Any suspicious lesion should be biopsied and carefully examined for its precise anatomical position with respect to the midline and distance from the vaginal introitus; this is crucial for planned surgical management. Imaging studies may be indicated to evaluate the extent of the disease. If there is suspicion of bladder or rectal involvement, cystoscopy and proctoscopy should be performed.[37] In Paget disease, screening should be done for other malignancies, including genitourinary, gastrointestinal, and breast cancer, especially considering that the most common cause of secondary vulvar Paget disease is anorectal and urothelial adenocarcinomas.[39]
Treatment / Management
Surgical excision is the standard therapy for vulvar cancer, but adjuvant radiation and chemotherapy may be recommended depending on the histopathology and extent of the disease.
Differential Diagnosis
The differential diagnosis for vulvar cancer is broad due to the sometimes nonspecific nature of this disease. Several diseases that can mimic vulvar cancer include cutaneous SCC, cutaneous BCC, cutaneous melanoma, atopic dermatitis, psoriasis, lichen sclerosus, lichen planus, lichen chronicus simplex, contact dermatitis, candidiasis, pemphigus vegetans, or mycosis fungoides. Image 8. Pathology, Kraurosis vulvae, lichen sclerosus, Chronic inflammatory dermatosis, white plaque, vulva, precancerous, squamous cell carcinoma, Urogenital, Genitalia, Vulvar Diseases (Contributed by Dr. N.J. Fiumara, The Centers for Disease Control and Prevention)
Surgical Oncology
Surgery is the primary treatment of early-stage disease.[40] The risk of recurrence is associated with tumor size, lymph node involvement, and positive margins.[41][42] For SCC with a depth of invasion ≤1mm, wide-local excision without lymphadenectomy is sufficient with a recommended surgical margin of 1 to 2 cm. If tumor depth is greater than 1mm or tumor diameter exceeds 2 cm, radical resection with margins extending to the perineal fascia and inguinal lymph node assessment should be performed. This more aggressive treatment is recommended due to the risk of occult nodal metastasis and increased risk of death from groin recurrence. For verrucous carcinoma, local excision is typically sufficient; however, the advanced disease may require radical resection.[43] Tumor-free margins decrease the risk of recurrence.[44]
Similarly, wide local excision with tumor-free margins is also recommended for vulvar melanoma as in cutaneous melanoma because radical surgery to treat vulvar melanoma does not improve survival and is associated with increased morbidity.[4] In vulvar Paget disease, local excision is the standard of care; however, given that multifocal disease is common, high rates of positive margins and recurrence are often observed. Inguinal lymphadenectomy should be considered if the invasion is greater than 1mm. Mohs surgery may benefit the successful resection of Pagetoid lesions and has been associated with a higher rate of negative margins.[45] In vulvar sarcoma, the standard treatment is radical local excision, with inadequate excision of margins being the most important predictor of recurrence.[31]
Lymph Nodes: The decision to perform staging lymphadenectomy should be carefully considered based on the risk of occult disease and morbidity. There is no successful treatment for vulvar cancer patients with groin recurrence.[46] while 14 to 48% of vulvar cancer patients experience clinically significant lymphedema after groin dissection.[47] Evaluation of lymph nodes should be performed for vulvar cancers with DOI >1mm.[48] Factors associated with the potential risk of lymphedema include the duration of follow-up, the surgical procedure used, the cancer stage, the presence of wound infection, elevated BMI, and adjuvant radiation or chemotherapy.[49]
The type of node assessment also impacts morbidity, with those undergoing complete lymphadenectomy at five times higher risk of lymphedema than those undergoing sentinel lymph node (SNL) biopsy.[49] The SLN concept has been proven safe and feasible in specific cancers such as breast cancer, melanoma, and some gynecologic cancers, including vulvar cancer. Studies of SLN mapping in early-stage vulvar cancer have been demonstrated as safe with a high detection rate and high sensitivity.[48][50] when performed by experienced surgeons and is recommended for newly diagnosed vulvar melanomas.[27] Adjuvant radiation for vulvar cancer patients with metastatic disease to the groin improves survival at the cost of potential lymphedema, making SLN mapping an ideal option for reducing morbidity without diminishing survival.
Suppose the primary lesion is unilateral (greater than 1cm from the vulvar midline). In that case, an ipsilateral inguinal lymphadenectomy can be performed for vulvar SCC because the risk for contralateral lymph node involvement is less than one percent.[51] For midline lesions, a bilateral lymphadenectomy is recommended. The GOG-173 study prospectively assessed the reliability of sentinel lymph node biopsy in vulvar cancer detection and reported a false-negative predictive value of only 2.0% with primary tumors < 4 cm in diameter vs 7.4% in tumors >4cm in diameter.[52] If technetium-99 is combined with intraoperative blue dye, the detection rate of sentinel lymph nodes is close to 100%.[51] In patients with unifocal tumors <4cm and clinically negative lymph nodes, SLN biopsy is recommended.[53]
For vulvar BCC, lymph node biopsy is generally not required. In patients with melanoma, SLN biopsy is recommended at the surgical resection of the primary tumor.[27] Bilateral versus unilateral lymph node assessment follows SCC criteria. In patients with verrucous carcinoma, lesions are locally invasive, with reports of tumors up to 15cm in size with little to no risk of lymph node metastasis. However, due to the possible coexistence of SCC with VC and the considerable differences in treatment, an adequately large and deep biopsy should be obtained to rule out concomitant disease. After SCC is excluded, routine lymph node dissection should be omitted for verrucous carcinoma. For vulvar sarcomas, lymph node dissection should be reserved for cases in which lymph nodes are clinically positive. Treatment recommendations for Bartholin gland carcinoma are similar to those for vulvar SCC.[34]
Radiation Oncology
Radiation is recommended for vulvar cancer as adjuvant therapy for histology-confirmed metastatic disease and as primary therapy for locally advanced disease, followed by radical resection of the residual tumor. When SLN biopsy is positive, it is acceptable to offer adjuvant radiation with or without chemotherapy or to perform a complete inguinal lymphadenectomy and offer adjuvant therapy only if high-risk features are identified, such as positive or close resection margins, multifocal disease, multiple involved nodes, or extracapsular extension. The latter approach is recommended, especially if there are ≥2 positive nodes or 1 positive node with >2 mm metastasis.[54][55]
Primary chemoradiation is recommended in the case of locally advanced disease, followed by radical resection of any residual disease.[56][57][58] In the event of distant metastasis, treatment is palliative, primarily focused on improved quality of life. In these cases, chemoradiation can be used for symptomatic relief at the primary tumor site and pelvis.[59] For vulvar melanoma, radiotherapy has a limited benefit, and the use of neoadjuvant radiotherapy has not been described.[5]
In vulvar Paget disease, radiotherapy or photodynamic therapy (PDT) can be considered as an alternative treatment option to surgical resection.[2][60] PDT is a clinically approved, minimally invasive procedure that involves treatment with a photosensitizing agent, followed by irradiation at a wavelength corresponding to the absorbance band of the sensitizer. PDT is an effective and safe alternative treatment more commonly used for VIN that preserves normal anatomy and sexual function without risk of disease progression.[61]
Medical Oncology
Prospective trials of chemotherapy as a treatment for vulvar cancers are lacking. Recommendations are largely extrapolated from therapeutic trials for metastatic cervical or anal cancers. The agents commonly used are platinum-based chemotherapeutic agents.[57][58][59] More recent studies have investigated targeted agents. One such agent, Erlotinib, is an anti-epidermal growth factor receptor tyrosine kinase inhibitor and has been tested in vulvar SCC. A partial response was observed in 27% of patients, and an additional 40% exhibited stable disease, but the progression-free survival was poor.[62] Cemiplimab, a PD-1 blocker, has undergone a Phase-II trial in patients with locally advanced or metastatic cutaneous squamous-cell carcinoma that reported a 47% response rate, of which more than half persisted greater than 6 months, and it is currently approved to treat metastatic cutaneous vulvar SCC.[63]
Suppose surgical excision of SCC or BCC is contraindicated. In that case, an alternative treatment option proven effective in HPV-associated VIN is topical 5% imiquimod cream applied 3 times weekly for 16 weeks.[64] Treatment with topical 5-fluorouracil and photodynamic therapy may also be considered in BCC. In patients with melanoma, medical management with novel CTLA-4-, PD-1-, BRAF-, and MEK-inhibitors such as nivolumab and ipilimumab has improved overall survival. These therapies are now considered first-line therapy in patients with stage III disease, but limited data is available for the response of vulvar melanoma. One of the differences between cutaneous melanoma and vulvar melanoma is the relatively high number of receptor tyrosine kinase proteins (KIT) mutations. This creates the potential to explore tyrosine kinase inhibitors as a treatment for vulvar melanoma in future studies.[65] While one retrospective study reported a 75% response rate to topical 5% imiquimod cream for the treatment of vulvar Paget disease, the results of a prospective trial are not yet available.[66][67][66]
Staging
The International Federation of Gynecology and Obstetrics (FIGO) performs vulvar cancer staging surgically.[68] The surgical stage is related to treatment and prognosis.
Stage I Tumors confined to the vulva or perineum, no nodal metastasis.
- IA: Tumor ≤2 cm with stromal invasion ≤1 mm
- IB: Tumor >2 cm or stromal invasion >1 mm
Stage II Tumor of any size with extension to adjacent perineal structures (lower urethra, lower vagina, anus), no nodal metastasis
Stage III Tumor of any size with or without extension to adjacent perineal structures (lower urethra, lower vagina, anus), with inguinofemoral nodal metastasis
- IIIA: 1 node metastasis (≥5 mm) or 1 to 2 node metastasis(es) (<5 mm)
- IIIB: ≥2 node metastases (≥5 mm) or ≥ 3 node metastases (<5 mm
- IIIC: node metastases with extra-capsular spread
Stage IV Tumor invades other regional or distal structures.
- IVA: Tumor invades any of the following: upper urethra or vaginal mucosa, bladder mucosa, rectal mucosa, or fixed to the pelvic bone, or fixed or ulcerated inguinofemoral nodes
- IVB: Any distant metastasis, including pelvic nodes
The exception of FIGO staging of vulvar cancers is melanoma, for which staging is the same as that of cutaneous melanoma and follows the TNM staging system used by the AJCC. The Breslow, Clark, and Chung micro staging systems have been historically used to evaluate pathological characteristics, with Breslow staging affording the most accurate prediction of survival and recurrence.[26] Breslow depth is defined as tumor thickness from the top of the epidermal granular layer to the deepest invasion point.
Prognosis
In vulvar cancer, the lymphatic spread is initially to homolateral superficial inguinal lymph nodes and then to deep femoroinguinal lymph nodes. Lymph node status is the most powerful prognostic factor for overall survival in patients with vulvar cancer. Survival rates are significantly lower for patients with lymph node metastasis: 65% vs 91% at 10 years, 52.5% vs 87.5% at 5 years, and 56.2% vs 90.2% at 3 years. Progression-free survival is better in node-positive patients who receive adjuvant radiotherapy. Overall, the recurrence rate of vulvar cancer is 37% at five years. Patients diagnosed with distant metastasis have a poor prognosis.[68][69][68] Vulvar melanoma prognosis is poor, with an estimated 5-year survival of between 10% to 63%.[70] Patients with centrally-located vulva melanoma have a reduced survival rate and a shorter recurrence-free interval.[26][71][26] The average time to recurrence in vulvar melanoma is 43.5 months, with an overall 50% recurrence rate.[72]
Vulvar Paget disease has a favorable prognosis despite a frequent recurrence rate. In patients with vulvar Paget disease and an underlying adenocarcinoma, the prognosis depends on the type of and treatment outcomes for the associated adenocarcinoma. Local recurrence of vulvar sarcoma is common. The most predictive factors for recurrence are inadequate resection margins and tumor diameter greater than 5mm, infiltrating margins, and high mitotic rate. Bartholin gland carcinoma is similar to vulvar SCC because the prognosis is stage-dependent.[34] HPV status is also relevant for prognosis as HPV-positive vulvar cancers have more favorable outcomes than HPV-negative and may be exploited to allow for more conservative treatment and post-treatment follow-up.[7]][73][7] Recurrent disease confined to the vulva can be successfully treated with surgical resection. However, patients with metastatic or locally-aggressive recurrence of vulvar cancer have a 2-year overall survival of only 57% after exenterative surgery.[74]
Complications
Complications of vulvar cancer include the results of the disease process itself and the results of the treatment and management. Complications of vulvar cancer include worsening pain and irritation and declining functional status as the malignancy spreads and begins to invade locally and regionally. Complications of surgery are many and can include infection, critical blood loss, lower extremity lymphedema, significant chronic pain, as well as associated organ complications in the event of additional procedures needed to resect disease optimally. Complications of chemotherapy and radiation include susceptibility to infection, multiple gastrointestinal reactions, as well as tissue fibrosis and lymphedema.
Postoperative and Rehabilitation Care
Due to the significant rate of recurrent patients, they should be followed closely after treatment. Follow-up surveillance should be every three months for two years, every six months for 3 years, and annually afterward.[75]
Deterrence and Patient Education
Patients should be educated on the nature of the disease process and the importance of close follow-up. Patients should also be educated on the best treatment options and the associated risks with each treatment modality. Finally, after treatment, patient education should focus on the need for routine surveillance and general healthcare maintenance and follow-up.
Enhancing Healthcare Team Outcomes
A multidisciplinary approach to vulvar cancer should commence upon diagnosis with input from gynecologic oncology, medical oncology, and radiation oncology teams. An emphasis should be placed on early diagnosis and treatment, with close post-treatment surveillance. Patients should be encouraged to receive the HPV vaccine early in life to reduce their overall risk of contracting the HPV virus and thus reduce their risk of contracting HPV-dependent vulvar cancer. The HPV vaccine may also have a therapeutic role in early VIN. However, more studies are needed to investigate the potential therapeutic impact of vulvar carcinoma.[76]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Khanna N, Rauh LA, Lachiewicz MP, Horowitz IR. Margins for cervical and vulvar cancer. Journal of surgical oncology. 2016 Mar:113(3):304-9. doi: 10.1002/jso.24108. Epub 2016 Feb 8 [PubMed PMID: 26852901]
Wohlmuth C, Wohlmuth-Wieser I. Vulvar malignancies: an interdisciplinary perspective. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2019 Dec:17(12):1257-1276. doi: 10.1111/ddg.13995. Epub 2019 Dec 12 [PubMed PMID: 31829526]
Level 3 (low-level) evidenceCanavan TP, Cohen D. Vulvar cancer. American family physician. 2002 Oct 1:66(7):1269-74 [PubMed PMID: 12387439]
Boer FL, Ten Eikelder MLG, Kapiteijn EH, Creutzberg CL, Galaal K, van Poelgeest MIE. Vulvar malignant melanoma: Pathogenesis, clinical behaviour and management: Review of the literature. Cancer treatment reviews. 2019 Feb:73():91-103. doi: 10.1016/j.ctrv.2018.12.005. Epub 2018 Dec 24 [PubMed PMID: 30685613]
Weinberg D, Gomez-Martinez RA. Vulvar Cancer. Obstetrics and gynecology clinics of North America. 2019 Mar:46(1):125-135. doi: 10.1016/j.ogc.2018.09.008. Epub [PubMed PMID: 30683259]
Faber MT, Sand FL, Albieri V, Norrild B, Kjaer SK, Verdoodt F. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. International journal of cancer. 2017 Sep 15:141(6):1161-1169. doi: 10.1002/ijc.30821. Epub 2017 Jun 21 [PubMed PMID: 28577297]
Zhang J, Zhang Y, Zhang Z. Prevalence of human papillomavirus and its prognostic value in vulvar cancer: A systematic review and meta-analysis. PloS one. 2018:13(9):e0204162. doi: 10.1371/journal.pone.0204162. Epub 2018 Sep 26 [PubMed PMID: 30256833]
Level 1 (high-level) evidenceClancy AA, Spaans JN, Weberpals JI. The forgotten woman's cancer: vulvar squamous cell carcinoma (VSCC) and a targeted approach to therapy. Annals of oncology : official journal of the European Society for Medical Oncology. 2016 Sep:27(9):1696-705. doi: 10.1093/annonc/mdw242. Epub 2016 Jun 20 [PubMed PMID: 27329249]
Allbritton JI. Vulvar Neoplasms, Benign and Malignant. Obstetrics and gynecology clinics of North America. 2017 Sep:44(3):339-352. doi: 10.1016/j.ogc.2017.04.002. Epub [PubMed PMID: 28778635]
Bornstein J, Bogliatto F, Haefner HK, Stockdale CK, Preti M, Bohl TG, Reutter J, ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. Journal of lower genital tract disease. 2016 Jan:20(1):11-4. doi: 10.1097/LGT.0000000000000169. Epub [PubMed PMID: 26704327]
Tan A, Bieber AK, Stein JA, Pomeranz MK. Diagnosis and management of vulvar cancer: A review. Journal of the American Academy of Dermatology. 2019 Dec:81(6):1387-1396. doi: 10.1016/j.jaad.2019.07.055. Epub 2019 Jul 23 [PubMed PMID: 31349045]
Dasgupta S, Ewing-Graham PC, Swagemakers SMA, van der Spek PJ, van Doorn HC, Noordhoek Hegt V, Koljenović S, van Kemenade FJ. Precursor lesions of vulvar squamous cell carcinoma - histology and biomarkers: A systematic review. Critical reviews in oncology/hematology. 2020 Mar:147():102866. doi: 10.1016/j.critrevonc.2020.102866. Epub 2020 Jan 15 [PubMed PMID: 32058913]
Level 1 (high-level) evidenceMicheletti L, Preti M, Radici G, Boveri S, Di Pumpo O, Privitera SS, Ghiringhello B, Benedetto C. Vulvar Lichen Sclerosus and Neoplastic Transformation: A Retrospective Study of 976 Cases. Journal of lower genital tract disease. 2016 Apr:20(2):180-3. doi: 10.1097/LGT.0000000000000186. Epub [PubMed PMID: 26882123]
Level 2 (mid-level) evidenceSchuurman MS, van den Einden LC, Massuger LF, Kiemeney LA, van der Aa MA, de Hullu JA. Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. European journal of cancer (Oxford, England : 1990). 2013 Dec:49(18):3872-80. doi: 10.1016/j.ejca.2013.08.003. Epub 2013 Sep 3 [PubMed PMID: 24011936]
Sinha K, Abdul-Wahab A, Calonje E, Craythorne E, Lewis FM. Basal cell carcinoma of the vulva: treatment with Mohs micrographic surgery. Clinical and experimental dermatology. 2019 Aug:44(6):651-653. doi: 10.1111/ced.13881. Epub 2019 Jan 7 [PubMed PMID: 30618159]
Chanda JJ. Extramammary Paget's disease: prognosis and relationship to internal malignancy. Journal of the American Academy of Dermatology. 1985 Dec:13(6):1009-14 [PubMed PMID: 3001158]
Level 2 (mid-level) evidenceLloyd J, Flanagan AM. Mammary and extramammary Paget's disease. Journal of clinical pathology. 2000 Oct:53(10):742-9 [PubMed PMID: 11064666]
Jones IS, Crandon A, Sanday K. Paget's disease of the vulva: Diagnosis and follow-up key to management; a retrospective study of 50 cases from Queensland. Gynecologic oncology. 2011 Jul:122(1):42-4. doi: 10.1016/j.ygyno.2011.03.033. Epub 2011 Apr 17 [PubMed PMID: 21501860]
Level 2 (mid-level) evidenceParker LP, Parker JR, Bodurka-Bevers D, Deavers M, Bevers MW, Shen-Gunther J, Gershenson DM. Paget's disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecologic oncology. 2000 Apr:77(1):183-9 [PubMed PMID: 10739709]
St Claire K, Hoover A, Ashack K, Khachemoune A. Extramammary Paget disease. Dermatology online journal. 2019 Apr 15:25(4):. pii: 13030/qt7qg8g292. Epub 2019 Apr 15 [PubMed PMID: 31046904]
Cai Y, Sheng W, Xiang L, Wu X, Yang H. Primary extramammary Paget's disease of the vulva: the clinicopathological features and treatment outcomes in a series of 43 patients. Gynecologic oncology. 2013 May:129(2):412-6. doi: 10.1016/j.ygyno.2013.02.029. Epub 2013 Feb 27 [PubMed PMID: 23454498]
Konstantinova AM, Shelekhova KV, Stewart CJ, Spagnolo DV, Kutzner H, Kacerovska D, Plaza JA, Suster S, Bouda J, Pavlovsky M, Kyrpychova L, Michal M, Guenova E, Kazakov DV. Depth and Patterns of Adnexal Involvement in Primary Extramammary (Anogenital) Paget Disease: A Study of 178 Lesions From 146 Patients. The American Journal of dermatopathology. 2016 Nov:38(11):802-808 [PubMed PMID: 26863064]
Ragnarsson-Olding BK, Nilsson BR, Kanter-Lewensohn LR, Lagerlöf B, Ringborg UK. Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females: predictors of survival. Cancer. 1999 Oct 1:86(7):1285-93 [PubMed PMID: 10506715]
Rouzbahman M, Kamel-Reid S, Al Habeeb A, Butler M, Dodge J, Laframboise S, Murphy J, Rasty G, Ghazarian D. Malignant Melanoma of Vulva and Vagina: A Histomorphological Review and Mutation Analysis--A Single-Center Study. Journal of lower genital tract disease. 2015 Oct:19(4):350-3. doi: 10.1097/LGT.0000000000000142. Epub [PubMed PMID: 26225944]
Hou JY, Baptiste C, Hombalegowda RB, Tergas AI, Feldman R, Jones NL, Chatterjee-Paer S, Bus-Kwolfski A, Wright JD, Burke WM. Vulvar and vaginal melanoma: A unique subclass of mucosal melanoma based on a comprehensive molecular analysis of 51 cases compared with 2253 cases of nongynecologic melanoma. Cancer. 2017 Apr 15:123(8):1333-1344. doi: 10.1002/cncr.30473. Epub 2016 Dec 27 [PubMed PMID: 28026870]
Level 3 (low-level) evidencePhillips GL, Bundy BN, Okagaki T, Kucera PR, Stehman FB. Malignant melanoma of the vulva treated by radical hemivulvectomy. A prospective study of the Gynecologic Oncology Group. Cancer. 1994 May 15:73(10):2626-32 [PubMed PMID: 8174062]
Leitao MM Jr. Management of vulvar and vaginal melanomas: current and future strategies. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting. 2014:():e277-81. doi: 10.14694/EdBook_AM.2014.34.e277. Epub [PubMed PMID: 24857113]
Nascimento AF, Granter SR, Cviko A, Yuan L, Hecht JL, Crum CP. Vulvar acanthosis with altered differentiation: a precursor to verrucous carcinoma? The American journal of surgical pathology. 2004 May:28(5):638-43 [PubMed PMID: 15105653]
Chen H, Gonzalez JL, Brennick JB, Liu M, Yan S. Immunohistochemical patterns of ProEx C in vulvar squamous lesions: detection of overexpression of MCM2 and TOP2A. The American journal of surgical pathology. 2010 Sep:34(9):1250-7. doi: 10.1097/PAS.0b013e3181ecf829. Epub [PubMed PMID: 20697251]
Gualco M, Bonin S, Foglia G, Fulcheri E, Odicino F, Prefumo F, Stanta G, Ragni N. Morphologic and biologic studies on ten cases of verrucous carcinoma of the vulva supporting the theory of a discrete clinico-pathologic entity. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2003 May-Jun:13(3):317-24 [PubMed PMID: 12801263]
Level 2 (mid-level) evidenceAartsen EJ, Albus-Lutter CE. Vulvar sarcoma: clinical implications. European journal of obstetrics, gynecology, and reproductive biology. 1994 Sep:56(3):181-9 [PubMed PMID: 7821491]
Nguyen AH, Detty SQ, Gonzaga MI, Huerter C. Clinical Features and Treatment of Dermatofibrosarcoma Protuberans Affecting the Vulva: A Literature Review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2017 Jun:43(6):771-774. doi: 10.1097/DSS.0000000000001113. Epub [PubMed PMID: 28323651]
Iavazzo C, Gkegkes ID, Vrachnis N. Dilemmas in the management of patients with vulval epithelioid sarcoma: a literature review. European journal of obstetrics, gynecology, and reproductive biology. 2014 May:176():1-4. doi: 10.1016/j.ejogrb.2014.02.013. Epub 2014 Feb 20 [PubMed PMID: 24636595]
Bhalwal AB, Nick AM, Dos Reis R, Chen CL, Munsell MF, Ramalingam P, Salcedo MP, Ramirez PT, Sood AK, Schmeler KM. Carcinoma of the Bartholin Gland: A Review of 33 Cases. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2016 May:26(4):785-9. doi: 10.1097/IGC.0000000000000656. Epub [PubMed PMID: 26844611]
Level 2 (mid-level) evidenceNazeran T, Cheng AS, Karnezis AN, Tinker AV, Gilks CB. Bartholin Gland Carcinoma: Clinicopathologic Features, Including p16 Expression and Clinical Outcome. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2019 Mar:38(2):189-195. doi: 10.1097/PGP.0000000000000489. Epub [PubMed PMID: 29406447]
Level 2 (mid-level) evidenceEdwards L. Pigmented vulvar lesions. Dermatologic therapy. 2010 Sep-Oct:23(5):449-57. doi: 10.1111/j.1529-8019.2010.01349.x. Epub [PubMed PMID: 20868400]
Koh WJ, Greer BE, Abu-Rustum NR, Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, Dizon DS, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, Han E, Higgins S, Huh WK, Lurain JR 3rd, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Ueda S, Valea FA, Wyse E, Yashar CM, McMillian N, Scavone J. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2017 Jan:15(1):92-120 [PubMed PMID: 28040721]
Level 1 (high-level) evidenceRigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA: a cancer journal for clinicians. 2000 Jul-Aug:50(4):215-36; quiz 237-40 [PubMed PMID: 10986965]
Schmitt AR, Long BJ, Weaver AL, McGree ME, Bakkum-Gamez JN, Brewer JD, Cliby WA. Evidence-Based Screening Recommendations for Occult Cancers in the Setting of Newly Diagnosed Extramammary Paget Disease. Mayo Clinic proceedings. 2018 Jul:93(7):877-883. doi: 10.1016/j.mayocp.2018.02.024. Epub 2018 May 24 [PubMed PMID: 29804724]
Ansink A, van der Velden J. Surgical interventions for early squamous cell carcinoma of the vulva. The Cochrane database of systematic reviews. 2000:2000(2):CD002036 [PubMed PMID: 10796849]
Level 1 (high-level) evidenceMaggino T, Landoni F, Sartori E, Zola P, Gadducci A, Alessi C, Soldà M, Coscio S, Spinetti G, Maneo A, Ferrero A, Konishi De Toffoli G. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer. 2000 Jul 1:89(1):116-22 [PubMed PMID: 10897008]
Level 2 (mid-level) evidenceMcCann GA, Taege SK, Boutsicaris CE, Phillips GS, Eisenhauer EL, Fowler JM, O'Malley DM, Copeland LJ, Cohn DE, Salani R. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecologic oncology. 2013 Jan:128(1):44-48. doi: 10.1016/j.ygyno.2012.10.028. Epub 2012 Nov 5 [PubMed PMID: 23138134]
Level 2 (mid-level) evidenceAng C, Bryant A, Barton DP, Pomel C, Naik R. Exenterative surgery for recurrent gynaecological malignancies. The Cochrane database of systematic reviews. 2014 Feb 4:2014(2):CD010449. doi: 10.1002/14651858.CD010449.pub2. Epub 2014 Feb 4 [PubMed PMID: 24497188]
Level 1 (high-level) evidenceZhang W, Wang Y, Chen W, Du J, Xiang L, Ye S, Yang H. Verrucous Carcinoma of the Vulva: A Case Report and Literature Review. The American journal of case reports. 2019 Apr 19:20():551-556. doi: 10.12659/AJCR.914367. Epub 2019 Apr 19 [PubMed PMID: 31002657]
Level 3 (low-level) evidenceLong B, Schmitt AR, Weaver AL, McGree M, Bakkum-Gamez JN, Brewer J, Cliby WA. A matter of margins: Surgical and pathologic risk factors for recurrence in extramammary Paget's disease. Gynecologic oncology. 2017 Nov:147(2):358-363. doi: 10.1016/j.ygyno.2017.09.008. Epub 2017 Sep 19 [PubMed PMID: 28935274]
Stehman FB, Bundy BN, Ball H, Clarke-Pearson DL. Sites of failure and times to failure in carcinoma of the vulva treated conservatively: a Gynecologic Oncology Group study. American journal of obstetrics and gynecology. 1996 Apr:174(4):1128-32; discussion 1132-3 [PubMed PMID: 8623839]
Wills A, Obermair A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecologic oncology. 2013 Nov:131(2):467-79. doi: 10.1016/j.ygyno.2013.07.082. Epub 2013 Jul 14 [PubMed PMID: 23863358]
Lawrie TA, Patel A, Martin-Hirsch PP, Bryant A, Ratnavelu ND, Naik R, Ralte A. Sentinel node assessment for diagnosis of groin lymph node involvement in vulval cancer. The Cochrane database of systematic reviews. 2014 Jun 27:2014(6):CD010409. doi: 10.1002/14651858.CD010409.pub2. Epub 2014 Jun 27 [PubMed PMID: 24970683]
Level 1 (high-level) evidenceHuang J, Yu N, Wang X, Long X. Incidence of lower limb lymphedema after vulvar cancer: A systematic review and meta-analysis. Medicine. 2017 Nov:96(46):e8722. doi: 10.1097/MD.0000000000008722. Epub [PubMed PMID: 29145314]
Level 1 (high-level) evidenceSkanjeti A, Dhomps A, Paschetta C, Tordo J, Giammarile F. Sentinel Node Mapping in Gynecologic Cancers: A Comprehensive Review. Seminars in nuclear medicine. 2019 Nov:49(6):521-533. doi: 10.1053/j.semnuclmed.2019.06.012. Epub 2019 Jun 29 [PubMed PMID: 31630736]
de Hullu JA, van der Zee AG. Surgery and radiotherapy in vulvar cancer. Critical reviews in oncology/hematology. 2006 Oct:60(1):38-58 [PubMed PMID: 16829120]
Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, Bell MC, De Geest K, Spirtos NM, Potkul RK, Leitao MM Jr, Bakkum-Gamez JN, Rossi EC, Lentz SS, Burke JJ 2nd, Van Le L, Trimble CL. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Nov 1:30(31):3786-91. doi: 10.1200/JCO.2011.41.2528. Epub 2012 Jul 2 [PubMed PMID: 22753905]
Covens A, Vella ET, Kennedy EB, Reade CJ, Jimenez W, Le T. Sentinel lymph node biopsy in vulvar cancer: Systematic review, meta-analysis and guideline recommendations. Gynecologic oncology. 2015 May:137(2):351-61. doi: 10.1016/j.ygyno.2015.02.014. Epub 2015 Feb 20 [PubMed PMID: 25703673]
Level 1 (high-level) evidenceGill BS, Bernard ME, Lin JF, Balasubramani GK, Rajagopalan MS, Sukumvanich P, Krivak TC, Olawaiye AB, Kelley JL, Beriwal S. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A National Cancer Data Base (NCDB) analysis. Gynecologic oncology. 2015 Jun:137(3):365-72. doi: 10.1016/j.ygyno.2015.03.056. Epub 2015 Apr 11 [PubMed PMID: 25868965]
Mahner S, Jueckstock J, Hilpert F, Neuser P, Harter P, de Gregorio N, Hasenburg A, Sehouli J, Habermann A, Hillemanns P, Fuerst S, Strauss HG, Baumann K, Thiel F, Mustea A, Meier W, du Bois A, Griebel LF, Woelber L, AGO-CaRE 1 investigators. Adjuvant therapy in lymph node-positive vulvar cancer: the AGO-CaRE-1 study. Journal of the National Cancer Institute. 2015 Mar:107(3):. pii: dju426. doi: 10.1093/jnci/dju426. Epub 2015 Jan 24 [PubMed PMID: 25618900]
Level 2 (mid-level) evidenceIgnatov T, Eggemann H, Burger E, Costa SD, Ignatov A. Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. Journal of cancer research and clinical oncology. 2016 Feb:142(2):489-95 [PubMed PMID: 26498775]
Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, Lentz SS, Averette HE. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. International journal of radiation oncology, biology, physics. 2000 Nov 1:48(4):1007-13 [PubMed PMID: 11072157]
Level 1 (high-level) evidenceMoore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. International journal of radiation oncology, biology, physics. 1998 Aug 1:42(1):79-85 [PubMed PMID: 9747823]
Moore DH, Ali S, Koh WJ, Michael H, Barnes MN, McCourt CK, Homesley HD, Walker JL. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecologic oncology. 2012 Mar:124(3):529-33. doi: 10.1016/j.ygyno.2011.11.003. Epub 2011 Nov 9 [PubMed PMID: 22079361]
Tagliaferri L, Casà C, Macchia G, Pesce A, Garganese G, Gui B, Perotti G, Gentileschi S, Inzani F, Autorino R, Cammelli S, Morganti AG, Valentini V, Gambacorta MA. The Role of Radiotherapy in Extramammary Paget Disease: A Systematic Review. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2018 May:28(4):829-839. doi: 10.1097/IGC.0000000000001237. Epub [PubMed PMID: 29538255]
Level 2 (mid-level) evidenceZhang R, Wang L. Photodynamic therapy for treatment of usual-type vulvar intraepithelial neoplasia: a case report and literature review. The Journal of international medical research. 2019 Aug:47(8):4019-4026. doi: 10.1177/0300060519862940. Epub 2019 Jul 31 [PubMed PMID: 31364444]
Level 3 (low-level) evidenceHorowitz NS, Olawaiye AB, Borger DR, Growdon WB, Krasner CN, Matulonis UA, Liu JF, Lee J, Brard L, Dizon DS. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecologic oncology. 2012 Oct:127(1):141-6. doi: 10.1016/j.ygyno.2012.06.028. Epub 2012 Jun 26 [PubMed PMID: 22750258]
Level 2 (mid-level) evidenceMigden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. The New England journal of medicine. 2018 Jul 26:379(4):341-351. doi: 10.1056/NEJMoa1805131. Epub 2018 Jun 4 [PubMed PMID: 29863979]
Terlou A, van Seters M, Kleinjan A, Heijmans-Antonissen C, Santegoets LA, Beckmann I, van Beurden M, Helmerhorst TJ, Blok LJ. Imiquimod-induced clearance of HPV is associated with normalization of immune cell counts in usual type vulvar intraepithelial neoplasia. International journal of cancer. 2010 Dec 15:127(12):2831-40. doi: 10.1002/ijc.25302. Epub [PubMed PMID: 21351262]
Level 2 (mid-level) evidenceWan MT, Ming ME. Nivolumab versus ipilimumab in the treatment of advanced melanoma: a critical appraisal: ORIGINAL ARTICLE: Wolchok JD, Chiarion-Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345-56. The British journal of dermatology. 2018 Aug:179(2):296-300. doi: 10.1111/bjd.16785. Epub 2018 Jun 5 [PubMed PMID: 29766492]
van der Linden M, Meeuwis K, van Hees C, van Dorst E, Bulten J, Bosse T, IntHout J, Boll D, Slangen B, van Seters M, van Beurden M, van Poelgeest M, de Hullu J. The Paget Trial: A Multicenter, Observational Cohort Intervention Study for the Clinical Efficacy, Safety, and Immunological Response of Topical 5% Imiquimod Cream for Vulvar Paget Disease. JMIR research protocols. 2017 Sep 6:6(9):e178. doi: 10.2196/resprot.7503. Epub 2017 Sep 6 [PubMed PMID: 28877863]
Edey KA, Allan E, Murdoch JB, Cooper S, Bryant A. Interventions for the treatment of Paget's disease of the vulva. The Cochrane database of systematic reviews. 2019 Jun 5:6(6):CD009245. doi: 10.1002/14651858.CD009245.pub3. Epub 2019 Jun 5 [PubMed PMID: 31167037]
Level 1 (high-level) evidenceTe Grootenhuis NC, van der Zee AG, van Doorn HC, van der Velden J, Vergote I, Zanagnolo V, Baldwin PJ, Gaarenstroom KN, van Dorst EB, Trum JW, Slangen BF, Runnebaum IB, Tamussino K, Hermans RH, Provencher DM, de Bock GH, de Hullu JA, Oonk MH. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecologic oncology. 2016 Jan:140(1):8-14. doi: 10.1016/j.ygyno.2015.09.077. Epub 2015 Sep 30 [PubMed PMID: 26428940]
Canlorbe G, Rouzier R, Bendifallah S, Chéreau E. [Impact of sentinel node technique on the survival in patients with vulvar cancer: analysis of the Surveillance, Epidemiology, and End Results (SEER) database]. Gynecologie, obstetrique & fertilite. 2012 Nov:40(11):647-51. doi: 10.1016/j.gyobfe.2012.07.014. Epub 2012 Sep 15 [PubMed PMID: 22985904]
Level 2 (mid-level) evidenceHeinzelmann-Schwarz VA, Nixdorf S, Valadan M, Diczbalis M, Olivier J, Otton G, Fedier A, Hacker NF, Scurry JP. A clinicopathological review of 33 patients with vulvar melanoma identifies c-KIT as a prognostic marker. International journal of molecular medicine. 2014 Apr:33(4):784-94. doi: 10.3892/ijmm.2014.1659. Epub 2014 Feb 14 [PubMed PMID: 24535703]
Level 2 (mid-level) evidenceScheistrøen M, Tropé C, Koern J, Pettersen EO, Abeler VM, Kristensen GB. Malignant melanoma of the vulva. Evaluation of prognostic factors with emphasis on DNA ploidy in 75 patients. Cancer. 1995 Jan 1:75(1):72-80 [PubMed PMID: 7804980]
Level 2 (mid-level) evidenceIacoponi S, Rubio P, Garcia E, Oehler MK, Diez J, Diaz-De la Noval B, Mora P, Gardella B, Gomez I, Kotsopoulos IC, Zalewski K, Zapardiel I, VULCAN Study collaborative group. Prognostic Factors of Recurrence and Survival in Vulvar Melanoma: Subgroup Analysis of the VULvar CANcer Study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2016 Sep:26(7):1307-12. doi: 10.1097/IGC.0000000000000768. Epub [PubMed PMID: 27465889]
Level 2 (mid-level) evidenceRasmussen CL, Sand FL, Hoffmann Frederiksen M, Kaae Andersen K, Kjaer SK. Does HPV status influence survival after vulvar cancer? International journal of cancer. 2018 Mar 15:142(6):1158-1165. doi: 10.1002/ijc.31139. Epub 2017 Nov 16 [PubMed PMID: 29090456]
Nooij LS, Brand FA, Gaarenstroom KN, Creutzberg CL, de Hullu JA, van Poelgeest MI. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Critical reviews in oncology/hematology. 2016 Oct:106():1-13. doi: 10.1016/j.critrevonc.2016.07.007. Epub 2016 Jul 25 [PubMed PMID: 27637349]
Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecologic oncology. 2017 Jul:146(1):3-10. doi: 10.1016/j.ygyno.2017.03.022. Epub 2017 Mar 31 [PubMed PMID: 28372871]
Bryan S, Barbara C, Thomas J, Olaitan A. HPV vaccine in the treatment of usual type vulval and vaginal intraepithelial neoplasia: a systematic review. BMC women's health. 2019 Jan 7:19(1):3. doi: 10.1186/s12905-018-0707-9. Epub 2019 Jan 7 [PubMed PMID: 30616555]
Level 1 (high-level) evidence