Introduction
The vitreous humor is a transparent, colorless, jelly-like, hydrophilic gel, which helps in maintaining the transparency and structure of the eye. Its volume in an adult eye is around 4ml, which is nearly 80% of the globe. It is composed of 98 to 99% water, and the rest is collagen, hyaluronic acid, and electrolytes.
Any blood in the vitreous cavity is known as vitreous hemorrhage (VH). By definition, it is the presence of extravasated blood within a space lined by posterior lens capsule anteriorly, internal limiting membrane (ILM) posteriorly, and non-pigmented epithelium of ciliary body laterally.[1]
Anatomically, it can be present in the following spaces:
- Preretinal
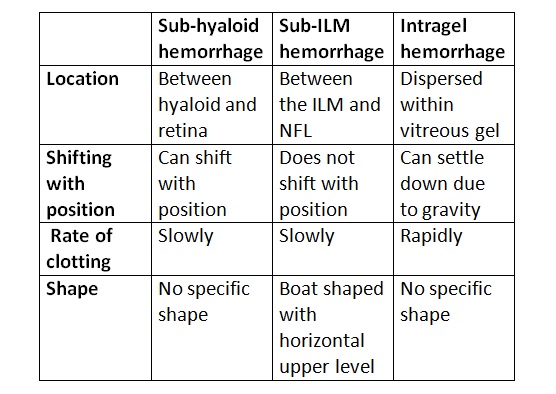
- Subhyaloid hemorrhage - The blood may lie between nondetached hyaloid and ILM. It occurs in a boat shape and is usually immobile. It is usually seen in proliferative diabetic retinopathy (PDR). The hemorrhage may occupy the space between extensively detached hyaloid and ILM also. It contains altered blood and can shift with the position.
- Sub-ILM hemorrhage - The hemorrhage lies between the nerve fiber layer of the retina and ILM. As clinical differentiation of sub-ILM hemorrhage and subhyaloid hemorrhage may not be possible in some cases, sub-ILM bleeding is also considered to be a type of vitreous hemorrhage.[1] Sub-ILM hemorrhage has a boat-shaped appearance with a horizontal upper level. It does not shift with the position. It is seen in Terson syndrome, retinal macroaneurysm (RAM), and Valsalva retinopathy.
- Intravitreal or intragel
- The blood is dispersed in the gel. It can settle down due to gravity and clots rapidly. The color of the blood may vary from red to yellow, depending on the extent of the degeneration of red blood cells (RBCs).
There are spaces filled with aqueous humor that lies anterior to the border of formed vitreous.[1]
- The canal of Hannover lies between orbiculo-anterocapsular and orbiculo-posterocapsular parts of zonular fibers.
- Berger's canal (retrolental space of Erggelet) and canal of Petit are located between the anterior hyaloid membrane and posterior capsular surface and orbiculo-posterocapsular part of zonular fibers. They are separated from each other by Wieger's ligament.
- Cloquet's canal and bursa premacularis are fluid-filled spaces within formed vitreous.
Hemorrhage into Berger's space, the canal of Petit, and Cloquet's canal have also been included in vitreous hemorrhage.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The mechanisms responsible for the pathogenesis of VH include retinal vascular disorders with or without associated ischemia, breakthrough VH, or rupture of blood vessels. Various causes include:
Proliferative Vascular Retinopathy
- The bleeding occurs from neovascularization due to retinal ischemia causing elevated angiogenic factors. Causes include proliferative diabetic retinopathy (PDR), retinal vein occlusion (RVO), vasculitis (Eales disease, and others), pars planitis, sickle cell retinopathy, and retinopathy of prematurity. Proliferative diabetic retinopathy is the most common cause of bilateral VH.[2]
Neovascular Age-related Macular Degeneration (AMD)
Idiopathic Polypoidal Choroidal Vasculopathy (IPCV)
In these cases, usually, the blood remains subretinal, but when it becomes massive, breakthrough VH can be seen breaking through the full thickness retina.
Trauma
It is the most common cause of VH in patients less than 40 years of age, more so in males. Associated serous or hemorrhagic choroidal detachments can be there.[2]
- Blunt trauma: It can often lead to occult globe rupture, at the site of which vitreous incarceration may be noted. VH, in such cases, can be associated with retinal dialysis, choroidal rupture, Berlin’s edema, optic nerve avulsion, and vitreous base avulsion.
- Penetrating, perforating trauma, and retained intraocular foreign body (RIOFB): Vitreous incarceration can be seen at the site of entry/exit over the sclera.
Intracranial Hemorrhage (Terson Syndrome)
Litten defined it as any intraocular hemorrhage occurring with subarachnoid hemorrhage (usually associated with an aneurysm), which later was found to be associated with any form of intracranial hemorrhage by Terson.[3] However, recently, it has been described as any intraocular hemorrhage associated with subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral brain injury.[4] According to a recent study, it occurred in 19.3% of patients with SAH, 9.1% of patients with intracerebral hemorrhage, and 3.1% of patients with traumatic brain injury.[4]
- It can be unilateral or bilateral. The intraocular hemorrhage can be in the form of VH, subhyaloid hemorrhage, intraretinal, and sub-ILM bleed. Dome-shaped hemorrhages, also known as a hemorrhagic macular cyst, can be seen over the macula. The macular double ring sign may be seen, which is formed by subhyaloid membrane on outside and sub-ILM bleed inside.[5]
- The pathogenesis of the intraocular hemorrhage is controversial. Some suggest it is the sub-arachnoid blood that comes into vitreous via optic nerve sheath.[4]The another concept is that of increased intracranial pressure causing an increase in optic nerve sheath pressure, which compresses the central retinal vein and increases its pressure leading to hemorrhages.[6]
Retinal Arterial Macroaneurysm (RAM)
Bleeding can be subretinal, intra-retinal, sub ILM, and intra-vitreal.
Posterior Vitreous Detachment (PVD)
VH can occur due to the avulsion of superficial or peripapillary retinal vessels or from the rupture of vessel/s bridging a tear. In cases with acute symptomatic PVD, if simultaneous VH is present, the risk of retinal tear increases by up to 70%.[7] Associated retinal detachment may be present.
Tumors
Rarely tumors may present with VH. Choroidal melanoma (after undergoing necrosis), vasoproliferative tumor, retinal cavernous hemangioma, and rarely choroidal metastasis may all present with VH. Careful ultrasonography may be helpful in such cases.
Blood dyscrasias
Leukemia, anemia, thrombocytopenia, hemophilia may all present with VH. Fever or other diseases associated with very low hemoglobin and thrombocyte counts may also be associated with multiple intraretinal, preretinal hemorrhages, and vitreous hemorrhages.
Valsalva retinopathy
Sudden elevation in intrathoracic or intraabdominal pressure against a closed glottis is known as the Valsalva maneuver.[8] Activities like heavy lifting, coughing, vomiting, straining may elicit it. Classically, it forms round or oval sub-ILM hemorrhage at or near the macula.[9]
Complications of Surgeries
These include peripheral iridectomy, buckle intrusion, high-intensity retinal laser burn, trabeculectomy, secondary intraocular lens implantation, and others.
Shaken Baby Syndrome
Most commonly noted lesions are retinal hemorrhages, cotton wool spots, premacular retinal folds, and VH. Retinal hemorrhages are whiplash induced.
- Wang et al. found out that of all the cases of VH, 43.3% were due to PDR, 11.4 % due to retinal detachment, 3.06% due to retinal vein occlusion, and 2.44% were due to AMD.[10]
- The role of anticoagulants in VH has been controversial, however various studies have shown that the risk of VH is not high in patients on anticoagulants. The landmark trial of Early Treatment Diabetic Retinopathy Study, found that use of aspirin did not increase the risk of VH in diabetic retinopathy cases.[11]
Epidemiology
The incidence of spontaneous vitreous hemorrhage as per Lindgren et al. is 7 cases per 1,00,000 population each year.[12] However, in the Chinese population, Wang et al. found its incidence to be much higher at 4.8 cases per 10,000 person-years.[10] The incidence was found to be more with age (mainly 40 to 59 years), male gender, and use of anticoagulants.
Pathophysiology
Catabolism of Blood in General
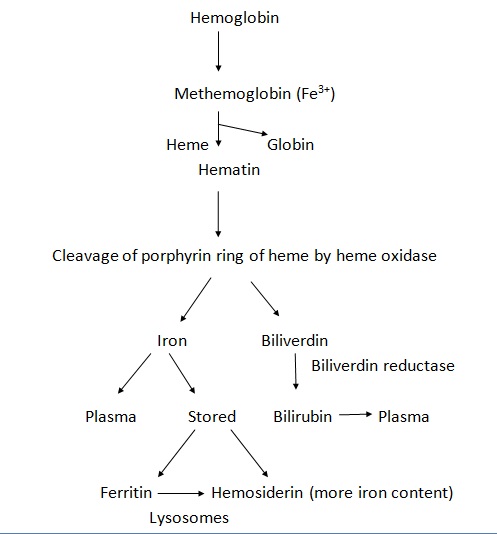
The extravasated blood in any tissue induces an inflammatory reaction, the extent of which in turn depends upon the amount of blood. Initially, polymorphonuclear neutrophils (PMN) come into the picture, which dissolute fibrin.[13] After 2 days, macrophages come and phagocytose red blood cells and cellular debris. Within 2 weeks, granulation tissue forms and hemorrhage resorbs and scar tissue is formed. These hemosiderin-laden macrophages can stay for months in scar tissue, giving it a golden yellow color.
The hemoglobin released from RBCs is first converted to methemoglobin (Fe3+) within mononuclear phagocytic cells, which is later cleaved into iron and biliverdin. The iron is either released into plasma or stored as ferritin or hemosiderin (more iron content than ferritin). Histologically, the hemosiderin can be stained blue with a Prussian blue reaction (Perl's reaction). The biliverdin (green), on the other hand, is converted into bilirubin (yellow) and released into the plasma. Hence over a period of time, the color of hemorrhage may vary from red-green to yellow.
The Course of VH and Pathophysiology of Blood Catabolism
Blood in the vitreous cavity behaves differently than when in other tissues
- Clot formation is rapid with sharp borders, as collagen in vitreous promotes platelet aggregation.
- Fibrin lysis is slower, due to a lack of early polymorphonuclear leukocyte response.
- RBCs are lysed extra-cellularly, unlike elsewhere, where lysis occurs after ingestion by macrophages.
- RBCs remain intact for months in the vitreous gel.
Iron in the blood promotes vitreous liquefaction.[14]
Hemorrhage into spaces outside formed vitreous or hemorrhage in vitrectomised eye clears faster than in the formed vitreous.[15] However, vitreous, subhyaloid, and sub-ILM hemorrhages also undergo similar changes in color with time. Fresh hemorrhage appears bright red, which becomes yellow to white with time.
Histopathology
In an experimental vitreous hemorrhage, induced by Forrester et al. by injecting 0.2 ml of whole blood in the rabbit eye, within 24 hours, a clotted mass of RBCs with fibrin formed pocket within the vitreous, displacing normal collagen fibrils.[16] Only a few PMNs, mononuclear inflammatory cells were present at this stage.
- On the second day, cellular response began.
- By 5th day, macrophages had come in the picture. Collagen formed an outer layer around the clot.
- By day 6, considerable vitreous liquefaction occurred.
- After 1 week, RBCs were released from the clot for phagocytosis.
- After 2 weeks, there was a surge in the number of macrophages, multinucleated giant cells.
- The lysis of these cells occurred within vitreous after 3 weeks, which was followed by another wave of mononuclear cells.[16]
- After 5 weeks up to 2 months, RBCs, its debris, fibrin, and recently migrating macrophages decreased, whereas older macrophages and collagen pseudocapsule remained. Iron staining in Perl’s reaction was pronounced.[16]
- After 5 months, much of RBC debris are removed, and only a few intact RBCs are present.
- Eighteen months later, there are hardly any RBCs left, with fine vitreous strands, large inactive macrophages with young mononuclear cells suggesting low-grade inflammation.[17]
Vitreous liquefaction occurs due to iron content in the blood. Iron generates hydroxyl radical, leading to depolymerization of hyaluronic acid.
Infrequent sequelae following intraocular hemorrhage include cholesterolosis bulbi. It may be present in the anterior or posterior chamber. Cholesterol particles formed as a result of the breakdown of RBCs, when present in the vitreous cavity is known as synchysis scintillans. These cholesterol crystals are usually found in subretinal space in long-standing retinal detachment in severely damaged eyes. The crystals in such cases deposit inferiorly at rest and can involve the whole vitreous with ocular movements (unlike asteroid hyalosis which remains suspended in the vitreous fibrils and do not deposit or gravitate). Synchysis scintillans/cholesterolosis bulbi is often associated with very poor vision or end-stage eye disease.
Accumulation of RBC and its debris, which are suspended in vitreous collagen, also form the so-called, Ochre membrane.
Hemoglobin spherulosis was noted in vitreous in some unusual cases with subretinal hemorrhage by Grossniklaus et al. [18] The free hemoglobin formed numerous slightly refractile brownish spherules in vitreous. Histologic examination showed eosinophilic spherules of 10 to 20 micrometers in size, associated with intact erythrocytes and macrophages, some of which had phagocytosed these spherules.
Similarly, detached vitreous lamellae sometimes condense and curl and form vitreous cylinders.[19] The extravasated blood causes disorganization of normal vitreous lamellae, leading to its detachment. These detached vitreous lamellae may form cylindroid structures composed of loose collagen fibers and spindle-shaped cells. The nodules noted over the surface of cylinders or detached retina are composed of lymphocytes and macrophages.
In the studies conducted on human eyes, Hogan found that blood clots were surrounded by a single or laminated endothelial membrane layer derived from the retina. Forrester et al. on experiments on enucleated eye found that all stages of extracellular hemolysis, including intact RBCs to ghost cells, were present by the 9th day.[17] By day 60, a considerable amount of RBC debris with old macrophages was present on the detached posterior hyaloid face, and no fibrin.
In long-standing recurrent hemorrhages, both extracellular and intracellular hemolysis with moderately sized macrophages is seen. In recurrent and fresh hemorrhages, young active macrophages are seen. Two different types of red blood cell lysis are seen, with granular type occurring in giant cells, and hemolytic in young macrophages.[17]
History and Physical
A patient with vitreous hemorrhage may present with complaints of floaters, haziness, perception of shadows, or cobwebs. It is usually painless. Visual acuity may be affected variably depending upon the amount of blood in the visual axis. Only a 12.5 microliters of diffuse blood in 5 ml aphakic eye and 10 microliters in 4 ml phakic eye can lead to a drop in visual acuity to hand motions.[20] If there are flashes, a posterior vitreous detachment, retinal detachment or retinal tear may be present.
The best-corrected visual acuity should be recorded. A relative afferent pupillary defect should be ruled out, the presence of which may be noted in cases with retinal detachment, macular disorder or optic nerve disease. On slit-lamp examination, the anterior segment may show neovascularization of iris and angle. Any keratic precipitates, flare, cells may suggest an inflammatory etiology. RBCs may be seen in the anterior vitreous face. Intraocular pressure must be recorded.
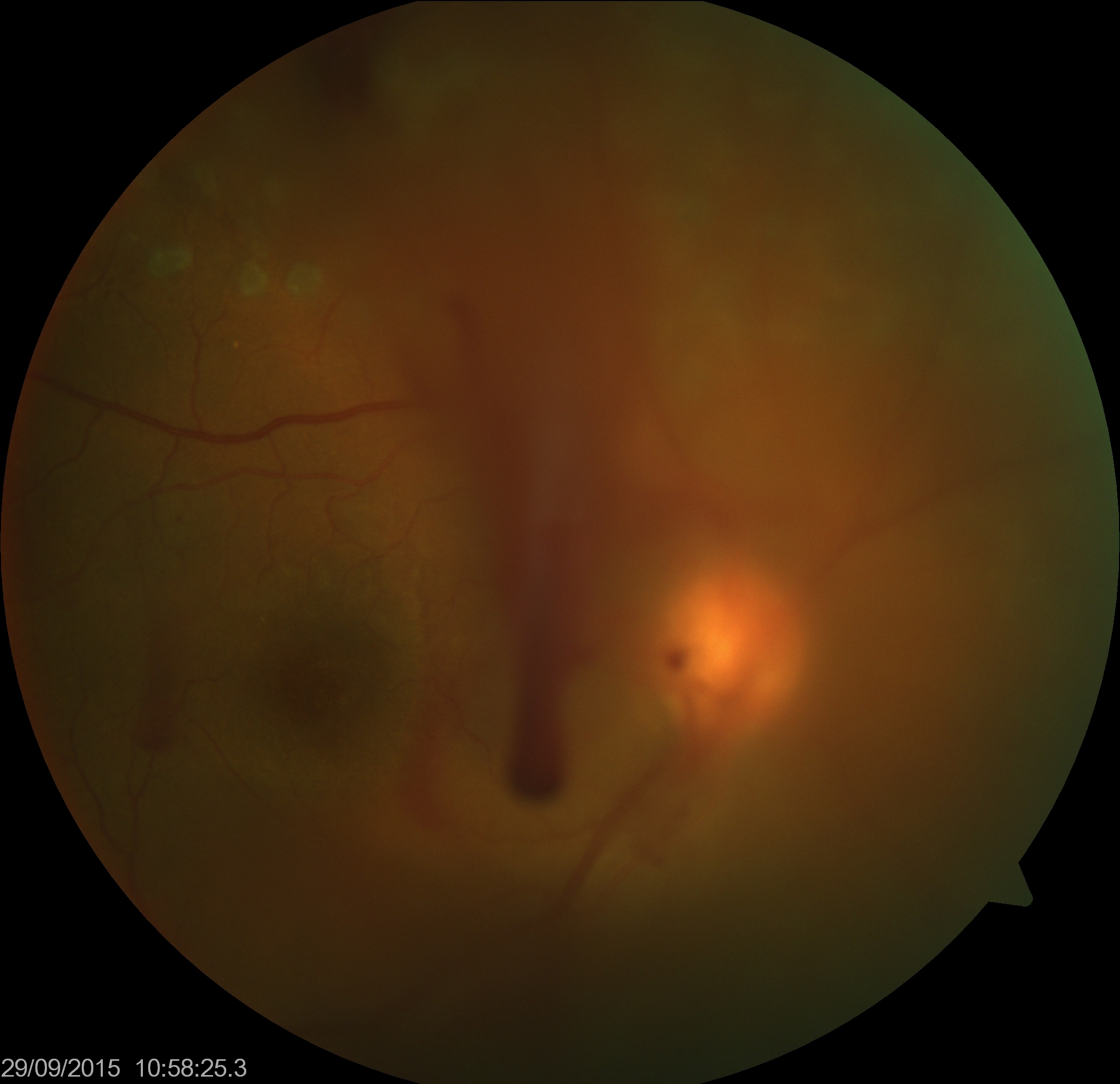
Vitreous hemorrhage is seen as blood floating in vitreous, occluding the view of retina variably. The typical boat shape of subhyaloid hemorrhage may be noted. In cases where retinal tear or detachment is suspected, thorough retinal evaluation with scleral depression is necessary. A thorough examination of the fellow eye is recommended as it may show diabetic retinopathy, retinal detachment, vasculitis, retinoschisis, or other features. Meticulous search should be done for the source of blood from abnormal blood vessels (retinal or optic disc neovascularization, or breakthrough hemorrhages from large choroidal neovascular membrane) or normal blood vessels (avulsion of the vessel due to vitreous traction, trauma, and other reasons).
History of systemic diseases like hypertension, diabetes mellitus, family history of any blood dyscrasias should be elicited. History of ocular trauma, violent cough, heavy weight lifting, previous ocular surgery should be asked.
Evaluation
Laboratory Investigations
Laboratory investigations include blood sugar, complete hemogram, complete blood count, bleeding and clotting time, platelet count, and peripheral blood smear.
Ocular Ultrasound
In cases where the posterior segment is not visible due to excessive vitreous hemorrhage, a B scan (brightness scan) with A-scan (amplitude scan) is done to find out the etiology as well as the characteristics of hemorrhage. Various characteristics of ultrasound have been described :
- Fresh VH / VH in vitrectomised eye: Multiple echolucent or low reflective dot-like echoes (RBCs) are seen, which may be better visualized in high gain. In a vitrectomized eye, the echoes may be very mobile in the vitreous cavity with the movement of the eye.
- Long-standing VH: Dot like echoes form a highly reflective membrane over a period of time, denser inferiorly. This must be differentiated from retinal detachment.
- Subhyaloid hemorrhage: It is seen as multiple dot-like echoes behind a mobile (with ocular movements) and thin membrane (posterior vitreous detachment, PVD).
- Other pathologies that may be detected using ocular ultrasound include underlying tractional or rhegmatogenous retinal detachment, retinal tear, mound in macula involving choroid suggestive of polypoidal choroidal vasculopathy, mass such as malignant melanoma, and intraocular foreign body.
- An area of vitreous attachment to the optic disc or peripheral retinal may denote the presence of neovascularization at the location of the vitreoretinal/vitreopapillary adhesion.
Optical Coherence Tomography (OCT)
This is important, especially in cases with premacular hemorrhage, to differentiate between subhyaloid or sub ILM bleed while planning treatment. [21]
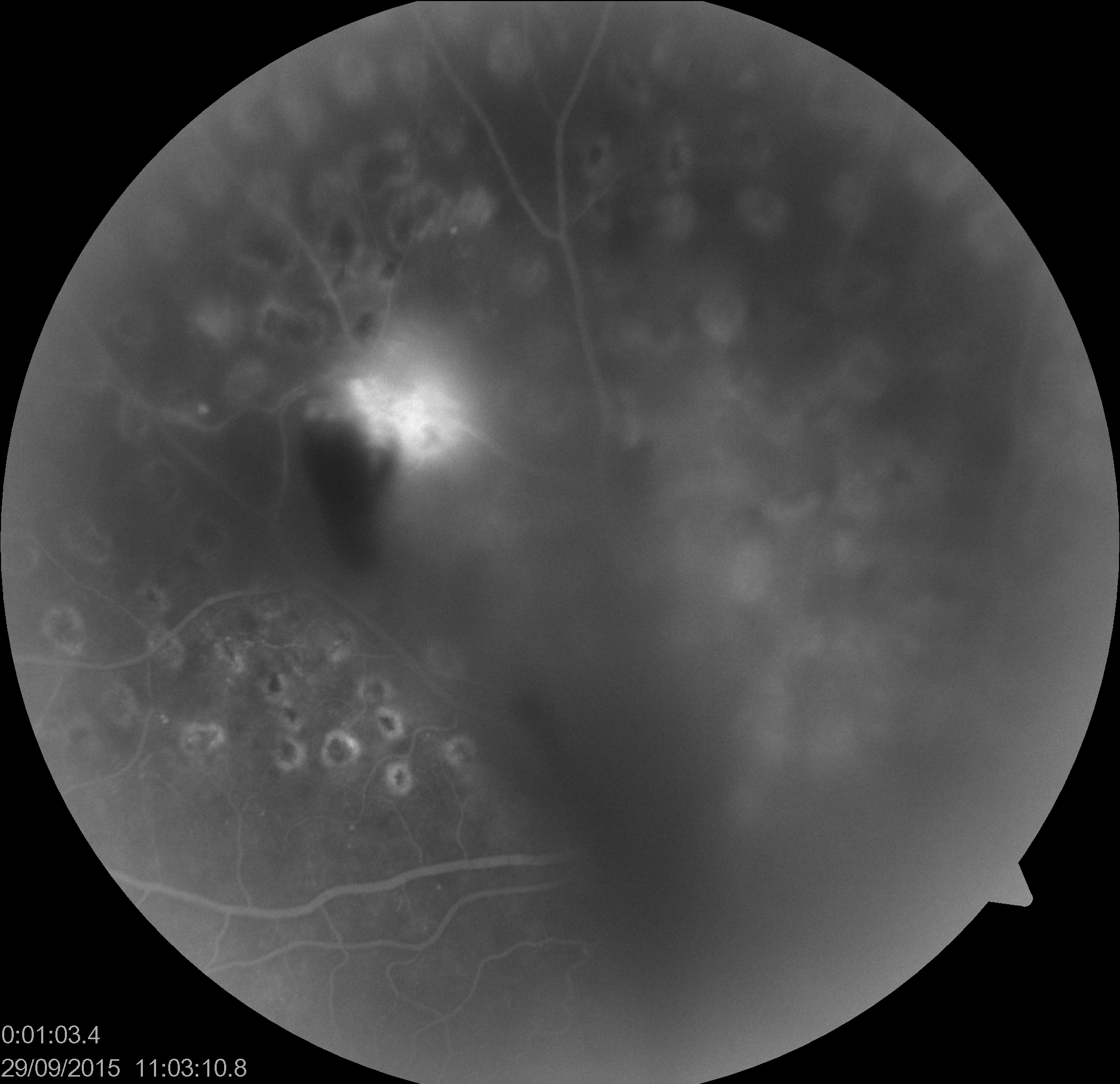
Fundus Fluorescein Angiography (FFA)/ Indocyanine Green Angiography (ICGA)
In cases where the view is there, with mild to moderate VH, FFA helps in localizing areas with capillary non-perfusion and neovascularisation, to plan laser therapy. ICGA may be helpful in cases with suspected IPCV/ AMD. ICGA has the advantage of better clarity in VH due to the longer wavelength used compared to FFA.
Neuroimaging
CT and MRI of the brain and orbit may be needed in cases with Terson syndrome to rule out intracranial bleeding. CT orbit may be necessary in cases with open globe injury to rule out any orbital fracture, intraocular foreign body, to assess the integrity of the ocular wall.
10 Hz Flash VEP / Bright Flash ERG
This is rarely done. It helps to predict the visual potential in cases with dense vitreous hemorrhages.
Treatment / Management
After establishing the cause of vitreous hemorrhage, the management is individually tailored.
Conservative
In cases with unknown causes and fresh VH with an attached retina, one can wait and advise the patient to maintain a head-end elevated position for the blood to settle inferiorly. We can re-evaluate after 3 to 7 days, to look for the cause with a clearer media. In cases where an etiology is known, like in post-laser, recurrent VH post vitrectomy, Terson syndrome, and an attached retina, one can observe for 2 to 4 weeks for resolution of VH and also monitor for any retinal detachment. Patients with active new vessels may be advised to avoid heavy works and exercise.
Photocoagulation
Laser photocoagulation is planned for proliferative retinopathies and should begin as soon as any retina is visible. Both indirect and slit lamp delivery systems can be used depending upon the clarity of the media.
Panretinal photocoagulation (PRP) is the mainstay treatment in PDR. It reduces the release of angiogenic factors (vascular endothelial growth factor/VEGF, platelet-derived growth factor/PDGF) by converting ischemic retina into anoxic.
The landmark Diabetic Retinopathy Study (DRS) found that in PDR cases, the risk of severe vision loss was 15.9% in control eyes, whereas it was 6.4% in eyes in which PRP was done. It concluded that PRP significantly reduced the chances of severe vision loss in PDR, mainly high-risk cases.[22] The DRS high-risk criteria included:[22](A1)
- NVD (neovascularization of the optic disc) greater than one-third to one-fourth of disc area even without vitreous hemorrhage (VH) or preretinal hemorrhage (PRH)
- Any NVD with VH or PRH
- Neovascularization elsewhere (NVE) greater than half of a disc area with VH or PRH
For severe NPDR or PDR without high-risk features, the study was inconclusive whether observation with close follow up or early treatment was better.[22](A1)
The Early Treatment Diabetic Retinopathy Study (ETDRS) suggested that in older patients with Type 2 DM with severe NPDR or early PDR, a prompt laser was better than deferral when compared to young patients with type 1 diabetes.[23](A1)
The Central Vein Occlusion Study (CVOS) recommended that scatter PRP has to be done in cases with CRVO only after the development of neovascularization of iris/angle and not prophylactically.[24] According to Branched Retinal Vein Occlusion Study (BVOS), scatter retinal photocoagulation has to be done only after the development of neovascularization, as 60% of cases may never develop neovascularisation and VH.[25](A1)
In cases with retinal breaks, a barrage laser is planned.[26]
Cryotherapy and Diathermy
Peripheral retinal cryotherapy may be done to ablate peripheral ischemic retina. After 360 degrees conjunctival peritomy, the four recti muscles are bridled. Under direct visualization of the retina with an indirect ophthalmoscope, the cryoprobe is kept at the level of insertions of recti. The endpoint is the direct visualization of retinal whitening. 2 to 3 such rows of cryo are applied posteriorly, one spot size apart, and are repeated 360 degrees. At the end of the procedure, bridle sutures are removed, and conjunctiva sutured.[27]
It disrupts the blood-retinal barrier and increases the clearance of blood and blood products. However, it causes a lot of inflammation and hence, is avoided in cases with a tractional component and in eyes where the laser has not been tried. Post vitrectomy recurrent hemorrhage from sclerotomy sites and anterior hyaloid proliferation remain the best indication for anterior retinal cryotherapy. [28] However, most vitreoretinal surgeons prefer peripheral laser photocoagulation to extensive 360-degree cryo with a curved endolaser probe due to the severity of postoperative inflammation with extensive cryotherapy. However, the port sites may be cryoed to prevent port-site neovascularization or anterior hyaloidal proliferation.
Diathermy has more side effects and is more difficult to perform than cryotherapy. Hence, it is obsolete these days.
Posterior Hyaloidotomy
For the rapid resolution of premacular subhyaloid hemorrhage, which can cause a sudden profound drop in vision, Nd: YAG hyaloidotomy has been tried. The etiologies mostly include Valsalva retinopathy, retinal macroaneurysm, retinal vein occlusion, and proliferative diabetic retinopathy. The hyaloidotomy is performed with the help of a Q switched Nd:YAG laser 1064 nm (photo-disruptive laser) with the help of Goldmann-Bussaca/area centralis lens. The laser beam is focused on the anterior surface of premacular hemorrhage at its inferior margin away from the fovea and retinal vessels. There should be an adequate separation of the posterior hyaloid face and underlying retina at the area of laser to prevent iatrogenic damage to the retina. This creates an opening over the surface, causing the rapid streaming of blood into the vitreous cavity. If only a little blood is released after the first shot, 2 to 3 more shots can be applied adjacent to it. The energy may vary from 2.1 to 11.5 mJ.[29] It causes the rapid dissolution of subhyaloid hemorrhage into the vitreous gel.[30] The patient may report improved vision but more floaters due to clearance of the blood in front of the fovea in subhyaloid space and dispersion of the blood in the vitreous cavity. The floaters usually reduce with time.(B3)
Pars Plana Vitrectomy (PPV)
Vitreous Haemorrhage associated with retinal detachment (RD), endophthalmitis, long-standing hemorrhage, open globe injury, VH due to neovascular AMD/IPCV, one-eyed patients, bilateral cases, secondary glaucoma (ghost cell, hemolytic, hemosiderotic glaucoma), children who are at risk of developing amblyopia are indications for surgery.[26][31] In eyes with Terson syndrome, post-cataract surgery VH, closed globe injuries, VH due to blood dyscrasias, one can wait to defer the surgery till a good PVD occurs.[26] In the setting of current advances and improved safety/efficacy of vitreoretinal surgery, the surgeon may plan early surgery depending on the visual needs of the patient.(B3)
In Proliferative diabetic retinopathy, vitrectomy, along with pan-retinal photocoagulation, helps in halting neovascularisation as they have no scaffold to grow. The Diabetic Vitrectomy study concluded that early vitrectomy was helpful in Type 1 DM (diabetes mellitus) patients with severe vision loss and non-clearing VH of at least 1 month.[32] Traction not involving macula can be observed unless there is severe proliferation. Early vitrectomy is also recommended for patients with good vision and advanced PDR.(A1)
In retinal vein occlusions, vitreous hemorrhage is more common in BRVO than CRVO. In cases where VH does not resolve within 1 to 3 months of follow up, vitrectomy with sectoral photocoagulation is helpful.
Anti VEGF
Many surgeons use anti-VEGF agents preoperatively (1 to 7 days prior) before vitrectomy to reduce the risk of an intraoperative and postoperative bleed and to help in intraoperative tissue dissection in proliferative retinopathy cases. It is also indicated in cases with VH due to nAMD/IPCV.
In cases with no view due to VH, where PRP is not possible, anti-VEGF injections have been tried to induce regression of neovascularization and reduce the risk of a fresh bleed.[33] With this, the VH may clear up faster, and chances of undergoing PPV may reduce by 30%.[34] The Protocol S (DRCR.net) has suggested in its 2 years follow up report, that visual acuity in PDR eyes treated with anti-VEGF (Ranibizumab) with deferred PRP (panretinal photocoagulation) was non-inferior to the ones promptly treated with PRP.[35]
In cases with neovascularization of iris/ angle with or without glaucoma, where PRP is difficult due to poor dilatation of the pupil, anti-VEGF injections may be used.
Experimental Treatment Strategies
A variety of treatments in the form of intravitreal injections of streptokinase, streptodornase, tissue plasminogen activator, gas, urokinase, induction of hemolysis by ultrasound has been tried. However, none of the methods has been used in clinical practice.[36] The role of oral vitamin C, oral serratiopeptidase, oral carbazochrome, intravitreal Vitrase (hyaluronidase), and oral iodized lecithin tablets in vitreous hemorrhage needs remains debatable and needs further study.(B3)
Management of the Cause and Systemic Diseases
Optimal control of systemic diseases and morbidities associated with VH needs to be achieved. Patients with active inflammation (retinal vasculitis/uveitis) with vitreous hemorrhage may need systemic, periocular, or intraocular steroids.
Differential Diagnosis
Vitritis: can be due to inflammation or infection.
- The patient usually has redness and pain in the eye.
- Slit-lamp examination shows signs of ocular inflammation, including keratic precipitates, anterior chamber cells, and flare, posterior synechia, complicated cataract, and anterior vitreous cells.
- The fundus may show area of retinitis or choroiditis or vascultiis.
- Low reflective dot or cobweb-like vitreous echoes may be noted on ocular ultrasound. In severe cases, thick, highly reflective membranes may also be seen.
- Other signs of inflammation like Tenon's space widening, thickening of ocular coats, choroidal detachment, exudative RD, optic disc elevation, may be present on B scan.
- It is seen in panuveitis, endophthalmitis, retino-choroiditis.
Primary intraocular lymphoma: This may masquerade as chronic vitreous hemorrhage.
Asteroid hyalosis: It mimics VH on ultrasound. It appears as multiple discrete, mobile, highly reflective echoes. An echolucent gap is seen between echoes and posterior globe wall. Patients usually do not have any complaints.
Retinal Detachment (RD): It may mimic blood lined PVD (posterior vitreous detachment) on B scan. RD is uniformly high reflective, of even thickness, and has restricted after movements. Blood lined PVD may show moderate to high reflectivity, is of uneven thickness, and is usually highly mobile.
Prognosis
The clearance of blood from vitreous occurs slowly, at the speed of 1% per day, especially in the formed vitreous.[37] The natural history and prognosis of a case with vitreous hemorrhage depend upon the underlying pathology.
- The diseases which do not cause recurrent bleeding like avulsion of a blood vessel due to posterior vitreous detachment or retina tear, usually have a good prognosis.[1]
- In diabetic retinopathy cases with vitreous hemorrhage, the prognosis is worse. Various studies have been conducted to explore the visual acuity improvement and worsening. In one such study, improvement in vision was noted in 30% cases, 28% remained unchanged, and it worsened in 42% cases.[38] 71% of eyes had visual acuity 5/200 or less, and the cause for this poor vision included the presence of persistent/ recurrent bleed, tractional retinal detachment, pre-retinal fibrosis, and neovascular glaucoma.[38]
- In retinal vein occlusions, central retinal vein occlusion has worst, branched retinal vein occlusion has best, and hemiretinal vein occlusion is somewhere in between in terms of prognosis.[1][39] Recurrent hemorrhages were noted in 28% of cases with branched retinal vein occlusion.[40]
- The proliferative sickle cell retinopathy cases have a better prognosis than diabetic retinopathy in terms of recurrent bleeding and vision loss, rarely causing permanent visual loss.[41]
- In Terson syndrome patients, the visual acuity was found to be better than 20/50 in 75% cases at the end of 3 years.[42]
- The prognosis in eyes with vitreous hemorrhage due to age-related macular degeneration is usually bad, with visual acuity rarely better than hand motions.[1][43] The reason for such a bad visual prognosis is a large subretinal hemorrhage at the macula.
- The cases with retinal macroaneurysm usually have a good prognosis in terms of visual acuity and rebleed.
Complications
Hemosiderosis Bulbi
It is very similar to siderosis bulbi due to an intraocular foreign body. Fe3+ is released from the catabolism of hemoglobin in RBCs. It takes place intracellularly in macrophages and is stored as ferritin and hemosiderin, which is later lysed and released into vitreous.[44] In vitreous, extracellular lysis occurs, and iron binds to iron-binding proteins- lactoferrin and transferrin.[44] Because of the slow hemolysis of RBCs and intact RBCs, the amount of hemoglobin iron released is a small fraction of what is available at a given time. [44] The lactoferrin and transferrin in vitreous can bind with this iron, making the incidence of hemosiderosis very low.[44]
Iron irreversibly binds to various ocular structures especially the ones derived from the outer layer of the optic cup–retinal pigment epithelium, lens epithelium, ciliary body, iris dilator and sphincter muscles, perivascular structures of the retina, optic nerve, and trabecular meshwork.[45] Retinal changes in hemosiderosis can be seen as the initial decreased amplitude of b wave in ERG. Followed by, peripheral vision loss, night blindness, loss of macular function, xanthopsia, and finally, extinction of ERG.[46]
Glial and Fibrivascular Proliferation
In animal experiments, it was found that intravitreal injection of blood, blood products or iron, led to a glial and fibrovascular proliferation in the vitreous and retinal surface. Its partly explained by induction of liquefaction and syneresis of blood in vitreous, leading to traction.[47] However, the VH occurring in normal human eyes is rarely followed by pre-retinal membranes, except Terson syndrome.[1] In Terson syndrome, the reason may be the damage to innermost retinal layers including ILM, leading to exuberant wound healing due to the presence of blood resorbing macrophages. [1]
Patients with a posterior penetrating ocular injury with vitreous hemorrhage are more likely to develop a tractional retinal detachment. In experimental rabbit eyes, it was found that penetrating injury leads to a breach in blood-retinal barrier leading to choroidal, scleral fibrocytes with retinal pigment epithelial cells gaining access into the vitreous cavity and leading to fibro cellular proliferation and secondary retinal detachments.[48]
Glaucoma
- Ghost Cell Glaucoma
This occurs only after VH. The RBCs lose their pliability and biconcavity over a period of 1 to 3 weeks to degenerate into ghost cells, which are spherical, smaller, khaki-colored, and rigid. These cells contain denatured hemoglobin attached to its membrane know as Heinz bodies.
These ghost cells reach the anterior chamber whenever there is a breach in the anterior hyaloid face as happens after trauma, vitrectomy, and cataract surgery. These cells obstruct the trabecular meshwork and cause secondary open-angle glaucoma.
On slit-lamp examination, khaki-colored cells can be seen in a deep anterior chamber, which may discolor trabecular meshwork. They may settle inferiorly and give the appearance of pseudohypopyon. If there is fresh bleeding, one can see khaki-colored cells over red, lying inferiorly in AC, the so-called candy stripe sign.[49]
- Hemolytic Glaucoma
RBC debris, hemoglobin laden macrophages may block the trabecular meshwork and cause hemolytic glaucoma.
- Hemosiderotic Glaucoma
This entity is very rare. It occurs following recurrent vitreous hemorrhage and is very similar to siderosis following an iron intra-ocular foreign body. The iron released from the RBCs reaches trabecular meshwork and damages the endothelial cells causing sclerosis and obstruction of the meshwork. In addition to raised intraocular pressure (IOP), other signs of ocular siderosis will also be present.
Amblyopia and Myopic Shift- This is noted in infants; hence early vitrectomy is needed to avoid amblyopia and myopic shift.[50]
Deterrence and Patient Education
Vitreous hemorrhage may present with symptoms of floaters, mild haziness, which patients often tend to ignore initially. They usually present when vision has considerably deteriorated, when surgery remains the only option. Early consultation with an ophthalmologist or optometrist may reduce the risk of unnecessary complications and surgery. In cases where observation is decided, the patients need to understand the need for maintaining a strict head end elevated position and be compliant. Very often, the cause is some uncontrolled systemic disease. Educating the patient regarding the need for proper control of disease is necessary.
Enhancing Healthcare Team Outcomes
Good communication between health professionals working as an interprofessional team is necessary for the management of patients with vitreous hemorrhage. As soon as a patient presents with complaints, which can be as minor as floaters, a proper history should be taken. Urgent ophthalmic reference should be given. The medical providers must check the best-corrected visual acuity, pupillary reactions before sending the patient. A retina specialist should be consulted for further management. It has been proven that early management leads to better outcomes in cases with vitreous hemorrhage.[51]
Vitreous hemorrhage is a sign and not a disease per se. To find the cause of VH can be quite a challenge if there are no associated comorbidities. Diabetes mellitus, hypertension, sickle cell anemia, prematurity are a few of the many causes that can lead to vitreous hemorrhage. Hence just treating the vitreous hemorrhage and not managing the cause will not cure it. Hence a multitude of medical disciplines like pediatricians, hematologists, radiologists, neurologists, oncologists are involved in its management. Even various ocular subspecialties like glaucoma, pediatric ophthalmology need close follow-ups. A regular follow up is needed to avoid any future complications.
Trained nursing staff is needed to check the vitals and run the necessary tests for surgical assistance, pre and postoperative care of patients, and to educate the patient and family. A pharmacist is needed in the postoperative period to help patients with appropriate medications and drops. Hence, it cannot be emphasized enough about the need for a good interprofessional team strategy in improving care, coordination, and communication to advance and improve outcomes in cases with vitreous hemorrhage.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Spraul CW, Grossniklaus HE. Vitreous Hemorrhage. Survey of ophthalmology. 1997 Jul-Aug:42(1):3-39 [PubMed PMID: 9265701]
Level 3 (low-level) evidenceDana MR, Werner MS, Viana MA, Shapiro MJ. Spontaneous and traumatic vitreous hemorrhage. Ophthalmology. 1993 Sep:100(9):1377-83 [PubMed PMID: 8371927]
Level 2 (mid-level) evidenceTripathy K. Dissociated optic nerve fiber layer in a case of Terson syndrome. European journal of ophthalmology. 2020 Sep:30(5):NP11-NP14. doi: 10.1177/1120672119853465. Epub 2019 Jun 3 [PubMed PMID: 31155955]
Level 3 (low-level) evidenceCzorlich P, Skevas C, Knospe V, Vettorazzi E, Richard G, Wagenfeld L, Westphal M, Regelsberger J. Terson syndrome in subarachnoid hemorrhage, intracerebral hemorrhage, and traumatic brain injury. Neurosurgical review. 2015 Jan:38(1):129-36; discussion 136. doi: 10.1007/s10143-014-0564-4. Epub 2014 Aug 31 [PubMed PMID: 25173620]
Srinivasan S, Kyle G. Subinternal limiting membrane and subhyaloid haemorrhage in Terson syndrome: the macular 'double ring' sign. Eye (London, England). 2006 Sep:20(9):1099-101 [PubMed PMID: 16227979]
Level 3 (low-level) evidenceGress DR, Wintermark M, Gean AD. A case of Terson syndrome and its mechanism of bleeding. Journal of neuroradiology = Journal de neuroradiologie. 2013 Oct:40(4):312-4. doi: 10.1016/j.neurad.2013.07.003. Epub 2013 Oct 4 [PubMed PMID: 24095292]
Level 3 (low-level) evidenceByer NE, Natural history of posterior vitreous detachment with early management as the premier line of defense against retinal detachment. Ophthalmology. 1994 Sep; [PubMed PMID: 8090453]
Simakurthy S, Tripathy K. Valsalva Retinopathy. StatPearls. 2023 Jan:(): [PubMed PMID: 31424803]
Tripathy K, Chawla R, Vekaria L, Sharma YR. Sub-internal Limiting Membrane Cavity Following Valsalva Retinopathy Resembling Central Serous Chorioretinopathy. Journal of ophthalmic & vision research. 2018 Jan-Mar:13(1):83-84. doi: 10.4103/jovr.jovr_192_16. Epub [PubMed PMID: 29403598]
Wang CY, Cheang WM, Hwang DK, Lin CH. Vitreous haemorrhage: a population-based study of the incidence and risk factors in Taiwan. International journal of ophthalmology. 2017:10(3):461-466. doi: 10.18240/ijo.2017.03.21. Epub 2017 Mar 18 [PubMed PMID: 28393040]
Chew EY, Klein ML, Murphy RP, Remaley NA, Ferris FL 3rd. Effects of aspirin on vitreous/preretinal hemorrhage in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report no. 20. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 Jan:113(1):52-5 [PubMed PMID: 7826294]
Level 1 (high-level) evidenceLindgren G,Sjödell L,Lindblom B, A prospective study of dense spontaneous vitreous hemorrhage. American journal of ophthalmology. 1995 Apr; [PubMed PMID: 7709970]
RIDDLE JM,BARNHART MI, ULTRASTRUCTURAL STUDY OF FIBRIN DISSOLUTION VIA EMIGRATED POLYMORPHONUCLEAR NEUTROPHILS. The American journal of pathology. 1964 Nov; [PubMed PMID: 14223582]
SQUIRE C,MCEWEN WK, The effect of iron compounds on rabbit vitreous. American journal of ophthalmology. 1958 Sep; [PubMed PMID: 13571344]
Level 3 (low-level) evidenceKerman BM,Kreiger AE,Straatsma BR, Resorption of intravitreal blood following vitrectomy. American journal of ophthalmology. 1976 Dec; [PubMed PMID: 826166]
Level 3 (low-level) evidenceForrester JV, Lee WR, Williamson J. The pathology of vitreous hemorrhage. I. Gross and histological appearances. Archives of ophthalmology (Chicago, Ill. : 1960). 1978 Apr:96(4):703-10 [PubMed PMID: 646700]
Level 3 (low-level) evidenceForrester JV, Grierson I, Lee WR. Comparative studies of erythrophagocytosis in the rabbit and human vitreous. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1978 Nov 8:208(1-3):143-58 [PubMed PMID: 310258]
Level 3 (low-level) evidenceGrossniklaus HE, Frank KE, Farhi DC, Jacobs G, Green WR. Hemoglobin spherulosis in the vitreous cavity. Archives of ophthalmology (Chicago, Ill. : 1960). 1988 Jul:106(7):961-2 [PubMed PMID: 3390061]
Level 3 (low-level) evidenceRoizenblatt J,Grant S,Foos RY, Vitreous cylinders. Archives of ophthalmology (Chicago, Ill. : 1960). 1980 Apr; [PubMed PMID: 7369912]
Level 3 (low-level) evidenceThompson JT, Stoessel KM. An analysis of the effect of intravitreal blood on visual acuity. American journal of ophthalmology. 1987 Oct 15:104(4):353-7 [PubMed PMID: 3661644]
Level 1 (high-level) evidenceShukla D,Naresh KB,Kim R, Optical coherence tomography findings in valsalva retinopathy. American journal of ophthalmology. 2005 Jul; [PubMed PMID: 16038658]
Level 3 (low-level) evidence. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981 Jul:88(7):583-600 [PubMed PMID: 7196564]
Level 1 (high-level) evidence. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991 May:98(5 Suppl):741-56 [PubMed PMID: 2062510]
Level 1 (high-level) evidence. Central vein occlusion study of photocoagulation therapy. Baseline findings. Central Vein Occlusion Study Group. The Online journal of current clinical trials. 1993 Oct 14:Doc No 95():[6021 words; 81 paragraphs] [PubMed PMID: 7508322]
Level 1 (high-level) evidenceMcIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007 May:114(5):835-54 [PubMed PMID: 17397923]
Level 1 (high-level) evidenceSaxena S, Jalali S, Verma L, Pathengay A. Management of vitreous haemorrhage. Indian journal of ophthalmology. 2003 Jun:51(2):189-96 [PubMed PMID: 12831156]
Mosier MA, Del Piero E, Gheewala SM. Anterior retinal cryotherapy in diabetic vitreous hemorrhage. American journal of ophthalmology. 1985 Sep 15:100(3):440-4 [PubMed PMID: 4037033]
Ross WH,Gottner MJ, Peripheral retinal cryopexy for subtotal vitreous hemorrhage. American journal of ophthalmology. 1988 Apr 15; [PubMed PMID: 3358430]
Raymond LA. Neodymium:YAG laser treatment for hemorrhages under the internal limiting membrane and posterior hyaloid face in the macula. Ophthalmology. 1995 Mar:102(3):406-11 [PubMed PMID: 7891977]
Level 3 (low-level) evidenceKhadka D,Bhandari S,Bajimaya S,Thapa R,Paudyal G,Pradhan E, Nd:YAG laser hyaloidotomy in the management of Premacular Subhyaloid Hemorrhage. BMC ophthalmology. 2016 Apr 18; [PubMed PMID: 27090882]
Brucker AJ,Michels RG,Green WR, Pars plana vitrectomy in the management of blood-induced glaucoma with vitreous hemorrhage. Annals of ophthalmology. 1978 Oct [PubMed PMID: 718045]
Level 3 (low-level) evidence. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial--Diabetic Retinopathy Vitrectomy Study Report 3. The Diabetic Retinopathy Vitrectomy Study Research Group. Ophthalmology. 1988 Oct:95(10):1307-20 [PubMed PMID: 2465517]
Level 1 (high-level) evidenceSalam A, Mathew R, Sivaprasad S. Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta ophthalmologica. 2011 Aug:89(5):405-11. doi: 10.1111/j.1755-3768.2010.02079.x. Epub 2011 Feb 5 [PubMed PMID: 21294854]
Huang YH,Yeh PT,Chen MS,Yang CH,Yang CM, Intravitreal bevacizumab and panretinal photocoagulation for proliferative diabetic retinopathy associated with vitreous hemorrhage. Retina (Philadelphia, Pa.). 2009 Sep [PubMed PMID: 19672218]
Sun JK, Glassman AR, Beaulieu WT, Stockdale CR, Bressler NM, Flaxel C, Gross JG, Shami M, Jampol LM, Diabetic Retinopathy Clinical Research Network. Rationale and Application of the Protocol S Anti-Vascular Endothelial Growth Factor Algorithm for Proliferative Diabetic Retinopathy. Ophthalmology. 2019 Jan:126(1):87-95. doi: 10.1016/j.ophtha.2018.08.001. Epub 2018 Aug 7 [PubMed PMID: 30096354]
Min WK,Kim YB,Lee KM, Treatment of experimental vitreous hemorrhage with tissue plasminogen activator. Korean journal of ophthalmology : KJO. 1990 Jun; [PubMed PMID: 2120486]
Level 3 (low-level) evidenceSanders D, Peyman GA, Fishman G, Vlchek J, Korey M. The toxicity of intravitreal whole blood and hemoglobin. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1975 Dec 4:197(3):255-67 [PubMed PMID: 813541]
Level 3 (low-level) evidenceZiemianski MC,McMeel JW,Franks EP, Natural history of vitreous hemorrhage in diabetic retinopathy. Ophthalmology. 1980 Apr; [PubMed PMID: 7393537]
A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995 Oct [PubMed PMID: 9097789]
Level 1 (high-level) evidenceHayreh SS, Rubenstein L, Podhajsky P. Argon laser scatter photocoagulation in treatment of branch retinal vein occlusion. A prospective clinical trial. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1993:206(1):1-14 [PubMed PMID: 7506400]
Level 1 (high-level) evidenceClarkson JG, The ocular manifestations of sickle-cell disease: a prevalence and natural history study. Transactions of the American Ophthalmological Society. 1992 [PubMed PMID: 1494832]
Schultz PN, Sobol WM, Weingeist TA. Long-term visual outcome in Terson syndrome. Ophthalmology. 1991 Dec:98(12):1814-9 [PubMed PMID: 1775315]
Level 2 (mid-level) evidenceel Baba F, Jarrett WH 2nd, Harbin TS Jr, Fine SL, Michels RG, Schachat AP, Green WR. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986 Dec:93(12):1581-92 [PubMed PMID: 2433658]
Level 3 (low-level) evidenceGoralska M, Fleisher LN, McGahan MC. Vitreous Humor Changes Expression of Iron-Handling Proteins in Lens Epithelial Cells. Investigative ophthalmology & visual science. 2017 Feb 1:58(2):1187-1195. doi: 10.1167/iovs.16-20610. Epub [PubMed PMID: 28245299]
Wise JB, Treatment of experimental siderosis bulbi, vitreous hemorrhage, and corneal blood staining with deferoxamine. Archives of ophthalmology (Chicago, Ill. : 1960). 1966 May; [PubMed PMID: 5937181]
Level 3 (low-level) evidenceWinter FC. Ocular hemosiderosis. Transactions - American Academy of Ophthalmology and Otolaryngology. American Academy of Ophthalmology and Otolaryngology. 1967 Sep-Oct:71(5):813-9 [PubMed PMID: 4168120]
Tolentino FI, Schepens CL, Freeman HM. Massive preretinal retraction. A biomicroscopic study. Archives of ophthalmology (Chicago, Ill. : 1960). 1967 Jul:78(1):16-22 [PubMed PMID: 6027731]
Cleary PE, Ryan SJ. Experimental posterior penetrating eye injury in the rabbit. I. Method of production and natural history. The British journal of ophthalmology. 1979 May:63(5):306-11 [PubMed PMID: 465404]
Level 3 (low-level) evidenceCampbell DG, Simmons RJ, Grant WM. Ghost cells as a cause of glaucoma. American journal of ophthalmology. 1976 Apr:81(4):441-50 [PubMed PMID: 1266922]
Level 3 (low-level) evidenceMiller-Meeks MJ, Bennett SR, Keech RV, Blodi CF. Myopia induced by vitreous hemorrhage. American journal of ophthalmology. 1990 Feb 15:109(2):199-203 [PubMed PMID: 2301532]
Fassbender JM, Ozkok A, Canter H, Schaal S. A Comparison of Immediate and Delayed Vitrectomy for the Management of Vitreous Hemorrhage due to Proliferative Diabetic Retinopathy. Ophthalmic surgery, lasers & imaging retina. 2016 Jan:47(1):35-41. doi: 10.3928/23258160-20151214-05. Epub [PubMed PMID: 26731207]