Introduction
Visual stimuli from our surroundings are processed by an intricate system of interconnecting neurons, which begins with the optic nerve in the eye and extends to the visual processing center in our forebrain, the visual cortex. All information travels through nerve impulses triggered by photosensitive chemical reactions occurring in the retina. Several separate and parallel pathways code its processing at multiple sites in the nervous system. Disruption in these pathways and their clinical manifestations offers crucial diagnostics for an underlying disease.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The visual system consists of 2 primary parallel pathways: optic and pupillary reflex pathways.[2][3]
Optic Pathway
The optic pathway begins in the retina, a complex structure of 10 layers, each serving a distinct function.
- The photoreceptor layers consist of rods and cones, which generate action potentials through photosensitive cycles with the help of rhodopsin.
- The ganglion cell layer and nerve fiber layer serve as the foundation of the optic nerve; the former contains the cell bodies, and the latter contains the axons as they stream across the retina. It consists of 2 types of fibers, namely temporal and nasal fibers, which control the nasal and temporal parts of the visual field, respectively. These fibers join together at the optic disc and are redirected posteriorly out of the eye to form the orbital part of the optic nerve. The dura surrounds the nerve, a continuation of the brain, allowing free movement of CSF between the eye and the intracranial vault.
- The axons exit the orbit through the orbital foramen, simultaneously with the ophthalmic artery and sympathetic fibers.
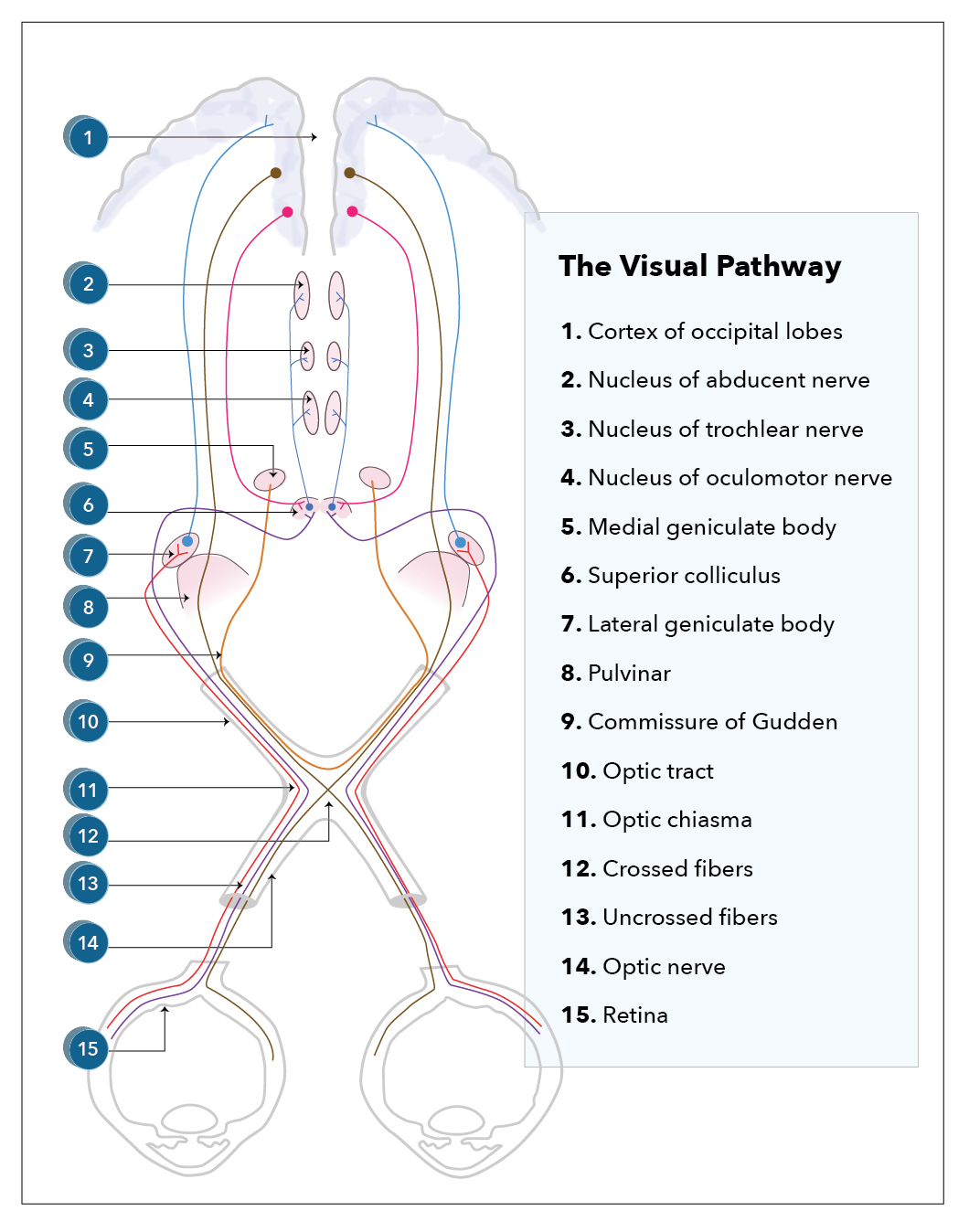
- It then enters the optic canal, a bone-encased tunnel to protect the nerve. It exits into the middle cranial fossa to form the intracranial part of the optic nerve. This continues till the 2 optic nerves join together to form the optic chiasm directly behind and above the pituitary stalk. Here, more than half of the nasal fibers from the left eye decussate to join the temporal fibers of the right eye and form the right optic tract and vice versa. This anomaly helps eye healthcare professionals in the assessment of the site of the lesion along the visual pathway, which produces well-described visual field defects, also known as hemianopias, posterior, or at the chiasm (see Image. Visual Pathway).
- Beyond the chiasm, the pathway continues as 2 distinct tracts, each carrying the temporal fibers from the other eye.
- The optic tract then passes posteriorly, where most of the axons synapse in the layers of the lateral geniculate body (LGB) of the midbrain, which is a posterolateral extension of the thalamus. A minority passes into the superior colliculus and Edinger-Westphal nuclei; these fibers allow for parasympathetic innervation of the pupil, i.e., pupillary constriction.
- The majority of the fibers pass posteriorly to become the genico-calcarine tracts, which have both parietal and temporal loops in the form of the dorsal optic radiation and Meyer's loop and terminate into the cuneus gyrus and lingual gyrus of the primary visual cortex, respectively (Broadmann area number 17).
Pupillary Light Reflex Pathway (parasympathetic innervation pathway)
- The parasympathetic system constricts the pupil to protect itself when light intensity increases to an uncomfortable level by decreasing the amount of light entering the eye.
- The optic nerve directs the afferent limb of the reflex pathway. Light stimulates the retinal ganglionic cells. The impulses travel through the optic nerve (CN II), which projects bilaterally to the midbrain's pretectal nucleus and then to the Edinger-Westphal nucleus.
- The efferent limb is directed by the oculomotor nerve (CN III). The Edinger-Westphal nucleus (preganglionic parasympathetic) relays to the ciliary ganglion (postganglionic sympathetic) via the oculomotor nerve (CN III), which then directs the pupillary sphincter muscle, completing the miotic reflex arc of the pupil.
- One interesting point is that the pretectal nucleus supplies the Edinger-Westphal nucleus bilaterally; a shining light in 1 eye causes ipsilateral and contralateral pupil constriction, known as the consensual light reflex.
The Sympathetic Visual System
- The sympathetic system governs the fight-or-flight response. In the case of vision, mydriasis and elevation of eyelids are the 2 primary responses.
- It originates in the hypothalamus, sending fibers into the cervical spinal cord to synapse through the brainstem in the upper thoracic spinal cord. Second-order neurons then pass out of the spinal cord, enter the thoracic ganglia, and run superiorly until they reach the superior cervical ganglion, where they again synapse. Third-order neurons form a latticework around the internal carotid artery, form the long ciliary nerves on entering the skull, and eventually enter the eye to innervate the pupil dilator and Muller’s muscle, which helps the levator palpebrae superioris to elevate the eyelid.
Conjugate Gaze
- Conjugate gaze is equally important to the visual system, as is the ability to see.
- The lateral rectus performs the abduction of the eye, the primary adductor of the eye, which is innervated by the abducens nerve.
- The adduction of the eye is brought about by the medial rectus muscle, innervated by the oculomotor muscle. Therefore, for right-sided horizontal gaze, both the right abducens nerve and the right lateral rectus muscle must be working to abduct the right eye, and the left oculomotor nerve and the left medial rectus muscle must be patent to adduct the left eye and vice versa.
- This action is controlled in the frontal eye field areas of the frontal lobes. Axons from this region project down to the abducens nucleus of the brainstem, where they synapse and decussate to form the medial longitudinal fasciculus (MLF), which then synapses with the oculomotor nucleus.
Embryology
At week 3 of gestation, the appearance of optic grooves from the developing forebrain marks the first sign of eye development. The optic grooves evaginate as the neural folds fuse, forming the optic vesicle. Subsequently, the optic vesicle invaginates and forms the optic cup at about 4 weeks of gestation; this becomes the retina. The inner and outer layers are due to the invagination process. They form the pigmented and the neural layers, respectively. Axons of the neural layer then proliferate into the optic stalk, causing the lumen to be obliterated; this forms the optic nerve. A multitude of factors, such as chondroitin sulfate proteoglycans (CSPGs), netrin signaling, and slit proteins, have been shown to play a role in guiding optic axons to their target nuclei within the brain.[4][5]
Blood Supply and Lymphatics
The blood supply and lymphatic factors for neuroanatomy of the visual pathway include the following:
- Branches of the internal carotid artery supply the majority of the visual system. The optic nerve's retina and extracranial part receive blood from the ophthalmic artery.
- The intracranial part and optic chiasm receive supply from the anterior cerebral, superior hypophyseal, and anterior communicating arteries.
- The posterior communicating and anterior choroidal arteries perfuse the optic tract.
- The anterior and posterior choroidal arteries supply the lateral geniculate nucleus.
- Both middle and posterior cerebral arteries perfuse otic radiation.
- The posterior cerebral artery primarily supplies the primary visual cortex (Brodmann area 17), with watershed areas processing peripheral information.
- The optic nerve is 1 of the ways followed by the glymphatic system to drain a part of the cerebrospinal fluid.
- The ophthalmic veins drain the orbit's back, top, and bottom. Their congestion and, therefore, their inadequate drainage produce retro-ocular headaches and heavy and pulsating eyes. They pass through the upper orbital and the sphenoid fissure and continue into the cavernous sinus.
Nerves
Cranial nerves III, IV, and VI control the motor output of the eyeball. These nerves supply the extraocular muscles and initiate eye movement. Afferent fibers to the cornea are by the ophthalmic branch of the trigeminal nerve and initiate the corneal reflex. Efferent fibers are by the zygomatic branch of the facial nerve that helps in the motor output of the reflex.
Muscles
All the extra-ocular muscles receive supply from the oculomotor nerve except the lateral rectus, supplied by the abducens nerve, and the superior oblique, supplied by the trochlear nerve. The oculomotor nerve also innervates levator palpebrae superioris.
- The lateral rectus helps in the abduction of the eyeball.
- The medial rectus is responsible for the adduction of the eyeball.
- The superior oblique and superior rectus primarily help in the intorsion of the eyeball.
- Inferior oblique and inferior rectus cause extortion of the eyeball.
- Levator palpebrae superioris elevates the eyelids.
Intrinsic eye muscles are present in the iris, a radial group called the dilator pupillae and a circular group called the sphincter pupillae. They control the dilation and constriction of the pupil, respectively.
Sphincter pupillae receive supply from the short ciliary nerve and help in pupillary constriction. Innervation of the dilator pupillae is done by the sympathetic fibers from the superior cervical ganglion, which helps dilate the pupil.
Physiologic Variants
Individuals vary substantially in the relative sizes of the components of the central visual system. In the blind population, various determinants could influence post-chiasmal visual anatomy. These include differences in the method of braille reading, involvement of the retinal ganglionic cells, and, most importantly, light sensitivity and visual fields.[6] In the path of the optic nerve, there may be anatomical variants. For example, there may be an accessory canal optic of the lesser wing of the sphenoid.
Surgical Considerations
The optic nerve is commonly injured during posterior ethmoidectomy and sphenoid dissection. Visual cortex injury occurs during the resection of tumors and hematomas in the brain matter.[7] Patient head positioning is important in preventing perioperative visual loss (POVL).[8]
Clinical Significance
Visual Field Defects
- Ipsilateral monocular visual loss: This is due to a lesion in the optic nerve, causing complete visual field loss in the ipsilateral eye.
- Bitemporal hemianopia: This can be due to a lesion of the optic chiasm or compression of the optic chiasm, as is seen in pituitary adenomas and craniopharyngiomas disturbing the medial portions of each optic nerve as they cross here. With 1 eye closed, the other eye loses vision in the temporal visual field.
- Unilateral anopia: This is due to a lesion in the optic tract on the side of the anopia.
- Homonymous hemianopia: This is due to a lesion in the optic radiations in the visual cortex on the contralateral side of the anopia
- Homonymous hemianopia with macular sparing: This is due to a posterior cerebral artery (PCA) stroke. The PCA supplies the occipital cortex, where visual processing for the contralateral side occurs. A PCA stroke, therefore, leads to contralateral homonymous hemianopia. The reason the macula is spared is that the macula has a dual blood supply from the middle cerebral artery (MCA) and the posterior cerebral artery.
- Upper quadrantanopia: This can be due to a lesion in the temporal lobe or a middle cerebral artery (MCA) stroke in the contralateral side of the anopia.
- Lower quadrantanopia: This can be due to a lesion in the parietal lobe or an MCA stroke in the contralateral side of the anopia.
- Central scotoma: This defect of central vision occurs in lesions of the macula, such as macular degeneration, cystoid macular edema, and inflammatory macular disease.
The ipsilateral monocular visual loss can be permanent or transient. In the latter case, we speak of "amaurosis fugax" or "transient monocular blindness." Amaurosis fugax is generally due to interruption of blood flow (ischemia) at the level of the optical pathways, for example, caused by retinal embolism or by severe homolateral carotid atheroma stenosis (usually near the common carotid artery bifurcation) or other causes of ischemia in the visual cortex or optic nerve. Possible causes are:
- Retinal embolism
- TIA (transient ischemic attack)
- Cerebrovascular accident
- Traumatic brain injury (eg, falls, motor vehicle collisions, etc)
- Dissection of the internal carotid artery
- Giant cell arteritis
- Emboligenic heart disease
- Coagulopathies
- Retinal migraine
- Carotid artery stenosis
- Inflammatory processes
- Optic atrophy
- Atherosclerosis
- Cerebral ischemia
- Essential thrombocythemia
- Degenerative changes in the optical pathways
- Tumors of the optical or brain pathways
In some cases, amaurosis fugax is idiopathic: it is not possible to highlight the cause of this manifestation, particularly in young subjects. In such cases, a spasm of the central artery of the retina is often thought of as an etiological factor.
Hemianopia or hemianopsia is a visual impairment characterized by the inability to perceive half of the visual field. The disorder can affect 1 eye or both; we can speak of lateral or vertical hemianopsia and superior or inferior hemianopsia (altitudinal or horizontal hemianopsia). The disorder can affect 1 eye or both. There is lateral or vertical hemianopsia and superior or inferior hemianopsia (altitudinal or horizontal hemianopsia). Other definitions include heteronymous bitemporal (loss of the temporal visual field of each eye due to a median lesion of the optic chiasma), binasal heteronymous hemianopsia (the left half of the visual field of the right eye and the right half of the visual field of the left eye is negatively affected due to bilateral lesions affecting both edges of the optic chiasm, which is rare; hemianopia homonymous (loss of the right/left visual field due to an injury to the left/right optic tract); and quadrantanopia (the loss of a single quadrant of the visual field).
The scotoma can be relative or absolute; in the first case, the alteration is related to a decrease in the sensitivity of the retina (one is no longer able to perceive some or all colors except for white), while in the second case, this sensitivity, in some areas, is of the all absent (the image is no longer perceived or in any case, perceived minimally). The disorder can affect 1 or both eyes. The term derives from the Greek ("skotos," darkness, dark). The scotoma can also be negative or positive; in the first case, it is a non-vision area within the visual field (the subject perceives a dark spot on the fixed objects). In the second case, there is the perception of an intermittent bright spot of variable color. A scotoma is generally referred to as a pathological alteration of vision, but it should be specified that there is also a physiological scotoma, the so-called blind spot or blind area of Mariotte; it is a point of the eye where vision is absent, the so-called optical papilla, an area where photoreceptors are absent. Examination of the visual field (campimetry), the scotoma is graphically represented as a black area located centrally or peripherally). Scotoma is 1 of the symptoms of various diseases affecting the functionality of the eye, and the ocular structures involved may be different; the main causes include:
- Macular pathologies
- Retinal detachment
- Cataract
- Glaucoma
- Optic nerve alterations
- Retinal hemorrhages
In some cases, the scotomas are secondary to brain tumors, ischemia, or intoxications; it is 1 of the main manifestations of migraine with aura. In many cases, it is the symptom that precedes the attack. In rare cases, the disorder has an iatrogenic origin. It is caused by taking some medicines (for example, streptomycin). There are other classifications of scotoma: sparkling scotoma (a dark spot surrounded by small colored stripes with intermittent brightness); central scotoma (a dark-colored spot in the center of vision and can be a senile maculopathy, an infectious or inflammatory process); central-cecal scotoma (affects both the central fixation point and the blind spot; normally the area involved is small but then tends to grow); peripheral scotoma; paracentral scotoma; annular scotoma; Bjerrum scotoma (the blind area has an arched shape).
Pituitary Adenomas: Pituitary adenomas are benign growths of the pituitary gland. The pituitary gland sits in the sella turcica, directly inferior to the optic chiasm. Pituitary adenomas can be functioning (producing hormones) or non-functioning (not producing hormones). Some examples of functioning pituitary adenomas can lead to hyperprolactinemia, acromegaly, or Cushing disease. Non-functioning pituitary adenomas often lead to a mass effect. They can compress the optic chiasm, leading to bitemporal hemianopia and headache.
Glaucoma: Glaucoma is a disease characterized by the degeneration of the optic nerve. The classic finding is optic disk atrophy with cupping, which means the outer portion of the optic nerve is thinning. The most common cause of glaucoma is an increase in intraocular pressure (IOP); however, a high IOP is not necessary to diagnose it.
Vigabatrin: Vigabatrin is an anti-epileptic drug used to treat refractory, complex partial seizures in adults who have failed. Vigabatrin has been shown to cause permanent peripheral visual field loss. Although the mechanism for how this happens is not fully understood, it most likely involves the toxicity of both retinal photoreceptors and ganglion cells.[9][10]
Other Issues
A capsule of connective tissue fuses with the optic sheath posteriorly and then meets the intermuscular septum anteriorly, forming an envelope. This structure is known as the capsule of tenon. It helps the globe to stay positioned in orbit.[11]
Media
References
Celesia GG, DeMarco PJ Jr. Anatomy and physiology of the visual system. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1994 Sep:11(5):482-92 [PubMed PMID: 7844239]
Level 3 (low-level) evidenceDe Moraes CG. Anatomy of the visual pathways. Journal of glaucoma. 2013 Jun-Jul:22 Suppl 5():S2-7. doi: 10.1097/IJG.0b013e3182934978. Epub [PubMed PMID: 23733119]
Kelts EA. The basic anatomy of the optic nerve and visual system (or, why Thoreau was wrong). NeuroRehabilitation. 2010:27(3):217-22. doi: 10.3233/NRE-2010-0600. Epub [PubMed PMID: 21098989]
Level 3 (low-level) evidenceBales TR, Lopez MJ, Clark J. Embryology, Eye. StatPearls. 2023 Jan:(): [PubMed PMID: 30860715]
Reese BE. Development of the retina and optic pathway. Vision research. 2011 Apr 13:51(7):613-32. doi: 10.1016/j.visres.2010.07.010. Epub 2010 Jul 18 [PubMed PMID: 20647017]
Level 3 (low-level) evidenceAguirre GK, Datta R, Benson NC, Prasad S, Jacobson SG, Cideciyan AV, Bridge H, Watkins KE, Butt OH, Dain AS, Brandes L, Gennatas ED. Patterns of Individual Variation in Visual Pathway Structure and Function in the Sighted and Blind. PloS one. 2016:11(11):e0164677. doi: 10.1371/journal.pone.0164677. Epub 2016 Nov 3 [PubMed PMID: 27812129]
Huff T, Mahabadi N, Tadi P. Neuroanatomy, Visual Cortex. StatPearls. 2024 Jan:(): [PubMed PMID: 29494110]
Roth S. Perioperative visual loss: what do we know, what can we do? British journal of anaesthesia. 2009 Dec:103 Suppl 1(Suppl 1):i31-40. doi: 10.1093/bja/aep295. Epub [PubMed PMID: 20007988]
Bruni J, Guberman A, Vachon L, Desforges C. Vigabatrin as add-on therapy for adult complex partial seizures: a double-blind, placebo-controlled multicentre study. The Canadian Vigabatrin Study Group. Seizure. 2000 Apr:9(3):224-32 [PubMed PMID: 10777431]
Level 1 (high-level) evidenceMcCoy B, Wright T, Weiss S, Go C, Westall CA. Electroretinogram changes in a pediatric population with epilepsy: is vigabatrin acting alone? Journal of child neurology. 2011 Jun:26(6):729-33. doi: 10.1177/0883073810390213. Epub 2011 Feb 22 [PubMed PMID: 21343605]
Shumway CL, Motlagh M, Wade M. Anatomy, Head and Neck, Eye Extraocular Muscles. StatPearls. 2024 Jan:(): [PubMed PMID: 30137849]