Introduction
Over the past 2 decades, video-assisted thoracoscopic surgery (VATS) has transformed the approach and treatment of various pulmonary, esophageal, and cardiac conditions. Initially introduced by Swedish physician Jacobeaus in 1912 for evaluating and managing pleural effusions in patients with tuberculosis, VATS has since evolved significantly.[1] A pivotal advancement was the introduction of fiber-optic light, which paved the way for safer, minimal-access surgeries.
Over time, VATS has become increasingly favored over traditional thoracotomy, offering benefits such as reduced postoperative pain, shorter hospital stays, and faster recovery of respiratory function—particularly beneficial for elderly or frail patients and those with chronic pulmonary conditions like chronic obstructive pulmonary disease. These advancements have expanded the scope of VATS applications and improved patient outcomes while lowering healthcare costs.[2][3][4]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
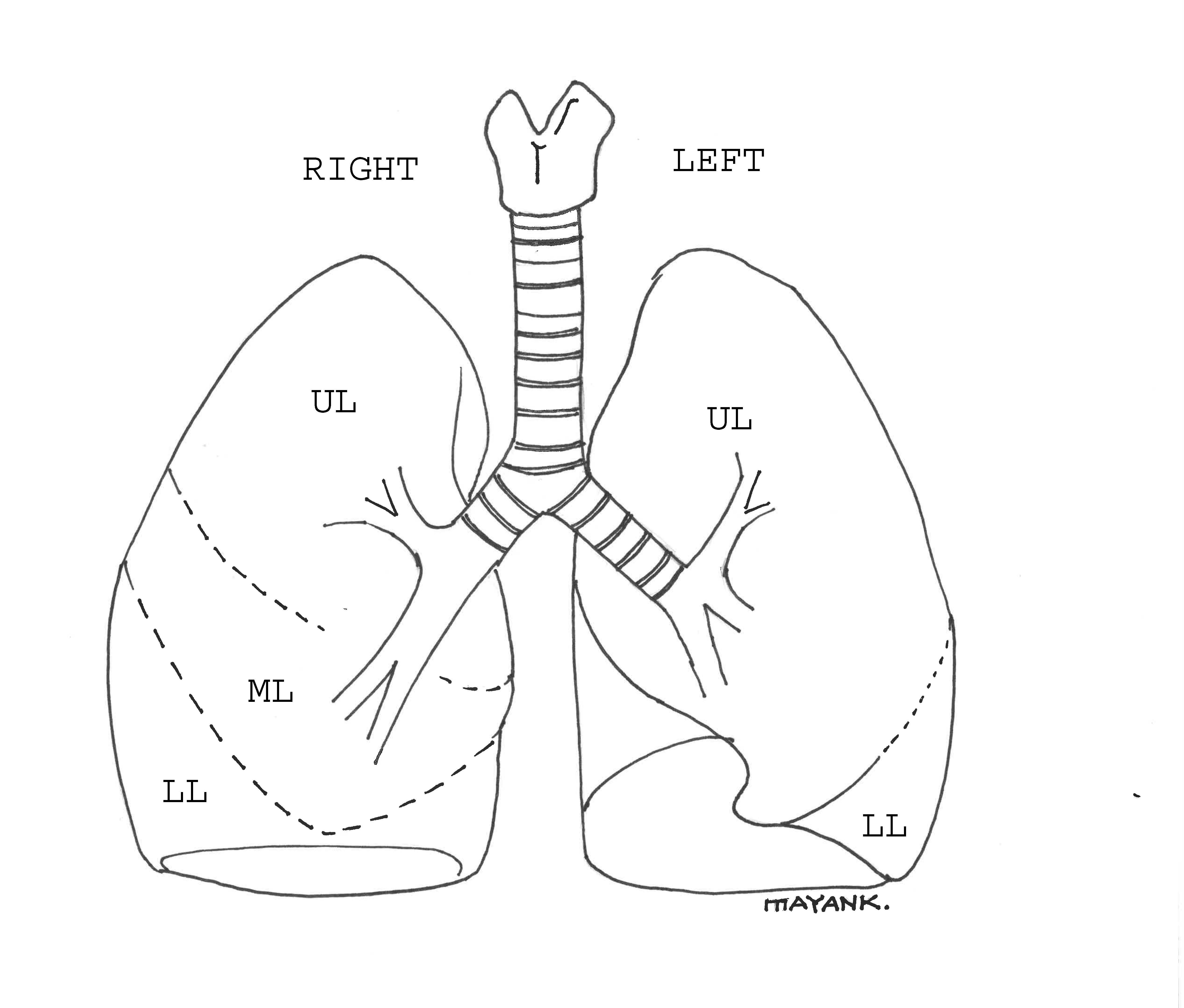
The adult trachea measures approximately 15 cm in length, starting from the lower edge of the cricoid cartilage at the level of C6, and divides into the right and left main bronchi around the T5 level. The right bronchus, broader and more aligned with the trachea, branches into upper, middle, and lower lobe segments, while the left bronchus, more horizontally oriented, divides into upper and lower segments (see Image. Trachea and Lung Lobes, Illustration). These segments are further divided into bronchopulmonary segments, each supplied by a segmental artery and bronchus, with veins between them. Surgeons performing VATS must be cognizant of these lung surgical units. Additionally, VATS-related procedures involve other mediastinal structures such as the esophagus, pleura, diaphragm, pericardium, thymus, sympathetic chain, thoracic duct, spine, and heart.
Indications
The following are diagnostic and therapeutic indications for VATS:
Diagnostic
- Mediastinal lymph node biopsy
- Pleuroscopy with or without pleural biopsy
- Tissue or lymph node biopsy for lung cancer
- Chest wall biopsy
- Cancer staging
Therapeutic
- Pulmonary resection (most common for lung cancer; eg, lobectomy, segmentectomy, and wedge resection)
- Pulmonary bleb and bullae resection
- Pleural drainage (eg, pneumothorax, hemothorax, and empyema)
- Pericardial effusion drainage
- Mechanical and chemical pleurodesis
- Excision or biopsy of mediastinal masses (eg, thymectomy) and nodules
- Thoracic duct ligation
- Sympathectomy
- Chest wall tumor resection
- Thoracoscopic laminectomy
- Spinal abscess drainage
- Esophagectomy
- Esophageal cyst removal
- Hiatal hernia
- Diaphragmatic procedures (eg, plication)
- Chest trauma (eg, diaphragm injuries)
- Truncal vagotomy
- Epicardial lead placement for pacemaker
Contraindications
Absolute contraindications to VATS include:
- Inability to tolerate single lung isolation ventilation
- Intraluminal airway mass (making double-lumen tube placement difficult or impossible)
- Severe adhesions in the pleural space
- Hemodynamic instability
- Prior talc pleurodesis
Relative contraindications to VATS include:
- Previous thoracotomies
- Prior radiation for thoracic malignancy
- Severe hypoxia
- Severe chronic obstructive pulmonary disease
- Severe pulmonary hypertension
- Coagulopathy
Equipment
The following equipment is used to perform a VATS:
- 5 or 10 mm high-resolution fiber-optic thoracoscope with a 0° or 30° lens
- A light source with a cable
- Video monitors
- Thoracoscopic instruments (scissors, hook or straight-blade cautery, biopsy forceps, grasper, and dissector)
- Endoscopic stapler if resection is planned
- Trocars
- Thoracotomy tray (to convert to open procedure if needed)
- Chest tubes and drainage devices
- Sterile gloves, gowns, and drapes
- Double-lumen endotracheal tube or single-lumen tube with bronchial blockers
Personnel
The following personnel are needed during a VATS:
- Surgeon
- Assistant
- Anesthesiologist
- Circulating nurse
- Surgical technologist
Preparation
Preoperative Evaluation
Patient selection significantly influences surgical outcomes, particularly in procedures requiring 1-lung ventilation. A thorough preoperative assessment, emphasizing cardiac and respiratory function, is crucial to identify candidates capable of tolerating 1-lung ventilation. This assessment includes evaluating the American Society of Anesthesiologists (ASA) physical status, spirometry, plethysmography, diffusing capacity of the lungs for carbon monoxide (DLCO) measurement, computed tomography (CT) imaging, and cardiopulmonary exercise testing. These comprehensive evaluations help determine the patient's suitability for the procedure and mitigate the risk of perioperative complications, ensuring optimal surgical outcomes.
Preoperative assessment evaluates lung mechanics, parenchymal function, and cardiopulmonary reserve to determine a patient's suitability for surgery. Lung mechanics, including forced expiratory volume in 1 second (FEV1), maximum voluntary ventilation, forced vital capacity, and residual volume/total lung capacity (RV/TLC) ratio, provide insights into respiratory function. An FEV1 greater than 60% indicates potential tolerance for anatomic lobe resection. An FEV1 of less than 30% suggests postoperative ventilator or supplemental oxygen dependence.[5] In FEV1s less than 60%, a ventilation-perfusion scan (VQ scan) may calculate a postoperative FEV1, with values above 35% (and up to 40%), suggesting adequate postoperative pulmonary reserve. DLCO, measuring gas diffusion, ideally exceeds 40% for surgery. Cardiopulmonary exercise testing assesses overall reserve, with a maximum rate of oxygen (O2) your body can use during exercise (VO2) greater than 10 mL/min/kg, indicating suitability for surgery. This comprehensive approach ensures optimal patient outcomes by preoperatively assessing respiratory and cardiovascular function.[6]
Preoperative assessment for VATS also involves evaluating blood counts for indications of polycythemia or infection. Chest x-rays and CT scans provide anatomical details for surgical planning. Arterial blood gases identify patients at risk for postoperative complications; a partial pressure of carbon dioxide (CO2) more than 50 mm Hg or a partial pressure of O2 less than 60 mm Hg indicates vulnerability. Optimization strategies may include smoking cessation, addressing infections, and pulmonary rehabilitation to enhance surgical outcomes. This comprehensive approach ensures patient readiness and minimizes risks associated with VATS procedures.
Operating Room Setup
The operating room setup for VATS includes monitors on each side of the table, with an extra screen for academic and staff viewing. The surgeon and assisting surgeon stand anteriorly, while the anesthesiologist is at the head of the bed. The scrub nurse stands opposite the assistant surgeon. This configuration allows all personnel in the operating room to visualize the procedure effectively.
The surgery typically begins with the patient positioned in a full lateral decubitus posture tailored to the side of the operation or in a supine position with appropriate support to access the chest. Incisions are made in the intercostal spaces parallel to their long axis, ensuring they are centered to avoid intercostal nerve injury. Once the camera is inserted, additional ports are placed under direct visualization.
Technique or Treatment
Patient Positioning and Ventilation
In VATS procedures, patients are typically positioned in the lateral decubitus position, facilitating rib separation and access to thoracic structures. Care is taken to avoid nerve injury through proper padding.
One-lung ventilation under anesthesia is standard practice during VATS. To ensure adequate oxygenation and maintain carbon dioxide levels akin to 2-lung ventilation, neuromuscular blockade, and controlled ventilation are essential during 1-lung ventilation. Optimal minute ventilation is achieved with lower tidal volumes (5-7 mL/kg) and increased respiratory rates, with peak inflation pressure ideally kept below 35 cm H20.[7][8] One-lung ventilation facilitates surgical access to the hemithorax by collapsing the nondependent lung, resulting in an intrapulmonary right-to-left shunt, widening the alveolar-arterial oxygen gradient and potentially causing hypoxemia.
The most common reason for hypoxemia in 1-lung ventilation is shunting, with its incidence having been reported to be up to 5%.[9] The lateral decubitus position, general anesthesia, mechanical ventilation, neuromuscular blockade, and surgical retraction significantly impact lung physiology during VATS procedures. Oxygenation relies on blood flow to the nonventilated lung, which is affected by hypoxic pulmonary vasoconstriction and reduces intrapulmonary shunting. Hypoxemia may necessitate increasing the inspired oxygen fraction (FiO2) or reverting to 2-lung ventilation. Positive end-expiratory pressure (PEEP) applied to the ventilated lung aids oxygenation, but careful titration is crucial to prevent perfusion compromise. Continuous positive airway pressure (CPAP) avoidance is recommended to maintain surgical access.
The techniques employed to achieve 1-lung ventilation encompass various approaches, including double-lumen tubes, single-lumen tubes with integrated bronchial blockers or separate bronchial blockers, and apneic oxygenation or high-frequency positive-pressure ventilation. Each method offers unique advantages and considerations, catering to the patient's needs and the surgical procedure's requirements.
Utilizing double-lumen endotracheal tubes offers the advantage of ventilating each lung independently or simultaneously during 1-lung ventilation. Proper double-lumen tube placement demands a specialized technique, ideally confirmed via fiberoptic bronchoscopy. Given their susceptibility to malpositioning and displacement, the tube position should be reassessed after final patient positioning for the procedure and if any changes in the patient's oxygen or carbon dioxide levels (or hemodynamics) occur.
Single-lumen endotracheal tubes equipped with bronchial blockers are an alternative to double-lumen tubes. Bronchial blockers with a high-volume/low-pressure cuff are inserted via fiberoptic visualization. One key advantage of using single-lumen tubes with bronchial blockers is their potential to remain in place after the procedure if the patient requires ongoing intubation. Like double-lumen tubes, proper positioning of bronchial blockers must be reaffirmed after placing the patient in the lateral decubitus position to ensure optimal ventilation during surgery.
Alternatively, apneic oxygenation or high-frequency positive-pressure ventilation can be employed for thoracic surgeries. However, their usage is limited due to potential drawbacks, including progressive respiratory acidosis and interference with surgery due to mediastinal bounce. While these techniques may offer specific advantages in certain scenarios, carefully considering their limitations is essential to ensure patient safety and procedural efficacy.
The most common cause of hypoxemia during 1-lung ventilation is shunting, with an incidence reported to be up to 5%. When hypoxemia occurs, a systematic evaluation of the anesthesia equipment and circuit should be conducted as the initial step. Increasing FiO2 to 100% and suctioning the dependent lung is recommended to manage hypoxemia. Additionally, recruitment maneuvers followed by applying PEEP on the ventilated lung can help improve oxygenation. If hypoxemia persists despite these maneuvers, the anesthesiologist may consider reverting to 2-lung ventilation. Again, CPAP should be avoided whenever possible, as it can complicate surgery on the operative side.
Surgical Technique
The standard VATS procedure typically involves making 3 to 4 incisions arranged in a triangular configuration to allow for scope and instrument insertion.[10] Alternatively, VATS with a single port has also been described.[11]
Initially, the patient is supine, and anesthesia is administered. A double-lumen endotracheal tube is commonly used for airway management. Once placed, its position is confirmed using a fiberoptic bronchoscope to ensure proper cuff placement. Following confirmation, the patient is placed in the lateral decubitus position with their arm positioned overhead. The operating table may be flexed to facilitate surgical exposure. After final positioning for the procedure, the double-lumen tube position is rechecked.
Three to 4 working incisions are typically made for the anterior approach, forming a triangular configuration with the utility incision at the apex. The camera is inserted through this incision to create additional entry ports safely. One port is created in the auscultatory triangle to accommodate the camera, while a third port is made in the midaxillary line at the level of the utility port incision.
Once the ports are established, the procedure progresses under the guidance of the video thoracoscope. Subsequent steps vary based on the specific surgery being performed. After the surgery, 1 or 2 pleural drains connected to an underwater seal drain are usually placed, depending on the procedure.
Postoperative Care
Postoperative management following VATS includes several key aspects to ensure optimal patient recovery and outcomes. They are:
- Pain management: Adequate pain control is essential to minimize discomfort and facilitate early mobilization and recovery. A multimodal approach often combines systemic analgesics, regional techniques, like epidural analgesia or intercostal nerve blocks, and nonpharmacological interventions, such as breathing exercises and positioning. Tailoring the pain management regimen to the patient's needs helps optimize pain relief while minimizing side effects.
- Respiratory care: Respiratory support prevents postoperative pulmonary complications and promotes lung expansion. Early mobilization, incentive spirometry, deep breathing exercises, and coughing techniques are encouraged to maintain lung function and avoid atelectasis. Continuous monitoring of respiratory status, including oxygen saturation and respiratory rate, allows for prompt identification and management of any respiratory issues.
- Chest tube management: Chest tubes are commonly placed during VATS procedures to drain air or fluid from the pleural space. Proper management of chest tubes involves monitoring drainage output, assessing for air leaks, and ensuring appropriate suction settings. Chest tube removal is typically guided by clinical parameters such as decreased drainage output and the absence of air leaks on a water seal. Careful observation for signs of pneumothorax or pleural effusion post-removal is crucial.
- Fluid therapy: Restrictive fluid therapy is often employed to minimize the risk of fluid overload and related complications. Fluid management strategies aim to maintain adequate intravascular volume while avoiding excessive fluid administration, which can contribute to pulmonary edema and other postoperative complications.
- Monitoring and surveillance: Close monitoring of vital signs, pain scores, fluid balance, and laboratory parameters (eg, electrolytes and blood counts) is essential during the postoperative period. Regular clinical assessments help detect and address any complications promptly.
- Early ambulation and rehabilitation: Encouraging early ambulation and participation in physical therapy and rehabilitation programs promotes faster recovery and reduces the risk of complications such as venous thromboembolism and deconditioning.
A comprehensive and individualized approach to postoperative management is critical to optimizing outcomes and promoting patient recovery following VATS procedures. Collaboration among the surgical team, anesthesia providers, nursing staff, and other healthcare professionals is essential to ensure coordinated and effective care.
Complications
The following are complications that can be associated with VATS:
- Postoperative air leak
- Postoperative pain
- Hypoxemia
- Atelectasis
- Bleeding
- Wound infection
- Postoperative reexpansion pulmonary edema
Bleeding due to vascular injury represents a critical complication during VATS for major pulmonary resection, necessitating emergent conversion to open thoracotomy. Surgeons, anesthesiologists, and the operating room team must remain vigilant and prepared to transition to an open procedure if vascular injury occurs swiftly. Establishing adequate intravenous access before commencing VATS procedures is crucial to facilitate prompt intervention in case of emergent conversion. While VATS with conversion and open thoracotomy yield similar early postoperative morbidity and mortality rates, VATS remains the preferred approach due to its associated benefits, even in cases requiring intraoperative conversion. Although conversion to open thoracotomy may be necessary to address vascular injury, VATS offers potential advantages, and patients may still benefit from a fully VATS-based resection despite conversion.[12]
In cases where one-lung ventilation cannot be maintained adequately, conversion to open thoracotomy may be necessary to ensure optimal ventilation and oxygenation. Extensive pleural adhesions can impede the visualization and manipulation of structures, making it challenging to complete the procedure safely and effectively via VATS alone. If the intended goal of the operation cannot be achieved adequately due to technical limitations or inadequate exposure, conversion to open thoracotomy allows for better visualization and access to the target area. Additionally, failure of video equipment during VATS procedures may warrant conversion to an open approach to ensure continued surgical progress and completion of the operation.
Clinical Significance
VATS has emerged as the preferred approach for thoracic surgeries, gradually replacing open thoracotomies in many centers worldwide due to its numerous clinical advantages. One of the most significant benefits of VATS is its safety profile, particularly in older patients, offering reduced perioperative morbidity and mortality compared to traditional open procedures. VATS also provides superior pain control, leading to faster recovery and shorter hospital stays.[13] Studies have consistently shown decreased length of hospital stay (mainly attributed to the shorter chest tube duration) and reduced rates of complications, such as postoperative bleeding and in-hospital mortality, in patients undergoing VATS compared to open thoracotomies.[14][15][16] Furthermore, VATS results in fewer blood transfusions, less postoperative pain, and improved quality of life for patients, contributing to its growing acceptance and utilization.[17][18][19]
Despite the initial higher cost associated with VATS procedures, the overall cost-effectiveness for the hospital system is enhanced due to shorter hospital stays and reduced rates of complications, making it an economically viable option.[20] Long-term survival outcomes are comparable between VATS and open procedures, with no statistically significant differences observed in overall 3-year survival rates among patients undergoing VATS lobectomy compared to open lobectomy.[21] Therefore, VATS has become the recommended standard of care for lobectomies, endorsed by a consensus of experts in thoracic surgery.[22] Additionally, VATS segmentectomy has demonstrated favorable outcomes, including fewer postoperative complications and shorter hospital stays, further supporting its preference over traditional thoracotomy.[23] VATS esophagectomy has also shown promising results regarding oncologic outcomes, safety, and effective surgical techniques, indicating its potential as a viable alternative to open esophagectomy.[24][25][26][27] Overall, the clinical significance of VATS lies in its ability to provide safer, more efficient, and cost-effective surgical interventions for a wide range of thoracic conditions, ultimately improving patient outcomes and quality of care.
Enhancing Healthcare Team Outcomes
Effective patient-centered care and optimal outcomes in video-assisted thoracoscopic surgery require a multidisciplinary approach with seamless coordination among various healthcare professionals. Physicians, advanced practitioners, nurses, pharmacists, and other team members play integral roles in ensuring the success of VATS procedures. Skillful execution of VATS demands proficiency in surgical techniques, anesthesia management, and perioperative care. Surgeons must possess advanced technical skills in performing minimally invasive procedures, while anesthesiologists and nurse anesthetists should be adept at managing 1-lung ventilation and ensuring hemodynamic stability throughout the surgery. Nurses are vital in patient preparation, intraoperative assistance, and postoperative monitoring. They provide keen attention to detail and effective communication with the surgical team.
Interprofessional communication is paramount in VATS to facilitate seamless coordination and enhance patient safety. Clear and concise communication among team members ensures alignment with the surgical plan, patient status, and any emergent concerns. Pharmacists play a crucial role in medication management, ensuring appropriate preoperative prophylaxis and postoperative pain management. Furthermore, collaborative care coordination ensures smooth transitions between preoperative, intraoperative, and postoperative phases, minimizing the risk of errors and optimizing patient outcomes. By fostering collaboration, mutual respect, and open communication, healthcare professionals can enhance team performance and provide patient-centered care that prioritizes safety and efficiency in VATS procedures.
Media
(Click Image to Enlarge)
References
Luh SP, Liu HP. Video-assisted thoracic surgery--the past, present status and the future. Journal of Zhejiang University. Science. B. 2006 Feb:7(2):118-28 [PubMed PMID: 16421967]
Bravo Iñiguez CE, Armstrong KW, Cooper Z, Weissman JS, Ducko CT, Wee JO, Martinez MP, Bueno R, Jaklitsch MT, Wiener DC. Thirty-Day Mortality After Lobectomy in Elderly Patients Eligible for Lung Cancer Screening. The Annals of thoracic surgery. 2016 Feb:101(2):541-6. doi: 10.1016/j.athoracsur.2015.08.067. Epub 2015 Oct 23 [PubMed PMID: 26603020]
Villamizar NR, Darrabie MD, Burfeind WR, Petersen RP, Onaitis MW, Toloza E, Harpole DH, D'Amico TA. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. The Journal of thoracic and cardiovascular surgery. 2009 Aug:138(2):419-25. doi: 10.1016/j.jtcvs.2009.04.026. Epub [PubMed PMID: 19619789]
Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, Stiles BM, Port JL, D'Amico TA. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. The Journal of thoracic and cardiovascular surgery. 2010 Feb:139(2):366-78. doi: 10.1016/j.jtcvs.2009.08.026. Epub [PubMed PMID: 20106398]
Mazzone PJ, Arroliga AC. Lung cancer: Preoperative pulmonary evaluation of the lung resection candidate. The American journal of medicine. 2005 Jun:118(6):578-83 [PubMed PMID: 15922686]
Roy PM. Preoperative pulmonary evaluation for lung resection. Journal of anaesthesiology, clinical pharmacology. 2018 Jul-Sep:34(3):296-300. doi: 10.4103/joacp.JOACP_89_17. Epub [PubMed PMID: 30386009]
Schultz MJ, Haitsma JJ, Slutsky AS, Gajic O. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007 Jun:106(6):1226-31 [PubMed PMID: 17525599]
Blank RS, Colquhoun DA, Durieux ME, Kozower BD, McMurry TL, Bender SP, Naik BI. Management of One-lung Ventilation: Impact of Tidal Volume on Complications after Thoracic Surgery. Anesthesiology. 2016 Jun:124(6):1286-95. doi: 10.1097/ALN.0000000000001100. Epub [PubMed PMID: 27011307]
Fukuhara K, Akashi A, Nakane S, Tomita E. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2008 Oct:34(4):875-7. doi: 10.1016/j.ejcts.2008.07.014. Epub 2008 Aug 15 [PubMed PMID: 18703345]
Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Annals of cardiothoracic surgery. 2012 May:1(1):70-6. doi: 10.3978/j.issn.2225-319X.2012.04.15. Epub [PubMed PMID: 23977470]
Bedetti B, Scarci M, Gonzalez-Rivas D. Technical steps in single port video-assisted thoracoscopic surgery lobectomy. Journal of visualized surgery. 2016:2():45. doi: 10.21037/jovs.2016.02.18. Epub 2016 Mar 14 [PubMed PMID: 29078473]
Fourdrain A, De Dominicis F, Iquille J, Lafitte S, Merlusca G, Witte-Pfister A, Meynier J, Bagan P, Berna P. Intraoperative conversion during video-assisted thoracoscopy does not constitute a treatment failure†. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2019 Apr 1:55(4):660-665. doi: 10.1093/ejcts/ezy343. Epub [PubMed PMID: 30325413]
Al-Ameri M, Bergman P, Franco-Cereceda A, Sartipy U. Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study. Journal of thoracic disease. 2018 Jun:10(6):3499-3506. doi: 10.21037/jtd.2018.05.177. Epub [PubMed PMID: 30069346]
Wolf A, Liu B, Leoncini E, Nicastri D, Lee DS, Taioli E, Flores R. Outcomes for Thoracoscopy Versus Thoracotomy Not Just Technique Dependent: A Study of 9,787 Patients. The Annals of thoracic surgery. 2018 Mar:105(3):886-891. doi: 10.1016/j.athoracsur.2017.09.059. Epub 2018 Feb 1 [PubMed PMID: 29397101]
Berfield KS, Farjah F, Mulligan MS. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer. The Annals of thoracic surgery. 2019 Feb:107(2):603-609. doi: 10.1016/j.athoracsur.2018.07.088. Epub 2018 Sep 29 [PubMed PMID: 30278164]
Gao HJ, Jiang ZH, Gong L, Ma K, Ren P, Yu ZT, Wei YC. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. The Annals of thoracic surgery. 2019 Oct:108(4):1072-1079. doi: 10.1016/j.athoracsur.2019.04.037. Epub 2019 Jun 1 [PubMed PMID: 31163131]
Mohajerzadeh L, Lotfollahzadeh S, Vosoughi A, Harirforoosh I, Parsay S, Amirifar H, Farahbakhsh N, Atqiaee K. Thoracotomy versus Video-Assisted Thoracoscopy in Pediatric Empyema. The Korean journal of thoracic and cardiovascular surgery. 2019 Jun:52(3):125-130. doi: 10.5090/kjtcs.2019.52.3.125. Epub 2019 Jun 5 [PubMed PMID: 31236371]
Level 2 (mid-level) evidenceZhang G, Fan J, Yu Z, Chai Y, Zhang S, Wu M, Shen G. Video-assisted thoracoscopic treatment as two-day surgery for lung neoplasms: a propensity-matched analysis. BMC cancer. 2022 Jul 30:22(1):832. doi: 10.1186/s12885-022-09938-x. Epub 2022 Jul 30 [PubMed PMID: 35907842]
Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. The Lancet. Oncology. 2016 Jun:17(6):836-844. doi: 10.1016/S1470-2045(16)00173-X. Epub 2016 May 6 [PubMed PMID: 27160473]
Level 2 (mid-level) evidenceChen W, Yu Z, Zhang Y, Liu H. Comparison of cost effectiveness between video-assisted thoracoscopic surgery (vats) and open lobectomy: a retrospective study. Cost effectiveness and resource allocation : C/E. 2021 Aug 28:19(1):55. doi: 10.1186/s12962-021-00307-2. Epub 2021 Aug 28 [PubMed PMID: 34454507]
Level 2 (mid-level) evidencePaul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ (Clinical research ed.). 2014 Oct 2:349():g5575. doi: 10.1136/bmj.g5575. Epub 2014 Oct 2 [PubMed PMID: 25277994]
Level 2 (mid-level) evidenceYan TD, Cao C, D'Amico TA, Demmy TL, He J, Hansen H, Swanson SJ, Walker WS, International VATS Lobectomy Consensus Group. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2014 Apr:45(4):633-9. doi: 10.1093/ejcts/ezt463. Epub 2013 Oct 14 [PubMed PMID: 24130372]
Level 3 (low-level) evidenceSabra MJ, Alwatari Y, Bierema C, Wolfe LG, Cassano AD, Shah RD. Five-Year Experience with VATS Versus Thoracotomy Segmentectomy for Lung Tumor Resection. Innovations (Philadelphia, Pa.). 2020 Jul/Aug:15(4):346-354. doi: 10.1177/1556984520938186. Epub 2020 Jul 27 [PubMed PMID: 32718194]
Nachira D, Congedo MT, Calabrese G, Tabacco D, Petracca Ciavarella L, Meacci E, Vita ML, Punzo G, Lococo F, Raveglia F, Chiappetta M, Porziella V, Guttadauro A, Cioffi U, Margaritora S. Uniportal-VATS vs. open McKeown esophagectomy: Surgical and long-term oncological outcomes. Frontiers in surgery. 2023:10():1103101. doi: 10.3389/fsurg.2023.1103101. Epub 2023 Feb 27 [PubMed PMID: 36923380]
Shen C, Li J, Che G. Video-Assisted Thoracic Surgery vs. Thoracotomy for the Treatment in Patients With Esophageal Leiomyoma: A Systematic Review and Meta-Analysis. Frontiers in surgery. 2021:8():809253. doi: 10.3389/fsurg.2021.809253. Epub 2022 Jan 11 [PubMed PMID: 35087862]
Level 1 (high-level) evidenceIguchi K, Kunisaki C, Sato S, Tanaka Y, Miyamoto H, Kosaka T, Sato K, Akiyama H, Endo I, Yukawa N, Rino Y, Masuda M, Yamanaka T. Efficacy of Video-assisted Thoracoscopic Esophagectomy for Stage II/III Esophageal Cancer: Analysis Using the Propensity Scoring System. Anticancer research. 2020 Mar:40(3):1587-1595. doi: 10.21873/anticanres.14106. Epub [PubMed PMID: 32132061]
Nayar R, Varshney V, Suman S, Soni S, Kumar N. Thoracolaparoscopic-Assisted Esophagectomy for Corrosive-Induced Esophageal Stricture. Cureus. 2020 May 1:12(5):e7909. doi: 10.7759/cureus.7909. Epub 2020 May 1 [PubMed PMID: 32494524]